Abstract
Aldehyde dehydrogenase 1 (ALDH1) has been identified as a breast cancer stem cell marker, but its value as a predictor of prognosis and chemoresistance is controversial. This study investigated the effect of ALDH1 on prognosis and chemoresponse by breast cancer subtype. We immunohistochemically analyzed 653 invasive breast cancer specimens and evaluated correlations among clinicopathological factors, survival status, response to neoadjuvant chemotherapy, and ALDH1 expression. Of 653 specimens, 139 (21.3 %) expressed ALDH1 in tumor cells. ALDH1 expression was correlated significantly with larger tumor size, node metastasis, higher nuclear grade, and with HER2+ and progesterone/estrogen receptor (HR)− subtypes. ALDH1 expression was significantly observed in HER2 type and triple-negative breast cancer (TNBC). Patients with ALDH1+ cancers had significantly shorter disease-free survival (P < 0001) and overall survival (P = 0.044). ALDH1 expression significantly affected prognosis of luminal types, but not TNBC and HER2-enriched types. For the 234 patients treated with neoadjuvant chemotherapy, pathological complete response (pCR) rate was significantly lower in ALDH1+ cases (13.5 vs. 30.3 %, P = 0.003). pCR and ALDH1 expression were significantly correlated in TNBC patients (P = 0.003). ALDH1+ breast cancers tended to be aggressive, with poor prognoses. Although ALDH1+ TNBC showed higher chemoresistance, ALDH1 had significant impact on prognosis in the luminal type but not in TNBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aldehyde dehydrogenase 1 (ALDH1) has been identified as a marker of breast cancer stem cells (CSCs) [1]. CSCs are rare tumor cells that are capable of self-renewal and are multipotent [2–5], and are thought to initiate cancer and propagate metastasis [6–8]. The CD44+/CD24− phenotype was initially used to identify CSCs in breast cancer. Although some cells from this population showed high tumorigenicity by initiating cancers in immunodeficient mice [9], in the original study, the CD44+/CD24− phenotype did not successfully identify CSCs in patients, which implied that other CSC markers existed. Subsequently, ALDH1 was suggested as a possible marker for breast CSCs [1].
Despite convincing data in cell lines and animals, the clinical significance of CSCs remains controversial [10–13]. ALDH1 expression is reportedly associated with poor clinical prognosis by some researchers [1, 14], but not others [15].
A meta-analysis of 12 studies with 898 ALDH1+ cases and 1853 controls found that ALDH1+ breast tumors were significantly associated with higher histological grade, estrogen receptor (ER)– and progesterone receptor (PR)− tumors, and HER2+ tumors, but not with tumor size or nodal status; ALDH1+ and CD44+/CD24− tumor cells were significantly associated with shorter overall survival (OS)[16]. In a later (2014) review and meta-analysis about ALDH1A1 (the isoform considered responsible for ALDH1-mediated stemness in breast cancer) that included 15 studies with 921 ALDH1A1+ cases and 2353 controls, higher ALDH1A1 expression was associated with larger tumor size, higher histological grade, higher rates of lymph node metastasis, greater expression of HER2, lower expression of ER/PR, and poorer prognosis [13].
Although both of these meta-analyses concluded that ALDH1 was a biomarker that could be used to predict tumor progression and poor OS in breast cancer patients, the influence of intrinsic subtype on its effect was not analyzed. In fact, in line with these two meta-analyses, most previous studies reported that HER2-enriched and triple-negative types, which are generally known to have poor prognoses, were more prevalent in ALDH+ cancers [1, 17].
The CSC hypothesis also implies a role of CSCs in chemoresistance [18]. Accumulating evidence indicates that CSCs are naturally resistant to chemotherapy because of their quiescence, more efficient DNA repair, resistance to apoptosis, and expression of drug-resistance proteins [19]. If so, a small population of chemoresistant CSCs may resist being killed by conventional chemotherapy and thereafter regrow into a more chemoresistant tumor. However, CSC-associated chemoresistance in human breast cancer has not been widely studied [20, 21], although several in vitro studies have supported this idea [22–25].
A possible reason for the clinical uncertainty about the value of ALDH1 in predicting prognosis and chemoresistance is the heterogeneity of breast cancer and the corresponding potential effect of intrinsic subtype. Whereas chemoresponse and prognosis in breast cancer largely depends on subtype, the effect of ALDH1 in each subtype has not been addressed, to our knowledge. This study investigated the impact of ALDH1 on chemoresistance and prognosis according to intrinsic subtype in invasive breast cancer.
Materials and methods
Patients and tumor specimens
A total of 653 patients with primary breast cancer who had been treated between November 2004 and June 2013 at the Yokohama City University Medical Center were enrolled in this study. All patients were diagnosed from core needle biopsies with invasive breast carcinoma prior to any treatment. Patient characteristics are shown in Table 1. Their ages ranged from 24 to 94 years of age. Disease-free survival (DFS) interval was the time from diagnosis to the date of breast cancer-derived relapse or metastasis; overall survival (OS) was the number of days from diagnosis to breast cancer-related death. The median follow-up period was 2491 days.
Of the 653 enrolled patients, 234 received neoadjuvant chemotherapy (NAC). Of those 234 patients, 171 also received anthracycline followed by taxane-based therapy. All HER2+ patients also received trastuzumab. Patients underwent breast-conserving surgery or mastectomy and axillary lymph node dissection about a month after final chemotherapy. Clinical data, tumor characteristics, lymph node status, and prognostic information were retrieved from the clinical database. The Ethics Committee of Yokohama City University approved the study. Informed consent was obtained from each patient.
Histopathological and immunohistological staining
Core needle biopsy specimens from patients were fixed in phosphate-buffered formalin and embedded in paraffin. Serial Sects. (3–4 µm) were mounted on silane-coated slides (Muto-Glass, Tokyo, Japan) and dried at 37 °C overnight. Hematoxylin and eosin (H&E)-stained sections from each block were prepared to determine histological diagnosis, histological type, and nuclear grade. Immunohistochemical (IHC) staining was performed using an automatic staining machine (Dako-Autostainer; Dako, Kyoto, Japan). Slides were incubated at 95 °C for 40 min and deparaffinized four times for 5 min in xylene, followed by 5 min each in 95, 90, and 70 % ethanol, and twice for 1 min in distilled water. Antigen retrieval was achieved by microwaving slides in citrate buffer (pH 6.0) for 40 min at 95 °C. Endogenous peroxidase was blocked with a peroxidase-blocking reagent (Dako). Tissue sections were incubated with primary antibodies for 30 min, and with secondary reagents for 30 min. 3,3′diaminobenzidine (DAB; Dako) was used as a chromogenic substrate; slides were lightly counterstained with Mayer’s hematoxylin.
Pathological evaluation
Three board-certified pathologists assessed the pathological diagnosis and IHC evaluation blindly. ER and PR status was evaluated using the Allred score [26]. HER2 status was examined using the Dako HercepTest™ (K5204; Dako) or PathVysion™, (Abbott). HER2 positivity (overexpression or amplification) was scored according to the American Society of Clinical Oncology/College of American Pathologists guidelines [27]. Tumors were also classified into four subtypes using IHC results as surrogates, i.e., luminal type: ER ≥ 3 (Allred score) and HER2−; luminal-HER2 type: ER ≥ 3 and HER2+; HER2-enriched type: ER ≤ 2 and HER2+; Triple-negative breast cancer (TNBC): ER ≤ 2 and HER2−.
Immunostaining for MIB-1 (Dako), topoisomerase IIα (Topo2) (Ki-S1; Dako), and P53 (DO-7; Dako) was evaluated on the basis of the percentage of tumor cells with positive nuclear staining. For MIB-1, Topo2, and P53, only unequivocal nuclear staining was considered to be a positive reaction. After scanning the slides at low magnification to determine the most evenly labeled tissue areas, a minimum of 1000 tumor cells were counted at high power (× 350), and the number of labeled cells was calculated as a percentage of the total cell count. Ki-67, Topo2, and p53 were each dichotomized to high- and low-expression groups using their respective mean values as cut-off points.
Immunostaining of cytokeratin 5/6 (CK5/6) (D5/16 B4; Dako) and epidermal growth factor receptor (EGFR) (3C6; Roche Diagnostics, Tokyo, Japan) was semiquantitatively graded according to the proportion of tumor cells with positive nuclear staining (0, 0 %; 1, 0–10 %; and 2, >10 %). A score of 0 was defined as negative, and 1 or 2 was positive. Specimens that were CK5/6+ and/or EGFR+ were considered to be basal-type cancers.
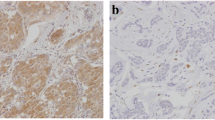
ALDH1 was evaluated by IHC with ALDH1A1 antibody (EP1933Y; Abcam). Specimens were considered ALDH1+ when ALDH1 staining was >1 % in cancer cells. Expression in only stromal cells was considered negative. Representative results are shown in Fig. 1.
In pathological evaluation of NAC response, pCR was defined as any combination of (a) necrosis; (b) disappearance of all tumor cells; and (c) replacement of cancer cells by granulation and/or fibrosis. Pathological effect was defined as lack of histologic sign of invasive cancer cells in the primary tumor, using the NSABP B-18 criteria [28].
Correlation of ALDH1 with clinicopathological factors
We analyzed associations among ALDH1 expression and other biomarkers with clinicopathological factors, including age, histologic type, tumor size, nodal status, ER/PR/HER2 status, nuclear grade (NG), Ki-67, Topo2, p53, CK5/6, and EGFR.
Statistical analysis
Findings were analyzed using the SPSS Statistics v.23 software (IBM SPSS Statistics for Windows, Version 23.0. IBM Corp, Armonk, NY, USA). We used χ 2 analysis (Spearman’s coefficient) to test for associations between ALDH1 and clinicopathological factors, the Mann–Whitney U test for associations between ALDH1 and patient age and tumor size, and Fisher’s exact test for ALDH1 associations with other biomarkers. Survival data were evaluated using the Kaplan–Meier method and the log-rank test. Multivariate analyses of prognosis were evaluated using the Cox proportional hazards model. P < 0.05 was considered significant.
Results
Correlation between ALDH1 and clinicopathological factors
We performed IHC staining to examine ALDH1 expression in 653 specimens of invasive breast carcinoma from core needle biopsies taken prior to treatment, and found ALDH1+ tumor cells in 139 cases (21.3 %). ALDH1 expression correlated significantly with larger tumor size, clinical node metastasis, higher clinical staging, higher nuclear grade, ER/PR positivity, and HER2 absence, but did not correlate with age, histologic type, expression of Ki67 or Topo2, or basal phenotype (Table 1). The percentages of subtypes with ALDH1+ cells were luminal: 12.2 %; luminal-HER2: 36.5 %; HER2-enriched: 37.9 %; and TNBC: 30.0 %. ALDH1 expression was significantly more common in HER2 type and TNBC.
Prognostic study
Patients with ALDH1+ specimens had significantly shorter DFS (P < 0001) and OS (P = 0.044) than the group with ALDH1− specimens (Fig. 2a). Cox multivariate analyses of DFS and OS, against tumor size, lymph node metastasis, clinical staging, nuclear grade, and ER, HER2, and ALDH1 expressions found that ALDH1 expression was an independent predictor for DFS (P = 0.033), but not for OS (P = 0.124; Table 2). Although ALDH1 expression significantly affected prognosis of patients with luminal-type cancers, it did not significantly affect those with TNBC and HER2+ types (Fig. 2b–d).
Neoadjuvant study
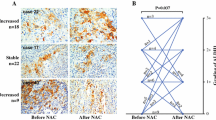
We analyzed specimens from 234 breast cancer patients treated with NAC, including 63 with luminal type, 20 with luminal-HER2 type, 45 with HER2-enriched type, and 106 with TNBC type. ALDH1 was expressed in 88 specimens (37.6 %). For all subtypes, 56 patients (23.9 %) achieved pCR. The pCR rate was significantly lower in patients with ALDH1+ tumors (13.5 vs. 30.3 %, P = 0.003). In univariate analysis, pCR was significantly correlated with ALDH1 expression (P = 0.003), ER negativity (P = 0.001), PR (P = 0.004) negativity, and higher expression of Ki67 (P = 0.04) and p53 (P = 0.017). In multivariate analysis, ALDH1 and ER correlated significantly with the pCR rate (Table 3).
Among the subtypes, pCR and ALDH1 expressions were very significantly associated in TNBC (P = 0.003). In the HER2-enriched and luminal-HER2 types, ALDH1 expression tended to correlate with low pCR rates, but not significantly so (Table 4). In the luminal (HER2−) group, two patients achieved pCR; ALDH1 was not detected in either specimen. A univariate analysis of pCR predictors in TNBC correlated ALDH1 and Ki67 with pCR, but only ALDH1 survived in multivariate analysis (P = 0.012; Table 5).
Discussion
Cancer stem cells (CSCs) are considered to cause the initiation, progression, and recurrence of cancers [6, 29, 30]. ALDH1 expression has been correlated with increased tumor size, lymph node metastasis, nuclear grade, and clinical staging, all of which are associated with progression and poor prognosis. Our results conform to those of a meta-analysis published in 2014 [13]. However, in another meta-analysis published in 2010, tumor size and nodal status did not correlate with ALDH1 expression, which differs from our results and the 2014 meta-analysis, in which both showed a positive correlation.
We found the clinical significance of ALDH1 to differ by subtype. ALDH1 positivity significantly varied with poor prognosis in luminal breast cancers, but not for the other subtypes. In ER+/PR+ breast cancers, CSCs rarely exist and their clinical significance is generally unknown. Although several reports concerning the prognostic importance of CSCs in luminal breast cancer have been published, they were limited by small sample sizes and yielded questionable results. Kim et al. reported that non-TNBC patients with ALDH1+ tumors had significantly shorter OS than those with ALDH1− tumors, whereas TNBC patients showed no statistical differences in DFS and OS according to ALDH1 expression [31]. Although the main limitation of this study is its small sample size (n = 70), we showed a similar tendency by subtype.
Hashimoto et al. reported that neither ALDH1 positivity nor CD44+/CD24− status of tumor cells was related to progression-free survival in luminal metastatic breast cancers [32]. In our study, ALDH1 positivity was found in 13.9 % of luminal-type tumors and CSCs were shown to be an especially pessimistic prognostic indicator in patients with this subtype. To our knowledge, this is the largest study to report on the prognostic impact of CSCs on luminal breast cancers. Luminal breast cancers that are refractory to chemotherapy and endocrine therapy—the so-called “unmet needs”—have become a focus of much recent study. ALDH1 could be a biomarker for this particular subgroup.
The next argument concerns the impact of CSCs on TNBC. Ohi et al. stated that ALDH1+ cancer cells were found in 59 % of TNBC cases and correlated significantly with high histological grade. We also found Cox multivariate analysis to show that ALDH1 expression was an independent prognostic indicator of relapse-free survival [33]. Although the relationship between ALDH1 and prognosis has been controversial in earlier studies [31, 34, 35], the present study found no significant association between prognosis and ALDH1 expression, although ALDH1+ TNBC patients had a significantly lower pCR rate. This result may have been caused by the ambiguity of using the pCR rate as a surrogate prognostic marker in TNBC. TNBC also encompasses a heterogeneous group within the subtype, which should be considered when performing future analyses of this particular subtype.
The relationship between HER2 and ALDH1 in HER2+ breast cancers has recently become a focus of study. Several studies have shown HER2 to be an important regulator of the CSC population in breast cancer [36–39]. These studies indicate that the clinical efficacy of trastuzumab may be based on its ability to target the CSC population in HER2-amplified tumors. However, the impact of ALDH1 on HER2+ breast cancer prognosis is not widely reported. In our current research, all HER2+ patients received trastuzumab; they showed no correlation between ALDH1 positivity and prognosis, regardless of ER status. If trastuzumab can indeed target CSCs, our clinical finding could be considered reasonable. As our HER2+ sample size was small, additional studies with larger cohorts are needed to verify our findings. Furthermore, the efficacy of trastuzumab has been suggested to rely on its ability to target CSCs in a process that does not depend on HER2 gene amplification [39]. If so, trastuzumab may be effective against CSC+/HER2− cancers. This hypothesis bears exploring and further research in this area is expected.
Our study had the advantages of having a moderate sample size and performing accurate analyses by breast cancer subtype. However, it was limited by its retrospective design; moreover, chemotherapy regimens were not the same for all patients, though most were anthracyclines followed by a taxane.
In conclusion, ALDH1 expression in tumor cells correlated significantly with tumor size, lymph node metastasis, nuclear grade, poor prognosis, and response to NAC. Although the correlation between ALDH1 expression and pCR rate was much higher in TNBC than other subtypes, ALDH1 expression was also correlated with prognosis in the luminal type. Our results indicate that ALDH1 is a potential biomarker for poor survival, and its significance varies by breast cancer subtype.
References
Ginester C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1(5):555–567. doi:10.1016/j.stem.2007.08.014
Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL (1997) Identification of a lineage of multipotent hematopoietic progenitors. Development 124:1929–1939
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Molofsky AV, Pardal R, Morrison SJ (2004) Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol 16:700–707
Ward RJ, Dirks PB (2007) Cancer stem cells: at the headwaters of tumor development. Annu Rev Pathol 2:175–189
Polyak K, Hahn WC (2006) Roots and stems: stem cells in cancer. Nat Med 12:296–300
Gupta PB, Chaffer CL, Weinberg RA (2009) Cancer stem cells: mirage or reality? Nat Med 15:1010–1012
Zhao D, Najbauer J, Annala AJ, Garcia E, Mets MZ, Gutova M, Polewski MD, Gilchrist M, Glackin CA, Kim SU, Aboody KS (2012) Human neural stem cell tropism to metastatic breast cancer. Stem Cells 30:314–325
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100:3983–3988. doi:10.1073/pnas.05302911000530291100
Mieog JS, de Kruijf EM, Bastiaannet E, Kuppen PJ, Sajet A, de Craen AJ, Smit VT, van de Velde CJ, Liefers GJ (2012) Age determines the prognostic role of the cancer stem cell marker aldehyde dehydrogenase-1 in breast cancer. BMC Cancer 12:42
Resetkova E, Reis-Filho JS, Jain RK, Mehta R, Thorat MA, Nakshatri H, Badve S (2010) Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res Treat 123:97–108
Yoshioka T, Umekita Y, Ohi Y, Souda M, Sagara Y, Rai Y, Tanimoto A (2011) Aldehyde dehydrogenase 1 expression is a predictor of poor prognosis in node-positive breast cancers: a long-term follow-up study. Histopathology 58:608–616
Liu Y, Lv D, Duan J, Xu S, Zhang J, Yang X, Zhang X, Cui Y, Bian X, Yu S (2014) ALDH1A1 expression correlates with clinicopathologic features and poor prognosis of breast cancer patients: a systematic review and meta-analysis. BMC Cancer 14:444
Morimoto K, Kim SJ, Tanei T, Shimazu K, Tanji Y, Taguchi T, Tamaki Y, Terada N, Noguchi S (2009) Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci 100:1062–1068. doi:10.1111/j.1349-7006.2009.01151.x
Neumeister V, Agarwal S, Bordeaux J, Camp RL, Rimm DL (2010) In situ identification of putative cancer stem cells by multiplexing ALDH1, CD44, and cytokeratin identifies breast cancer patients with poor prognosis. Am J Pathol 176:2131–2138. doi:10.2353/ajpath.2010.090712
Zhou L, Jiang Y, Yan T, Di G, Shen Z, Shao Z, Lu J (2010) The prognostic role of cancer stem cells in breast cancer: a meta-analysis of published literatures. Breast Cancer Res Treat 122:795–801. doi:10.1007/s10549-010-0999-4
Ricardo S, Vieira AF, Gerhard R, Leitão D, Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F, Paredes J (2011) Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol 64:937–946. doi:10.1136/jcp.2011.090456
Kakarala M, Wicha MS (2008) Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol 26:2813–2820. doi:10.1200/JCO.2008.16.3931
Dean M, Fojo T, Bates S (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5:275–284
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100:672–679
Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y, Noguchi S (2009) Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res 15:4234–4241
Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS (2006) Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 5:67
Ghods AJ, Irvin D, Liu G, Yuan X, Abdulkadir IR, Tunici P, Konda B, Wachsmann-Hogiu S, Black KL, Yu JS (2007) Spheres isolated from 9L gliosarcoma rat cell line possess chemoresistant and aggressive cancer stem-like cells. Stem Cells 25:1645–1653
Fillmore CM, Kuperwasser C (2008) Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 10:25
Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y, Noguchi S (2009) Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res 15:4234–4241
Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11:155–168
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical Oncology; College of American Pathologists (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013. doi:10.1200/JCO.2013.50.9984
Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB Jr, Fisher ER, Wickerham DL, Wolmark N, DeCillis A, Hoehn JL, Lees AW, Dimitrov NV (1997) Effect of preoperative chemotherapy on local- regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15:2483–2493
Gupta PB, Chaffer CL, Weinberg RA (2009) Cancer stem cells: mirage or reality? Nat Med 15:1010–1012
Zhao D, Najbauer J, Annala AJ, Garcia E, Metz MZ, Gutova M, Polewski MD, Gilchrist M, Glackin CA, Kim SU, Aboody KS (2012) Human neural stem cell tropism to metastatic breast cancer. Stem Cells 30:314–325
Kim YS, Jung MJ, Ryu DW, Lee CH (2014) Clinicopathologic characteristics of breast cancer stem cells identified on the basis of aldehyde dehydrogenase 1 expression. J Breast Cancer 17:121–128. doi:10.4048/jbc.2014.17.2.121
Hashimoto K, Shimizu C, Tsuda H, Saji S, Osaki A, Shigekawa T, Aogi K (2012) Immunohistochemical detection of breast cancer stem cells in hormone receptor-positive breast cancer and their role in response to endocrine therapy and clinical outcome. Oncology 82:168–174. doi:10.1159/000336078
Ohi Y, Umekita Y, Yoshioka T, Souda M, Rai Y, Sagara Y, Sagara Y, Sagara Y, Tanimoto A (2011) Aldehyde dehydrogenase 1 expression predicts poor prognosis in triple-negative breast cancer. Histopathology 59:776–780. doi:10.1111/j.1365-2559.2011.03884.x
Zhou L, Li K, Luo Y, Tian L, Wang M, Li C, Huang Q (2013) Novel prognostic markers for patients with triple-negative breast cancer. Hum Pathol 44:2180–2187. doi:10.1016/j.humpath.2013.03.021
De Brot M, Rocha RM, Soares FA, Gobbi H (2012) Prognostic impact of the cancer stem cell related markers ALDH1 and EZH2 in triple-negative and basal-like breast cancers. Pathology 44:303–312. doi:10.1097/PAT.0b013e3283534bcb
Paik S, Kim C, Wolmark N (2008) HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med 358:1409–1411
Korkaya H, Wicha M (2013) HER2 and breast cancer stem cells: more than meets the eye. Cancer Res 73:3489–3493. doi:10.1158/0008-5472.CAN-13-0260
Korkaya H, Paulson A, Iovino F, Wicha MS (2008) HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 27:6120–6130. doi:10.1038/onc.2008.207
Ithimakin S, Day KC, Malik F, Zen Q, Dawsey SJ, Bersano-Begey TF, Quraishi AA, Ignatoski KW, Daignault S, Davis A, Hall CL, Palanisamy N, Heath AN, Tawakkol N, Luther TK, Clouthier SG, Chadwick WA, Day ML, Kleer CG, Thomas DG, Hayes DF, Korkaya H, Wicha MS (2013) HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res 7:1635–1646. doi:10.1158/0008-5472.CAN-12-3349
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kida, K., Ishikawa, T., Yamada, A. et al. Effect of ALDH1 on prognosis and chemoresistance by breast cancer subtype. Breast Cancer Res Treat 156, 261–269 (2016). https://doi.org/10.1007/s10549-016-3738-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3738-7