Abstract
Being a candidate of BCS class II, dolutegravir (DTG), a recently approved antiretroviral drug, possesses solubility issues. The current research was aimed to improve the solubility of the DTG and thereby enhance its efficacy using the solid dispersion technique. In due course, the miscibility study of the drug was performed with different polymers, where Poloxamer 407 (P407) was found suitable to move forward. The solid dispersion of DTG and P407 was formulated using solvent evaporation technique with a 1:1 proportion of drug and polymer, where the solid-state characterization was performed using differential scanning calorimetry, Fourier transform infrared spectroscopy and X-ray diffraction. No physicochemical interaction was found between the DTG and P407 in the fabricated solid dispersion; however, crystalline state of the drug was changed to amorphous as evident from the X-ray diffractogram. A rapid release of DTG was observed from the solid dispersion (>95%), which is highly significant (p<0.05) as compared to pure drug (11.40%), physical mixture (20.07%) and marketed preparation of DTG (35.30%). The drug release from the formulated solid dispersion followed Weibull model kinetics. Finally, the rapid drug release from the solid dispersion formulation revealed increased Cmax (14.56 μg/mL) when compared to the physical mixture (4.12 μg/mL) and pure drug (3.45 μg/mL). This was further reflected by improved bioavailability of DTG (AUC: 105.99±10.07 μg/h/mL) in the experimental Wistar rats when compared to the AUC of animals administered with physical mixture (54.45±6.58 μg/h/mL) and pure drug (49.27±6.16 μg/h/mL). Therefore, it could be concluded that the dissolution profile and simultaneously the bioavailability of DTG could be enhanced by means of the solid dispersion platform using the hydrophilic polymer, P407, which could be projected towards improved efficacy of the drug in HIV/AIDS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the past few decades, acquired immunodeficiency syndrome (AIDS) has been considered to be one of the world’s most devastating health challenges, which is known to cause by a specific etiologic agent human immunodeficiency virus (HIV). This causative retrovirus directly or indirectly destroys the vital components of the human immune system, which includes depletion of native and memory CD4+ T cells, inhibiting T helper cell, macrophages and dendritic cell activity (1). The World Health Organization intensified their advocacy in recently published guidelines on the provision of antiretroviral therapy for managing persons living with HIV (2). According to the 2018 reports by Joint United Nations Programme on HIV/AIDS, the numbers of global population infected with HIV were 36.9 million, including 1.8 million children under the age of 15 years. Out of the huge number, approximately 25% of them were newly infected in 2017 (3). Patients with HIV are at high risk for different numbers of non-infectious comorbid diseases, such as osteoporosis, type 2 diabetes mellitus, chronic kidney disease, cardiovascular disease and cancer (4). Currently available antiretroviral treatments include protease inhibitors (e.g. indinavir, ritonavir, saquinavir, nelfinavir, amprenavir), nucleoside reverse transcriptase inhibitors (e.g. zidovudine, didanosine, zalcitabine, stavudine, lamivudine, abacavir) and non-nucleoside reverse transcriptase inhibitors (e.g. nevirapine, delavirdine and efavirenz) (5). It has been reported that insufficient drug exposure to the susceptible cells is probably one of the main factors responsible for the inefficacy of conventional antiretroviral therapy to completely eliminate the virus from the host body (6). Furthermore, it is imperative to maintain the steady-state level above the target drug concentration during the entire treatment period to obtain a successful treatment of an antiretroviral therapy in HIV (7). Most of the antiretroviral drugs (e.g. HIV protease inhibitors) inadequately penetrate the blood-brain barrier (8). Reduced bioavailability of these drugs in the cellular and tissue reservoirs can also be affected by various factors, such as the efflux transporters (e.g. P-glycoprotein/P-gp), drug-metabolizing enzymes (e.g. cytochrome P-450), rapid elimination and limited permeability (9). Thus, there is an immediate requirement to bridge the gap between the availability of most potent antiretrovirals and effective disease management in a cost-effective manner.
The integrase strand transfer inhibitors are the currently approved class of drug that signify a major role in HIV treatment. It blocks the functioning of HIV integrase, which is an important component for HIV replication. In particular, dolutegravir (DTG) is the second-generation integrase strand transfer inhibitor with remarkable benefits, like a high genetic obstacle to drug resistance, once-a-day dose and low or no risk of any drug-drug interactions (10). Thus, DTG is generally well tolerated and well thought out to be metabolically friendlier compare to other classes of drug-like protease inhibitors. Initial investigations revealed that absorption of sodium salt of DTG is primarily restricted by its poor solubility and dissolution rate. It is a BCS class IIa drug with water solubility below 50 μg/mL between the pH ranges of 1 and 7 (11). Solubility enhancement of such drugs is an important area of drug delivery research due to the inherent difficulty of developing it as a successful dosage form. To overcome the solubility issues of drug components, many formulation techniques were developed during the last few decades such as miniaturization (12), surface modification (13), complexation (14), solid lipid nanoparticles (15) and solid dispersion (16). Among these methods, solid dispersion is considered as an excellent, low cost and feasible approach to improve the drug solubility, which allows a decrease in particle size which equals to molecular size, thereby enhancing particle wettability with increase in porosity, reduction in aggregation, transformation to amorphous state and subsequent improvement in drug solubility (17). In the present investigation, solid dispersion formulation of DTG was formulated with Poloxamer 407 (P407) using the conventional solvent evaporation technique. P407 is a widely used polymer in the pharmaceutical field, possessing advantages of thermoreversible properties, increasing bioadhesion, compatibility with other substances, immunomodulation, cytotoxic promotion, enhanced solubilization of poorly soluble drugs and extended-release profiles in drug delivery applications and low toxicity (18). Phase solubility study between DTG and P407 was performed to measure the impact of polymer and its concentration on drug solubility. Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and differential scanning calorimetry (DSC) analyses were carried out to characterize the solid-state properties of DTG and the formulated solid dispersion formulation. Finally, the oral bioavailability of the developed solid dispersion system was performed in male Wistar rats.
MATERIALS AND METHODS
Materials
DTG, Lutrol F 68, P407, Kollidon 90F, Kollidon CL and polyethylene glycol 6000 were provided as a gift sample from Emcure Pharmaceuticals Ltd., Ahmedabad, India. HP-β-cyclodextrin was supplied as a gift sample from Gangwal Chem, Boisar, India. Quinoxaline was acquired from Sigma Aldrich, Mumbai, India. Acetonitrile and 2-propanol were procured from SD Fines Chemicals Limited, Mumbai, India. All other chemicals and reagents employed in the research were analytical grade of purity and quality.
High-performance Liquid Chromatography (HPLC) Analysis of Dolutegravir
Quantification of DTG in various samples was done by Jasco HPLC (Jasco LC–4000, Easton, MD, USA) system containing degasser unit, a pump, an automatic injector and an MD-4010 UV-Visible detector, at wavelength of 260 nm for the detection of DTG and internal standard (quinoxaline). Chromatographic separation of DTG was carried out on Discovery C18 HPLC column (250 mm× 4.6 mm, i.d, 5 μm). Gradient elution was carried out using mobile phase prepared using acetonitrile and 50 mM solution of acetate buffer (pH 4.5) and the flow rate was adjusted to 1 mL/min by means of gradient dilution method with 50-μL volume injected (19). The gradient elution was done with acetonitrile:acetate buffer (40:60) until 4 min and followed 70:30. DTG content in plasma was determined after protein precipitation by adding identical amount of acetonitrile and 2-propanol (20). Moreover, the validation of analytical method was also performed in accordance with the procedure referred to in the International Council on Harmonization (ICH) guidelines. The validated method showed a retention time of 6.2 min and has linearity from 50 to 2000 ng/mL (R2 = 0.9992). The limit of detection of the method was found to be 20 ng/mL, while the limit of quantitation noticed was 50 ng/mL after the system suitability test.
Screening of Polymer
Briefly, 5 mg of drug plus 25 mg of polymers (P407, Lutrol F 68, HP-β-cyclodextrin, Kollidon 90F, Kollidon CL, Polyethylene glycol 6000) was mixed in 5 mL of phosphate buffer (pH 6.8). It was kept in a thermostatically controlled (25±1°C) shaker incubator for a period of 24 h (21). After the equilibration period, the dispersion was filtered using syringe membrane filter having a pore size of 0.2 μm and immediately estimated for DTG by the HPLC method.
Estimation of Dolutegravir-Poloxamer 407 Miscibility
Solubility of DTG in P407 was assessed by the procedure mentioned by Higuchi and Lach (22). An excess quantity of DTG was taken in a separate glass tube, and 20 mL of phosphate buffer (pH 6.8) having different concentrations of P407 (up to 0.18 Moles) was added. All dispersions were rotated in water-jacketed flasks at constant temperature (25±0.5°C) for 24 h. Thereafter, the suspensions were filtered through a syringe membrane filter (pore size of 0.2 μm) and diluted with the media to determine the amount of drug dissolved using the developed HPLC method. The stability constant (Kc) was estimated by plotting the moles of DTG dissolved against the moles of polymer as described in the literature (23).
Preparation of Physical Mixture
Samples of a DTG and P407 were initially sieved from a 40-mesh (425 μm) and then blended, weighed and stored in a desiccator maintained at room temperature condition (25±1°C and 70±5%) (24).
Preparation of solid dispersions by solvent evaporation method
Being higher soluble in methanol, sodium salt of DTG was used for preparation of solid dispersion. The composition of prepared solid dispersion is summarized in Table I. Briefly, DTG and P407 were taken and dissolved in a suitable quantity of methanol until the solution becomes completely clear. Methanol was evaporated using rotary evaporator under vacuum at 32±1°C to form a highly viscous mass and air-dried at room temperature (25±1°C) to obtain a solid fragile powder. It was pulverized, sieved through different sieves and sized and finally stored in a desiccator kept under the same conditions as discussed in preparation of physical mixture section above until further investigations.
Characterization of Solid Dispersion
Saturation Solubility Study
This study was performed to measure the improvement in drug solubility in the prepared solid dispersions. Excess quantity of powder from the prepared solid dispersions was mixed with distilled water (10 mL) in glass vials. The prepared solutions were kept on a shaker water-bath for 48 h and the temperature was maintained at 37±0.5°C. Thereafter, the mixture was filtered out through 0.2-μm membrane filters, further diluted and then analyzed by the developed HPLC method.
Drug Content and Percent Yield
Weighed quantity of solid dispersions equivalent to 50 mg was dissolved in a suitable quantity of methanol and then filtered through a 0.2-μm filter. The received filtrate was diluted with phosphate buffer (pH 6.8) and amount of DTG was quantified by HPLC method. The percentage yield of DTG solid dispersions is computed using Eq. 1.
Solid-state Characterization
FTIR
Spectra of DTG, P407, physical mixture (drug and polymer) and the formulated solid dispersion were noted using an FTIR spectrometer (FT/IR-6100, Jasco, Tokyo, Japan). Previously ground and sieved samples were prepared by intimately mixing with potassium bromide (1:10) and the discs were formed by compression employing a hydraulic press (25), and were scanned at a resolution of 2 cm−1 and the IR spectra were developed in the range of 400–4000 cm−1.
DSC
The thermal behaviour of DTG, P407, physical mixture (drug and polymer) and the formulated solid dispersion were determined by DSC (Perkin-Elmer DSC7, USA). Weighed samples (6.5–10 mg) were kept in aluminium curled pans and sealed non-hermetically, while a blank pan was taken as a standard reference during study. Recording of thermogram was carried out at a heating speed of 10°C/min in presence of nitrogen and at a heating range of 25–400°C.
XRD
Powder XRD patterns of DTG, P407, physical mixture (drug and polymer) and the formulated solid dispersion were studied on an XD-6000 (Shimadzu, Tokyo, Japan) powder X-ray diffractometer. The sieved samples were filled in an aluminium sample container and analyzed with radiation under a voltage of 40 kV and a current of 40 mA (26). The scanning rate was maintained 1°/min over a 2θ range of 1–50°.
Dissolution studies
The in vitro dissolution of DTG from the formulated solid dispersion was determined using type-2 USP dissolution apparatus with a slight modification of earlier method reported (27). The study was carried out using 900 mL of phosphate buffer (pH 6.8) as dissolution medium at temperature 37 ± 0.5°C and stirring speed at 50 rpm for 2 h. Weighted amount of solid dispersion equivalent to 50 mg of DTG was used for the study. The samples (5 mL) were taken at specific intervals and analysed for the amount of DTG release. Samples collected at specific time points were passed through a 0.2-μm syringe membrane filter (Millipore Corporation, Bedford, MA, USA) and readily quantified using the developed method by HPLC. Dissolution profile of DTG solid dispersion was compared with marketed product, physical mixture and pure DTG.
Furthermore, the cumulative release data were checked to determine release rate kinetics by employing various mathematical models including zero, first, Hixson Crowell, Higuchi diffusion and Korsmeyer-Peppas using the equation described in the literature (28, 29). The release mechanism and model that best fitting to the release data is chosen by taking into consideration of the higher regression coefficient (r2), low squares of residuals and minimum Fischer ratio. The squares of residuals and Fischer ratio are two added factors that are widely used to decrease the inaccuracy in calculating a good fit of the model (30). Furthermore, the measurement of dissolution efficiency of various samples like pure DTG, physical mixture and solid dispersion is done using Eq. 2 (31).
where dissolution efficiency is the area under the dissolution curve within time points; t1 and t2 are defined as a percentage of the curve at maximum dissolution, y100, over the same time period.
Stability
Solid dispersion was kept in a sealed polyethylene vial and stored in a desiccator for 3 months at stability condition maintained at 40±2°C and 75%±5% RH (32). Stability of the solid dispersion was checked by assessing the drug content and drug release during storage. The release of DTG from the solid dispersion prior to the storage and after storage was compared using similarity factors (f2). The formulation was also studied for its DSC and XRD pattern after the stability period.
Oral pharmacokinetic studies
The pharmacokinetic studies were performed on male Wistar rats (225–250 g) to examine the oral bioavailability differences between the optimized DTG solid dispersion with the pure drug (control). A separate room with proper ventilation was used to house experimental animals. The animals were kept in standard polypropylene cages at 25±5°C with a 12-h day/12-h night cycle with free access to food and water (33). Animals refrain from eating for 12 h were split into three groups, each comprised of six rats. Animal studies were conducted according to the standards and guidelines mentioned in the institutional animal ethical committee (Protocol No. IP/PCEU/FAC/23/2018/030). A dose equal to 5 mg/kg of pure DTG or physical mixture or solid dispersions was suspended separately in 0.5% w/v carboxymethyl cellulose. The dose was calculated from the standard daily human dose of 50 mg using the equation mentioned in the scientific literature (34). The respective suspensions were administered orally as a single dose by intragastric gavage in respective experimental groups. Blood sample (200 μL) was collected from retro-orbital plexus of each experimental rat under anesthesia (thiopentone sodium 30 mg/kg) using dry heparinized tubes at the specified time intervals post dose. The collected samples were protein precipitated and were centrifuged (10,000 rpm for 10 min) and the top layer was filtered (0.2 μm). The filtrate (50 μL) was immediately injected into HPLC while considering the sample withdrawn at zero time point as the baseline value.
Data analysis
The software used for Statistical data analysis was GraphPad Prism software (version 6, GraphPad, San Diego, CA, USA). The difference in values at p < 0.05 is considered statistically significant.
RESULTS AND DISCUSSION
Development of Dolutegravir Solid Dispersion and Characterization
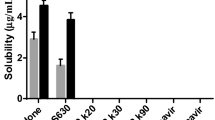
The extent of miscibility among the pharmaceutical active (drug) and the excipient (polymer) can be judged based on the variation in the solubility parameters of drug and polymer. It has been reported that the miscible compounds are possessing equivalent solubility parameters, whereas compounds with significant solubility differences are unable to become miscible (35, 36). Solubility profile of the drug in different polymers is presented in Fig. 1, where the highest solubility of sodium salt of DTG was obtained with P407 (123 μg/mL) followed by Kollidon 90F (99 μg/mL). Other tested polymers, Lutrol F 68, HP-β-cyclodextrin, Kollidon CL and PEG 6000, showed a slight increase in solubility, ranging between 60 and 65 μg/mL. The highest solubility in P407 might be explained by the surfactant property and their inherent tendency to self-agglomerate, hence generating micelles as well as liquid crystalline phases with enhanced hydrophilicity, an important factor responsible for the solubilization of sparingly water-soluble drugs compared to Kollidon 90F (37, 38).
As per the data obtained from the miscibility study, it could be said that the P407 has a positive solubilizing influence on DTG. The phase solubility curve of DTG with different concentrations of P407 is presented in Fig. 2. The aqueous solubility of DTG at room temperature (⁓25°C) is less than 50 μg/mL and thus this agent is considered a water-insoluble agent (11). From Fig. 2, it is evident that the apparent solubility of DTG was increased with the rising concentration of the carrier (P407). Here, the carrier shows an AL type of linear graph (with higher r2=0.9953 and slope of 0.0095) referring to Higuchi and Connors solubility figures (14). Moreover, the linear curve observed also signifies 1:1 (mol/mol) ratio of drug to polymer which is suitable for solid dispersion preparation. The observed stability constant (Kc) value (81 M−1) suggests drug and polymer dispersion possess adequate stability, though have very weak binding (interaction) between DTG and P407 (14). On the other hand, the weak binding is advantageous as it helps the rapid release of drug molecules and thereby facilitates drug absorption (23).
Development of Solid Dispersion of Dolutegravir
Technology of solid dispersion could be employed between hydrophobic drugs and hydrophilic polymers, where the ultimate goal of these formulations is to quickly release the entrapped drug (39). We have employed methanol as a common solvent. Furthermore, the solvent evaporation method used to fabricate the solid dispersion of DTG in the present study using a drug to polymer ratio of 1:1, which can be considered as a simple and straightforward approach to prepare solid dispersion with uniform distribution of drug, with nearly 100% recovery of the yield (40). The drug content of fabricated solid dispersion formulations was established in the range of 98.2 ± 0.5%.
Solid-state Characterization
FTIR
FTIR is the most widely used technique in the pharmaceutical field to establish drug-carrier interaction in any physical mixture or in solid dispersion formulation where the comparison on the spectra of the individual components could be made with the mixtures of the components (25). Absence of shift, or reduction or abolition of spectra usually refers to have molecular interaction between the components (41). FTIR is the most extensively used technique to find drug-carrier interaction in solid dispersion. It was revealed in literature that absorption bands of the group involved in hydrogen bonding, shift to lower wavenumber and if the bonded group are high then new bend appears and if non-bonded groups are dominating then slight broadening is observed due to superposition of the two bands (42). FTIR of sodium salt of DTG showed (Fig. 3) two predominant peaks, one apparently assigned to the secondary amide (3273 cm−1) and the other linked to carbonyl functional group (1696 cm−1). The spectra also contain additional peaks at 3165 and 1645 cm−1 characterizing amide and carbonyl groups, respectively, and C=C stretching in the structure at 2874 cm−1 and O-H at 2500–3000 cm−1. Between the spectral region of 3000 and 2900 cm−1, vibrational group frequencies depict the symmetrical and asymmetric stretching of methyl and methylene groups. The frequency wavenumber between the 1350- and 1000-cm−1 regions displays axial distortion and band overlay between C–N and C–H due to skeletal vibrations of methylene group. The infrared spectroscopy is used to exhibit the vibrational spectrum of the molecule and used for identification by comparing with either a reference spectrum or basic interpretation from the first principle resulting in characterization of the sample. The non-covalent bonding due to functional group interactions between the polymer and drug typically show characteristic peak shifts or change in the pattern of the spectra. Apparently, the absorption spectra of physical mixtures and formulated solid dispersions are very similar; nevertheless, there is a minor variation between them regarding intensities. Solid dispersions showed low intensity in 1000–2000 cm−1. The decreased intensities in those peaks can be interpreted due to the hydrogen bond formation between carrier and drug. P407 consists of hydroxyl groups that could result in hydrogen bonding where the formation of bonding can shift to different wave numbers, thereby they have reduced intensities and their peaks can disappear.
DSC
The thermal characteristics of pure drug, P407, physical mixture and solid dispersion were examined utilizing DSC and the individual thermograms are illustrated in Fig. 4. Thermogram of pure DTG has shown a distinctive melting endotherm at 335.7°C demonstrating its characteristic crystalline nature. Furthermore, the DSC thermogram for P407 indicated endothermic peak at 57.05°C. Thermogram of physical mixture exhibited characteristic peak of P407 while the drug peak was slightly broadened with reduced intensity and shifted to a little lower temperature. The single endothermic peak due to the fusion of DTG was not evident in the case of solid dispersion, indicating the absence of crystalline form of the drug and further confirmed that inclusion of drug in the crust of polymer. It is presumed that the thermal property of DTG was changed in the solid dispersion. The available data disclosed that the pure drug and polymers are non-interacting and therefore compatible. Moreover, this study also confirms the transformation of drug physical state from crystalline to amorphous.
XRD
In order to evaluate the change in crystalline characteristics of the drug as a resulting effect of the solid dispersion technique, an XRD experiment was conducted (23). The diffraction spectra of DTG in Fig. 5 demonstrate many characteristic peaks with two sharp peaks 6° and 9° implying that the drug was present predominantly in a crystalline state. The spectra of P407 displayed two dominant peaks at 20° and 22°. The absence of the many unique peaks of the drug and peak broadening in the solid dispersion formulation confirming that a significant amount of the drug was molecularly dispersed in the carrier matrix as amorphous form. The diffraction patterns of all the tested samples have retained typical peaks linked to P407 but the standard crystalline peak of the drug was completely absent, hence proving the amorphous state of DTG in solid dispersions.
In Vitro Dissolution Studies
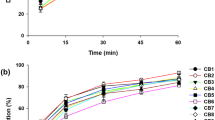
Obtaining faster release of the entrapped hydrophobic drugs in the solid dispersion formulation is of prime interest (40). It is a well-known fact for oral drug products, dissolution data is considered as a crucial pharmaceutical factor particularly for low aqueous soluble drug. This is mainly because the absorption of these compounds is mainly limited by dissolution rate (43). Therefore, the in vitro dissolution experiments were conducted for pure drug, solid dispersion, physical mixture of 1:1 ratio of drug and P407, and marketed product (InstgraTM tablet containing 50 mg of drug) in phosphate buffer (pH 6.8) medium containing 0.2% Tween 80 (to keep sink condition) at temperature of 37±1°C. The dissolution profiles of DTG, solid dispersion, physical mixture and marketed preparation are shown in Fig. 6. The dissolution rate of pure DTG was low during the first hour demonstrating a maximum percentage released as 11.40%. The rationale for the limited dissolution rate of pure drug could be directly attributed to the non-wettability and subsequent poor aqueous solubility. In vitro release studies disclosed that there was a pronounced dissolution rate enhancement of DTG, about 95%, from the formulated solid dispersion. Such increase in release was statistically significant when compared to the release of pure drug (p<0.05). Physical mixture showed slightly higher release (20.07% in 60 min) than the pure drug. The marketed formulation also showed moderately higher release (35.30% in 60 min) when compared with the pure drug or physical mixture; however, the release of DTG from the marketed formulation was significantly low in comparison to the formulated solid dispersion (p<0.05). In general, the solid dispersion exhibits a faster dissolution rate at the end of 1 h (99.15%) than pure drug. This could be interpreted by the fact that drug was molecularly dispersed in the fabricated solid dispersion and resultant amorphization, where the hydrophilic polymer assisted rapid release of the drug from the dispersion preparation. Drug release data of solid dispersion showed higher r2 (0.9937), lowest squares of residuals value (19.9768) and minimum Fischer ratio (3.9954), with Weibull model kinetics, suggesting the DTG release from solid dispersion followed Weibull diffusion-controlled mechanism. Moreover, the dissolution efficiency of solid dispersion (73.65) was significantly higher than (p<0.0001) the marketed formulation (60.71), physical mixture (64.75) and pure drug (56.14). The probable reasons for the higher dissolution efficiency in prepared solid dispersion could be due to the creation of easily soluble matrix, reduction in the crystallinity of DTG and/or decrease in particle size (44).
Stability
Stability studies provide adequate indication of the quality of the formulation with duration at various environmental conditions. There was no significant difference in drug content or release profile during storage for 3 months. The observed f2 value (79) guarantees uniformity/equivalency/performance of solid dispersion (as the value is greater than 50) according to the literature (45). The DSC and XRD studies performed after stability period showed that the formulation was stable and maintained the same spectra (Fig. 7) and prevent any recrystallization of amorphous form of drug, observed before the stability period. Overall, the stability study demonstrated the prepared solid dispersion was stable under the current experimental conditions.
Oral Pharmacokinetic Studies
The pharmacokinetic evaluation of amorphous solid dispersion was carried out in Wistar rats in fasted condition and compared against both physical mixture (control) and pure drug comprised of a single dose. The plasma drug concentration-time profiles of solid dispersion, physical mixture and pure DTG are illustrated in Fig. 8, while the estimated pharmacokinetic parameters are summarized in Table II. The pharmacokinetic parameters of interest included area under the plasma concentration-time curve (AUC0−t), maximum concentration achieved (Cmax) and time to reach peak concentration (Tmax) (46). The pharmacokinetic parameters obtained from non-compartmental analysis indicated Cmax of physical mixture (4.12 μg/mL) as well as crystalline DTG (3.45 μg/mL) simultaneously achieved at 3 h (Tmax) while optimized amorphous DTG solid dispersion demonstrated significantly higher Cmax (14.56 μg/mL) at the same time. In addition, formulated solid dispersion of DTG showed a higher rate and extent of absorption as indicated by higher AUC (105.99±10.07 μg/h/mL) when compared to the physical mixture (54.45±6.58 μg/h/mL) and plain drug (49.27±6.16 μg/h/mL). Slight improvement of bioavailability was noticed in physical mixture in comparison to pure DTG but was statistically insignificant. Thus, it could be inferred from the above findings that the prepared solid dispersion could significantly enhance the oral bioavailability of the drug when compared to both physical mixture and pure crystalline drug. It further endorsed the data from in vitro dissolution studies done in phosphate buffer (pH 6.8). Alternatively, the fast elimination of the drug from the plasma could be explained by the rapid absorption of the drug because of immediate release in the aqueous environment from the formulated solid dispersion. Following instantaneous absorption, the drug was eliminated rapidly, where there is no further absorption of the drug from the gastrointestinal tract.
CONCLUSION
The present study was investigated on miscibility of sodium salt of DTG and P407, where concentration-dependent miscibility of DTG was found. The solid dispersion of DTG was formulated using conventional solvent evaporation technique. The solid-state characterization of the solid dispersion revealed no interaction between DTG and P407, where XRD analysis revealed transformation of crystalline state to amorphous form, when formulated as solid dispersion. A faster release of the drug was found in phosphate buffer within the time frame of 1 h, which was further reflected by the improved bioavailability of the drug in experimental animals. In summary, solid dispersion of DTG using P407 would provide a potential platform for improving the solubility of the drug towards improved bioavailability and therapeutic efficacy against HIV/AIDS.
References
Wong K, Nguyen J, Blair L, Banjanin M, Grewal B, Bowman S, et al. Pathogenesis of human immunodeficiency virus-mycobacterium tuberculosis co-infection. J Clin Med. 2020;9(11). https://doi.org/10.3390/jcm9113575.
WHO Guidelines Approved by the Guidelines Review Committee. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva: World Health Organization Copyright © World Health Organization 2017.; 2017.
Akimbekov NS, Ortoski RA, Razzaque MS. Effects of sunlight exposure and vitamin D supplementation on HIV patients. J Steroid Biochem Mol Biol. 2020;200:105664. https://doi.org/10.1016/j.jsbmb.2020.105664.
Heron JE, Norman SM, Yoo J, Lembke K, O’Connor CC, Weston CE, et al. The prevalence and risk of non-infectious comorbidities in HIV-infected and non-HIV infected men attending general practice in Australia. PLoS One. 2019;14(10):e0223224. https://doi.org/10.1371/journal.pone.0223224.
Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hammer SM, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2000;283(3):381–90. https://doi.org/10.1001/jama.283.3.381.
Endsley AN, Ho RJ. Enhanced anti-HIV efficacy of indinavir after inclusion in CD4-targeted lipid nanoparticles. J Acquir Immune Defic Syndr. 2012;61(4):417–24. https://doi.org/10.1097/QAI.0b013e3182653c1f.
Kakuda TN, Page LM, Anderson PL, Henry K, Schacker TW, Rhame FS, et al. Pharmacological basis for concentration-controlled therapy with zidovudine, lamivudine, and indinavir. Antimicrob Agents Chemother. 2001;45(1):236–42. https://doi.org/10.1128/aac.45.1.236-242.2001.
Ene L, Duiculescu D, Ruta SM. How much do antiretroviral drugs penetrate into the central nervous system? J Med Life. 2011;4(4):432–9.
Robillard KR, Chan GN, Zhang G, la Porte C, Cameron W, Bendayan R. Role of P-glycoprotein in the distribution of the HIV protease inhibitor atazanavir in the brain and male genital tract. Antimicrob Agents Chemother. 2014;58(3):1713–22. https://doi.org/10.1128/aac.02031-13.
Zhao XZ, Smith SJ, Maskell DP, Metifiot M, Pye VE, Fesen K, et al. HIV-1 integrase strand transfer inhibitors with reduced susceptibility to drug resistant mutant integrases. ACS Chem Biol. 2016;11(4):1074–81. https://doi.org/10.1021/acschembio.5b00948.
Lakshman D, Chegireddy M, Hanegave GK, Sree KN, Kumar N, Lewis SA, et al. Investigation of drug-polymer miscibility, biorelevant dissolution, and bioavailability improvement of Dolutegravir-polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer solid dispersions. Eur J Pharm Sci. 2020;142:105137. https://doi.org/10.1016/j.ejps.2019.105137.
Pandey M, Choudhury H, Verma RK, Chawla V, Bhattamisra SK, Gorain B, et al. Nanoparticles based intranasal delivery of drug to treat Alzheimer’s disease: a recent update. CNS Neurol Disord Drug Targets. 2020;19:648–62. https://doi.org/10.2174/1871527319999200819095620.
Choudhury H, Pandey M, Chin PX, Phang YL, Cheah JY, Ooi SC, et al. Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: a review of recent advancements and emerging trends. Drug Deliv Transl Res. 2018;8(5):1545–63. https://doi.org/10.1007/s13346-018-0552-2.
Jacob S, Nair AB. Cyclodextrin complexes: perspective from drug delivery and formulation. Drug Dev Res. 2018;79(5):201–17. https://doi.org/10.1002/ddr.21452.
Kamboj S, Bala S, Nair AB. Solid lipid nanoparticles: an effective lipid based technology for poorly water soluble drugs. Int J Pharm Sci Rev Res. 2010;5(2):78–90.
Paudwal G, Rawat N, Gupta R, Baldi A, Singh G, Gupta PN. Recent advances in solid dispersion technology for efficient delivery of poorly water-soluble drugs. Curr Pharm Des. 2019;25(13):1524–35. https://doi.org/10.2174/1381612825666190618121553.
Huang Y, Dai WG. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B. 2014;4(1):18–25. https://doi.org/10.1016/j.apsb.2013.11.001.
Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23(12):2709–28. https://doi.org/10.1007/s11095-006-9104-4.
Charbe N, Baldelli S, Cozzi V, Castoldi S, Cattaneo D, Clementi E. Development of an HPLC-UV assay method for the simultaneous quantification of nine antiretroviral agents in the plasma of HIV-infected patients. J Pharm Anal. 2016;6(6):396–403. https://doi.org/10.1016/j.jpha.2016.05.008.
Nair AB, Kumria R, Al-Dhubiab BE, Attimarad M, Harsha S. Development of transdermal delivery system of vildagliptin and its comparison with oral therapy. Indian J Pharm Educ Res. 2016;50(1):130–7. https://doi.org/10.5530/ijper.50.1.17.
Nair AB, Attimarad M, Al-Dhubiab BE, Wadhwa J, Harsha S, Ahmed M. Enhanced oral bioavailability of acyclovir by inclusion complex using hydroxypropyl-β-cyclodextrin. Drug Delivery. 2014;21(7):540–7. https://doi.org/10.3109/10717544.2013.853213.
Higuchi T, Lach JL. Investigation of some complexes formed in solution by caffeine. IV. Interactions between caffeine and sulfathiazole, sulfadiazine, p-aminobenzoic acid, benzocaine, phenobarbital, and barbital. J Am Pharm Assoc Am Pharm Assoc. 1954;43(6 1):349–54. https://doi.org/10.1002/jps.3030430609.
Shah J, Vasanti S, Anroop B, Vyas H. Enhancement of dissolution rate of valdecoxib by solid dispersions technique with PVP K 30 & PEG 4000: preparation and in vitro evaluation. J Incl Phenom Macrocycl Chem. 2009;63(1-2):69–75. https://doi.org/10.1007/s10847-008-9490-9.
Jacob S, Shirwaikar A, Nair A. Preparation and evaluation of fast-disintegrating effervescent tablets of glibenclamide. Drug Dev Ind Pharm. 2009;35(3):321–8. https://doi.org/10.1080/03639040802337021.
Harsha SN, Aldhubiab BE, Nair AB, Alhaider IA, Attimarad M, Venugopala KN, et al. Nanoparticle formulation by Büchi b-90 nano spray dryer for oral mucoadhesion. Drug Des Devel Ther. 2015;9:273–82. https://doi.org/10.2147/DDDT.S66654.
SreeHarsha N, Hiremath JG, Sarudkar S, Attimarad M, Al-Dhubiab B, Nair AB, et al. Spray dried amorphous form of simvastatin: preparation and evaluation of the buccal tablet. Indian J Pharm Educ Res. 2020;54(1):46–54.
Choudhury H, Gorain B, Karmakar S, Biswas E, Dey G, Barik R, et al. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int J Pharm. 2014;460(1-2):131–43. https://doi.org/10.1016/j.ijpharm.2013.10.055.
Shah H, Nair AB, Shah J, Bharadia P, Al-Dhubiab BE. Proniosomal gel for transdermal delivery of lornoxicam: optimization using factorial design and in vivo evaluation in rats. Daru : journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 2019;27(1):59–70. https://doi.org/10.1007/s40199-019-00242-x.
Nagaraja SH, Al-Dhubiab BE, Tekade RK, Venugopala KN, Ghorpade RV, Meravanige G, et al. Novel preparation and effective delivery of mucoadeshive nanoparticles containing anti-diabetic drug. Indian J Pharm Educ Res. 2019;53(2):S43–S9. https://doi.org/10.5530/ijper.53.2s.47.
Nair AB, Al-Dhubiab BE, Shah J, Jacob S, Saraiya V, Attimarad M, et al. Mucoadhesive buccal film of almotriptan improved therapeutic delivery in rabbit model. Saudi Pharm J. 2020;28(2):201–9. https://doi.org/10.1016/j.jsps.2019.11.022.
Anderson NH, Bauer M, Boussac N, Khan-Malek R, Munden P, Sardaro M. An evaluation of fit factors and dissolution efficiency for the comparison of in vitro dissolution profiles. J Pharm Biomed Anal. 1998;17(4-5):811–22. https://doi.org/10.1016/s0731-7085(98)00011-9.
Jhaveri M, Nair AB, Shah J, Jacob S, Patel V, Mehta T. Improvement of oral bioavailability of carvedilol by liquisolid compact: optimization and pharmacokinetic study. Drug Deliv Transl Res. 2020;10(4):975–85.
Akrawi SH, Gorain B, Nair AB, Choudhury H, Pandey M, Shah JN, et al. Development and optimization of naringenin-loaded chitosan-coated nanoemulsion for topical therapy in wound healing. Pharmaceutics. 2020;12(9). https://doi.org/10.3390/pharmaceutics12090893.
Nair A, Morsy MA, Jacob S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev Res. 2018;79(8):373–82. https://doi.org/10.1002/ddr.21461.
Tian Y, Booth J, Meehan E, Jones DS, Li S, Andrews GP. Construction of drug-polymer thermodynamic phase diagrams using Flory-Huggins interaction theory: identifying the relevance of temperature and drug weight fraction to phase separation within solid dispersions. Mol Pharm. 2013;10(1):236–48. https://doi.org/10.1021/mp300386v.
Marsac PJ, Li T, Taylor LS. Estimation of drug-polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm Res. 2009;26(1):139–51. https://doi.org/10.1007/s11095-008-9721-1.
Sankari T, Al-Hariri S. Preparation and characterization of cefuroxime axetil solid dispersions using poloxamer 188. Braz J Pharm Sci. 2018;54(4).
Bodratti AM, Alexandridis P. Formulation of Poloxamers for Drug Delivery. J Funct Biomater. 2018;9(1). https://doi.org/10.3390/jfb9010011.
Chaudhary AS, Chaudhary BA, Mehta AT. Formulation development and optimization of polyox based quick dissolving film of quetiapine. J Pharm Bioallied Sci. 2012;4(Suppl 1):S19–20. https://doi.org/10.4103/0975-7406.94123.
Janssens S, Van den Mooter G. Review: physical chemistry of solid dispersions. J Pharm Pharmacol. 2009;61(12):1571–86. https://doi.org/10.1211/jpp/61.12.0001.
Tran TTD, Tran PHL. Molecular interactions in solid dispersions of poorly water-soluble drugs. Pharmaceutics. 2020;12(8). https://doi.org/10.3390/pharmaceutics120807458.
Gonjo T, Futami Y, Morisawa Y, Wojcik MJ, Ozaki Y. Hydrogen bonding effects on the wavenumbers and absorption intensities of the OH fundamental and the first, second, and third overtones of phenol and 2,6-dihalogenated phenols studied by visible/near-infrared/infrared spectroscopy. J Phys Chem A. 2011;115(35):9845–53. https://doi.org/10.1021/jp201733n.
Takano R, Furumoto K, Shiraki K, Takata N, Hayashi Y, Aso Y, et al. Rate-limiting steps of oral absorption for poorly water-soluble drugs in dogs: prediction from a miniscale dissolution test and a physiologically-based computer simulation. Pharm Res. 2008;25(10):2334–44. https://doi.org/10.1007/s11095-008-9637-9.
Singh G, Sharma S, Gupta GD. Extensive diminution of particle size and amorphization of a crystalline drug attained by eminent technology of solid dispersion: a comparative study. AAPS PharmSciTech. 2017;18(5):1770–84. https://doi.org/10.1208/s12249-016-0647-3.
Jacob S, Nair AB. An updated overview with simple and practical approach for developing in vitro-in vivo correlation. Drug Dev Res. 2018;79(3):97–110. https://doi.org/10.1002/ddr.21427.
Nair AB, Al-Dhubiab BE, Shah J, Attimarad M, Harsha S. Poly(Lactic acid-co-glycolic acid) nanospheres improved the oral delivery of Candesartan Cilexetil. Indian J Pharm Educ Res. 2017;51(4):571–9. https://doi.org/10.5530/ijper.51.4.86.
Acknowledgments
The authors are highly grateful to Emcure Pharmaceuticals Ltd., Ahmedabad, India, for donating gift samples of drugs and polymers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaudhary, S., Nair, A.B., Shah, J. et al. Enhanced Solubility and Bioavailability of Dolutegravir by Solid Dispersion Method: In Vitro and In Vivo Evaluation—a Potential Approach for HIV Therapy. AAPS PharmSciTech 22, 127 (2021). https://doi.org/10.1208/s12249-021-01995-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-01995-y