Abstract
Purpose
The development of pH-independent drugs is difficult because it involves improvements in their solubility and dissolution (%) in solubilizer. In the present study, the pH-independent cilostazol (CLT) was formulated for enhanced solubility and dissolution (%) and for maintaining stability with meglumine by the solid dispersion (SD) technique.
Methods
The CLT formulations were prepared with weak acid or weak base by solvent evaporation method. Moreover, the physical properties of the optimal SD formulation that were evaluated were thermal property, crystallinity, and drug-excipient interaction.
Results
Based on the pre-dissolution test, meglumine and Aerosil®200 were selected as the weak base and carrier, respectively. The SD1 formulation (CLT:meglumine:Aerosil®200 at 1:1:0.5, w/w) enhanced the dissolution (%) of CLT in distilled water/pH 1.2/pH 6.8 buffer by 1.20-/1.13-/1.18-fold, respectively, compared with those of Pletal®. Moreover, SD1 formulation was stable at room temperature for 3 months. A slight change in the crystallinity of CLT was observed in the SD1 formulation, which may be due to intermolecular interaction between CLT and meglumine.
Conclusions
The SD1 formulation showed improved dissolution (%) and might improve the bioavailability and efficacy of CLT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cilostazol (CLT) is a phosphodiesterase inhibitor that improves treadmill walking performance by approximately 25–40% in people with symptomatic peripheral arterial disease [1]. A recent systematic review including 15 randomized clinical trials and involving 3718 patients with peripheral arterial disease and intermittent claudication concluded that CLT can significantly improve treadmill walking performance in these patients [2]. The CLT commercial product as Pletal® was approved as an antiplatelet agent in 1999 for the treatment of ischemic symptoms related to peripheral arterial occlusive disease and intermittent claudication [3, 4]. CLT administered orally is characterized by absorption primarily in the upper gastrointestinal tract and low absorption in the lower gastrointestinal tract [5]. CLT is a Biopharmaceutical Classification System class II (BCS II) drug that has low water solubility, high permeability, and highly variable oral bioavailability [3, 6]. Thus, studies on improving the solubility of CLT are important.
Several studies have been developed for improving the solubility of CLT, such as solid dispersion (SD) [4, 7,8,9,10,11], inclusion complex [6], hydrogel matrix [12], fattigation platform [13], salt formation [14], and nanocrystals [15]. The SD formulation is the most researched formulation. Among all formulations, it is easy to solubilize a solid drug using SD formulation, which involves a simple manufacturing method with advantageously high yields and powder-type products [16]. Reportedly, CLT-micronizing formulations have been developed with methylene chloride and glacial acetic acid using a supercritical antisolvent (SAS) process [4]. Sub-micron CLT (median diameter 0.26 μm) has been developed using a bead mill with 82.7% CLT, 16.5% hydroxypropyl cellulose, and 0.8% docusate sodium in water. The milled suspension was solidified with sugar alcohol as the water-soluble carrier by the spray-drying method [17]. CLT-SD formulations were developed with poloxamer 188, poloxamer 407, TPGS 1000, Gelucire® 44/14, and Gelucire® 50/13 (CLT/polymers solutions, [99:1, 100 mg/mL]) by the SAS process [7]. Moreover, these formulations prepared with hydroxypropyl methylcellulose (HPMC) (CLT:HPMC, 1:4, w/w) involved the spray-drying process [8]. The CLT-loaded MCM-48-type and MCM-41-type mesoporous silica in acetic acid have also been developed [9]. Moreover, CLT and β-cyclodextrin complexes have been developed [6]. CLT-Eudragit SD formulations have been prepared using the spray-drying method [10]. CLT mesylate and CLT besylate have been synthesized from CLT by acid addition reaction with methane sulfonic acid and benzene sulfonic acid, respectively [14]. Three different types of CLT-loaded solid dispersion systems, including solvent-evaporated, solvent-wetted, and surface-attached solid dispersions with polyvinylpyrrolidone K30® and sodium lauryl sulfate (SLS), have been developed [18]. CLT-SD formulations with SLS and lactose (CLT:SLS:lactose, 10:1:20, w/w) have been prepared by the solvent evaporation method [11]. CLT-SD formulations with fattigation platform have been developed by the hot melting method [13]. These aforementioned SD formulations successfully have improved the dissolution (%) of CLT; however, the stability test of SD formulations did not perform except for salt formation.
The aim of this study was to improve the solubilization and stabilization of CLT using a SD technique. CLT-SD formulations were developed with seven solubilizers, seven pH agents, and nine carriers. The dissolution medium for CLT tablet (Pletal®) specified in the United States Food and Drug Administration (U.S. FDA) dissolution methods database is distilled water (DW) containing 0.3% SLS; moreover, the database reports that the drug is not affected by pH. However, we hypothesized that we could increase the solubility of CLT with weak acids or weak bases by forming hydrogen bonds or intermolecular interactions. The manufacturing process followed involved (1) solubilizer selection by solubility test, (2) pH agent selection by pre-dissolution test (base formulations, B), (3) carrier selection by pre-dissolution test (carrier base, CB), and (4) application of the polymer. CLT formulations were prepared using the solvent evaporation method. Usually, polymers (solubilizing agents) were used to increase the solubility of the drug. However, polymer-free formulation does not use polymers and uses other excipients. Optimal CLT polymer-free SD formulation was evaluated by dissolution tests in various media and by stability tests for 3 months. Moreover, the physical properties of the optimal SD formulation that were evaluated were thermal property, crystallinity, and drug-excipient interaction.
Materials and Methods
Materials
CLT was obtained from Chemagis Co. Ltd (Ramat Hovav, Israel). Citric acid monohydrate (99.5%), fumaric acid (99.0%), dl-malic acid (99.0%), l(+)tartaric acid (99.5%), succinic acid (99.3%), and universal buffer solutions (pH 1, 4, 7, and 10) were purchased from Samchun Pure Chemical Co., Ltd (Pyeongtaek, Korea). Meglumine was obtained from Merck (Darmstadt, Germany). MgO was obtained from Tomita Pharm Co., Ltd. (Tokyo, Japan). Kolliphor® (P188® and P407®), PEG6000, Kolliphor® HS-15, Kollicoat® IR (polyvinyl alcohol-polyethylene glycol graft-copolymer), polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (Soluplus®), d-α-tocopheryl polyethylene glycol-1000-succinate (TPGS), and Kollidon® (K12®) were obtained from BASF (Ludwigshafen, Germany). PVP/VA S-630 was purchased from ISP Technologies Inc. (Wayne, NJ, USA). Ethyl alcohol was purchased from Samchun Pure Chemical Co., Ltd (Pyeongtaek, Korea). Aerosil® 200 and Aerosil® 300 were obtained from Evonik (Essen, Germany). Mannitol (200SD) was purchased from Roquette Frères (Lestrem, France). Lactose (Flowlac® 100) was purchased from DFE pharma (Goch, Germany). Microcrystalline cellulose (MCC, Avicel® PH-102), starch, dicalcium phosphate anhydrous granular (DCP-A), and dicalcium phosphate dihydrate granular (DCP-D) were obtained from Whawon Pharm (Seoul, Korea).
Screening of Solubilizers
To know the solubility characteristics of CLT, solubility of CLT in polymer solutions and pH universal buffers were solubility tested. The solubility tests for CLT were performed in various polymeric solutions (3%, w/v) including P188®, P407®, PVP/VA S630, K12®, PEG6000, IR®, and HS-15®, and universal buffers including pH 1, pH 7, and pH 10 (Fig. S1). However, TPGS®, Soluplus®, and pH 4 universal buffer were excluded due to interference in the UV–vis spectrophotometer. CLT (10 mg) was added to the polymeric solutions, universal buffers, and DW (10 mL) in 20-mL vials with cap under the string at 200 rpm using a multi-channel stirrer (MS-33MH, JEIO TECH, Daejeon, Korea) at 37 ± 1 °C for 24 h [16, 19]. The samples were collected at 0.5, 1, and 24 h. The insoluble CLT was removed by centrifugation (10,000×g, 10 min) (CF-10 microcentrifuge; Daihan Scientific, Wonju, Korea). The supernatants were evaluated by UV–vis spectrophotometer (n = 3).
Preparation of CLT Formulations
Preparation of CLT-Base Formulations (B1-21)
The CLT-base formulations (B) were prepared by solvent evaporation method [20]. Various weak acids or weak bases were used to differentiate the CLT formulations from previous studies. Briefly, CLT (100 mg) and pH agents (weak acid or weak base) (100, 200, and 300 mg) were dispersed in ethyl alcohol (3 mL) for 60 min. The organic solvents in the samples were removed by drying in an oven overnight at 60 °C (Table S1). The obtained powder samples were milled with a mortar and pestle and then passed through a 20-mesh sieve (0.841 mm).
The carrier base formulations (CB) were prepared using the same method as used for the B formulations. From the pre-dissolution test, the B19 formulation (the ratio of CLT vs meglumine [1:1]) with highest per-dissolution (%) was selected. Briefly, CLT (100 mg) and meglumine (100 mg) were dispersed in ethyl alcohol (3 mL) and then mixed with the carrier (such as, Aerosil®200, Aerosil®300, Mannitol, MCC, Flowlac, starch, DCP-A, and DCP-D) for 60 min (Table 1). The organic solvents in the samples were removed by drying in an oven overnight at 60 °C. The obtained powder samples were milled with a mortar and pestle and then passed through a 20 mesh sieve (0.841 mm).
Preparation of CLT-SD Formulations
The SD formulations were prepared using the same method as the CB formulations. From the pre-dissolution test of the CB formulations, CB2 formulation (Aerosil®200) with highest pre-dissolution (%) was selected. To evaluated the effect of polymer, we prepared the SD formulations with polymer based on CB2 formulation. Briefly, CLT (100 mg) and meglumine (100 mg) were dispersed in ethyl alcohol (3 mL) and then the Aerosil®200 and solubilizer were mixed for 60 min (Table 2). The organic solvents in the samples were removed by drying overnight in the oven at 60 °C. The obtained powder samples were milled with a mortar and pestle and then passed through a 20-mesh sieve (0.841 mm). The physical mixtures (PMs) were prepared using the above method without organic solvents.
Pre-dissolution Test of CLT Formulations
The pre-dissolution test of CLT formulations was performed using a multi-channel stirrer (MS-33MH, JEIO TECH, Korea) at 37 ± 1 °C. The CLT formulations (equivalent to 5 mg of CLT) were added to DW (90 mL) containing 0.3% (w/v) SLS with constant stirring at 500 rpm for 60 min [21, 22]. The samples were removed at 5, 15, 30, 45, and 60 min, and the undissolved CLT was removed by centrifugation (10,000×g, 10 min). The supernatants were evaluated by UV–vis spectrophotometer (n = 3).
UV–vis Spectrophotometry
The drug content, pre-dissolution (%), and dissolution (%) of CLT were evaluated at 254 nm using a spectrophotometer (X-ma 1000, Human Co., Korea) [15]. Each measurement was performed in triplicate and compared with a calibration curve (R2 = 0.9992). Blank formulations were prepared and analyzed to rule out any interference because of the excipients.
Dissolution Test
The dissolution tests of pure CLT, commercial product (Pletal®), physical mixture (PM1), and SD1 formulations were performed using a dissolution tester (Distek 6300, New Brunswick, NJ, USA) in DW, and pH 1.2 and pH 6.8 buffers (900 mL) containing 0.3% (w/v) SLS at 37 ± 0.5 °C at 100 rpm, in accordance with the United States Pharmacopeia Apparatus II paddle method. The samples were withdrawn at 5, 15, 30, 45, and 60 min, and the undissolved CLT was removed by centrifugation (10,000×g, 10 min). The supernatants were evaluated using UV–vis spectrophotometer (n = 3).
Physicochemical Properties of CLT-SD Formulation
The thermal analysis of pure CLT, meglumine, Aerosil®200, physical mixture (PM1), and SD1 formulations was evaluated using a differential scanning calorimeter (DSC) 60A (Shimadzu, Japan) [23]. The samples (2–3 mg) were enveloped in hermetically sealed aluminum pans and heated from 5 to 200 °C at a scanning rate of 10 °C/min under a nitrogen purge of 40 mL/min.
The crystallinity of pure CLT, meglumine, Aerosil®200, PM1, SD1, and SD (3 M) formulations was evaluated using a high-resolution X-ray diffractometer (X’pert Pro MRD, PANalytical, Holland). The samples were scanned in 0.02° steps from 0° to 70° (diffraction angle of 2θ) at 40 kV with 150-mA Cu-Kα radiation.
The chemical structures of pure CLT, meglumine, Aerosil®200, PM1, and SD1 formulations were confirmed by Fourier transform infrared (FT-IR) spectrometer (Nicolet6700, Thermo scientific, USA). The spectra were recorded using a frequency range from 4000 to 500 cm−1, with a resolution of 2 cm−1.
Stability Test
The stabilities of CB1 (without Aerosil®200) and CB2 (SD1, with Aerosil®200) were evaluated for drug content and pre-dissolution (%) for 3 months. The samples were stored in capped glass vials at room temperature (20 ± 5.0 °C and relative humidity (RH) 50–60%). Moreover, CB1, CB2 (SD1), and Pletal® were evaluated for solubility. Briefly, CLT (equivalent to 25 mg) in the samples was added to 10 mL of DW containing 0.3% (w/v) SLS in 20-mL vials and then stirred at 500 rpm for 24 h. After 24 h, the undissolved CLT was removed by centrifugation (10,000×g, 10 min). The supernatants were evaluated using UV–vis spectrophotometer (n = 3).
Statistical Analysis
The statistical analysis was performed using Student’s t test using SigmaPlot (ver. 12.5; SYSTAT, Inc., Chicago, IL). The data are expressed as mean ± standard deviation (SD). In all analyses, p < 0.005 (***), p < 0.01 (**), and p < 0.05 (*) were considered statistically significant.
Results and Discussion
Characterization of CLT Formulations
CLT belong to BCS class II drug with poor water solubility. Thus, the solubility test is very important as a starting stage for CLT formulation development. The following solubilizers were screened in 3% aqueous solution: P188®, P407®, PVP/VA S-630, Soluplus®, TPGS, K12®, PEG6000, IR®, and HS-15®. However, Soluplus® and TPGS solutions were excluded because they exhibited severe UV interference. The solubility of CLT in various solutions after 24 h was as follows: P188®, 14.7 ± 2.0μg/mL; P407®, 50.1 ± 3.1 μg/mL; PVP/VA S-630, 38.3 ± 2.5 μg/mL; K12®, 25.8 ± 3.0 μg/mL; PEG6000, 25.7 ± 3.0 μg/mL; IR®, 23.1 ± 2.0μg/mL; and HS 15®, 129.7 ± 6.0 μg/mL (Fig. S1). Among these solubilizers, HS 15® solution showed the highest solubility. Moreover, the solubility of CLT in the universal buffers showed similar results (5–14 μg/mL) with low solubility. However, pH 4 universal buffer was excluded because it exhibited severe UV interference. The solubility of CLT in HS 15® solution was significantly different from that of the other polymer solutions at 24 h (p < 0.005).
The solubility test confirmed that CLT had no effect on pH. However, we hypothesized that the solubility of CLT can be increased with weak acids or weak bases by hydrogen bonding or inter-molecular interaction between CLT and excipients. Thus, the base formulations (B) were designed with weak acids or weak bases (Table S1). The B formulations (1–21) were prepared with different ratios of weak acids or weak bases of CLT (Fig. 1a). Fumaric acid (B4-6 base), MgO (B16-18 base), and meglumine (B19-21 base) were not dissolved in ethyl alcohol but were dispersed during the preparation of the formulations. The B formulations (B1-9 and B12) were excluded from the analysis because they were sticky after preparation and were difficult to collect to powders. The pre-dissolution (%) of CLT in B formulations for 60 min was as follows: tartaric acid (B10 [66.8 ± 3.8%], B11 [61.0 ± 2.5%]), succinic acid (B13 [71.9 ± 6.6%], B14 [69.1 ± 5.3%], B15 [85.8 ± 9.9%]), MgO (B16 [84.3 ± 3.4%], B17 [84.1 ± 2.0%], B18 [82.3 ± 3.5%]), meglumine (B19 [88.1 ± 2.5%], B20 [86.9 ± 3.3%], and B21 [89.6 ± 3.3%]). These results suggest that the weak base formulations were more suitable as a pH agent for CLT than the weak acid. The B19 formulation (meglumine based) was selected because it has a low weight ratio of CLT to pH agent (1:1, weight ratio) high pre-dissolution (%) of CLT. The pre-dissolution (%) of CLT in B19 formulation (meglumine) was significantly different from that of other B formulations (B10–B14) for 60 min (p < 0.005).
Meglumine in B19 formulation was selected as a pH agent by pre-dissolution (%), and the CB formulations were developed to select the appropriate carrier. The CB formulations (1–9) were prepared with various carriers (Table 1). Briefly, CLT and meglumine were fixed at a 1:1 weight ratio and were prepared with one of the carriers (Aerosil®200 [CB2], Aerosil®300 [CB3], Mannitol [CB4], MCC [CB5], Flowlac [CB6], Starch [CB7], DCP-A [CB8], or DCP-D [CB9]). All the CB formulations were successfully obtained as powders. The CB1 formulation was a control without carrier, and the pre-dissolution (%) varied depending on the carrier. The pre-dissolution (%) of CLT in CB formulations for 60 min were as follows: CB1 (84.1 ± 2.0%), CB2 (92.9 ± 1.5%), CB3 (86.0 ± 5.1%), CB4 (88.7 ± 5.7%), CB5 (86.5 ± 1.2%), CB6 (81.2 ± 0.7%), CB7 (87.0 ± 5.0%), CB8 (86.4 ± 4.7%), and CB9 (84.1 ± 1.3%) (Fig. 1b). The pre-dissolution (%) of most of the CB formulations was higher than CB1 formulation (except CB6 [MCC based] and CB9 [DCP-D based]). The CB2 formulation showed the highest pre-dissolution (%) among them. It is thought that Aerosil®200 increased the surface area of CLT and improved the dispersibility and wettability, thereby increasing the pre-dissolution (%) of CLT. In addition, since Aerosil®200 has a large surface area, it is thought that it has a good effect on preventing recrystallization of drugs. In our group, Aerosil®200 had a large surface area, and it has been used earlier as a carrier to increase the solubility and stability of drugs including tadalafil, dutasteride, naftopidil, celecoxib, coenzyme Q10, paclitaxel, and piroxicam [16, 19,20,21,22, 24, 25]. The pre-dissolution (%) of CLT in CB2 formulation was significantly different from that of the other CB formulations (CB4-5 and CB9) for 60 min (p < 0.005).
Characterization of SD Formulations
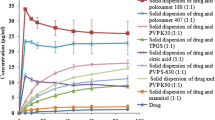
The CB2 formulation was selected as a result of pre-dissolution test in CB formulations (fixed with CLT:meglumine:Aerosil®200 = 1:1:0.5, weight ratio). Since the CB formulations were polymer-free formulations, the SD formulations were developed by adding several polymers to the CB2 formulation. In brief, the SD formulations were composed of CLT:meglumine:Aerosil®200:polymers at a weight ratio of 1:1:0.5:0.5. Most of the SD formulations showed pre-dissolution (%) below 85%. The pre-dissolution (%) of CLT in the SD formulations was as follows: SD1 (92.9 ± 1.5%), SD2 (80.3 ± 4.7%), SD3 (77.3 ± 13.0%), SD4 (86.4 ± 7.2%), SD5 (77.7 ± 4.6%), SD6 (76.4 ± 4.4%), SD7 (72.5 ± 12.0%), SD8 (68.7 ± 8.3%), SD9 (83.0 ± 6.3%), and SD10 (83.3 ± 9.3%) for 60 min (Fig. 2). The addition of the polymer was expected to increase the wettability, but the results were unexpected. The pre-dissolution (%) of half of the SD formulations (80%) were lower than that of the control (92.9%) as SD1 (CB2). These results suggested that the powder hardness of SD formulations (2–10) was higher and their disintegration time was slower than that of the SD1 formulation. Therefore, the polymer-free SD1 formulation was finalized, and dissolution test and the physicochemical evaluations were performed. The pre-dissolution (%) of CLT in SD1 formulation was significantly different from that of the other SD formulations (5–9) for 60 min (p < 0.005).
Dissolution Study
Dissolution tests were evaluated with different dissolution media by a dissolution tester (Fig. 3). The samples (pure CLT, Pletal®, PM1, and SD1; equivalent to 50 mg of CLT) were added into the media with 0.3% SLS (w/v, 900 mL) and stirred at 100 rpm for 60 min. The dissolution (%) of CLT in DW containing 0.3% SLS for 60 min was as follows: pure CLT (76.6 ± 6.2%), Pletal® (82.0 ± 0.9%), PM1 (79.7 ± 3.5%), and SD1 (95.6 ± 0.5%). The dissolution (%) of CLT in pH 1.2 media containing 0.3% SLS for 60 min was as follows: pure CLT (75.8 ± 4.0%), Pletal® (80.1 ± 0.9%), PM1 (77.4 ± 3.9%), and SD1 (91.2 ± 1.7%). The dissolution (%) of CLT in pH 6.8 buffer containing 0.3% SLS for 60 min was as follows: pure CLT (75.9 ± 3.9%), Pletal® (81.2 ± 1.1%), PM1 (77.5 ± 4.5%), and SD1 (93.3 ± 4.0%). The SD1 formulation improved the dissolution (%) of pure CLT, Pletal®, and PM1 by 1.24-/1.16-/1.20-fold, 1.20-/1.13-/1.18-fold, and 1.24-/1.16-/1.20-fold in DW/pH 1.2/pH 6.8 buffer, respectively, compared with those. The dissolution (%) of CLT in the SD1 formulation was significantly different from that of the other formulations at 60 min in all media (p < 0.005).
In previous studies, using the SAS process, the dissolution (%) of CLT-micronizing formulations (50 mg/900 mL) were observed to be about 95% as compared to that of the pure CLT (20%) in pH 1.2 media containing 0.3% SLS (w/v) for 60 min [4]. The dissolution (%) of sub-micron-sized CLT formulations were about 80% as compared to that of the commercial tablet (about 85%) in DW containing 0.3% SLS (w/v) [17]. The dissolution (%) of CLT-SD formulations (50 mg/900 mL) were above 80% as compared to that of the pure CLT (20%) in pH 1.2 media containing 0.3% SLS (w/v) for 60 min [7]. In spray-drying process, the dissolution (%) of CLT-SD formulations (CLT:HPMC, 1:4, w/w) (50 mg/900 mL) were above 75% as compared to that of the physical mixture (30%) in pH 1.2 media containing 0.3% SLS (w/v) for 60 min [8]. The dissolution of CLT-Eudragit SD formulations were above 12% as compared to that of Pletal® (3%) in pH 6.8 media for 60 min [10]. The dissolution (%) of CLT complex was about 80% as compared to that of the physical mixture (30%) in DW containing 0.3% SLS (w/v) for 60 min [6]. The CLT loaded MCM-48-type and MCM-41-type mesoporous silica in acetic acid were prepared by the solvent evaporation process. The dissolution (%) of the CLT-SD formulations (CLT-MCM-48, 1:3, w/w) were above 80% as compared to that of the pure CLT (10%) in DW containing 0.3% SLS (w/v) for 60 min [9]. The dissolution (%) of CLT-SD formulation in polyvinylpyrrolidone (K30®) and SLS by solvent-evaporation process were determined to be above 60% as compared to that of the pure CLT (20%) in DW containing 0.3% Tween® (w/v) for 60 min [18]. The CLT-SD formulations with SLS and lactose (CLT:SLS:lactose, 10:1:20) were prepared using the solvent evaporation method. The dissolution (%) of CLT-SD formulation (solvent-evaporation) were above 90% as compared to that of Pletal® (70%) in pH 6.8 buffer containing 0.2% SLS (w/v) for 60 min [11]. In salt process, the dissolution (%) of CLT-mesylate and CLT-besylate was about 40% compared with that of the pure CLT (0%) in pH 1.2 media for 60 min [14]. In fattigation process, the dissolution (%) of CLT-SD formulation (100 mg/900 mL) was above 70% as compared to that of PM (50%) in pH 6.8 buffer containing 0.2% SLS (w/v) for 60 min [13].
In the abovementioned previous studies, the dissolution (%) of CLT-micronizing formulation with the SAS process showed the highest dissolution (%) (95%) in pH 1.2 media containing 0.3% SLS (w/v) for 60 min [4]. Our SD1 formulation was facile preparation and did not require expensive equipment and could be manufactured with only a stirrer. Moreover, the dissolution (%) of CLT in the SD1 formulation was observed to be 95.6 ± 0.5% (DW), 91.2 ± 1.7% (pH 1.2 media), and 93.3 ± 4.0% (pH 6.8 buffer), containing 0.3% SLS (w/v) for 60 min. The previous paper has not conducted formulation evaluation of CLT-SD formulations in various dissolution media. Thus, Our SD1 formulation is a clear progress formulation compared with that reported in previous studies.
Thermal Properties of CLT in SD1 Formulation
The thermal property of CLT in SD1 formulation was evaluated by DSC (Fig. 4). The melting peaks of the samples were found to be CLT (161 °C), meglumine (132.5 °C), Aerosil®200 (none), PM1 (132.5 and 155.5 °C), and SD1 (131.1 and 155.6 °C). PM1 showed two melting peaks for meglumine (132.5 °C) and CLT (155.5 °C). SD1 showed two melting peaks for meglumine (131.1 °C) and CLT (155.6 °C). Both PM1 and SD1 showed slightly shifted to low temperature compare to that of pure CLT. However, the melting peaks of SD1 showed similar to those of PM1. These results showed that the thermal property of CLT in SD1 were almost unchanged.
PXRD
The crystallization of CLT in SD1 was evaluated by powder X-ray diffraction (PXRD). The PXRD patterns of CLT, meglumine, Aerosil®200, PM1, and SD1 are shown in Fig. 5a, b). The main 2θ peaks of CLT were found at 9.2, 12.7, 14.1, 15.6, 17.9, 18.7, 19.3, 20.2, 20.7, 22.1, 23.4, 24.0, 24.8, 25.6, 27.2, and 31.5. The main 2θ peaks of meglumine were found at 8.9, 9.4, 12.4, 17.3, 17.9, 19.5, 19.9, 21.9, 24.1, 27.0, 28.5, and 34.5. The main 2θ peaks of PM1 were found at 9.0, 12.4, 12.7, 15.7, 17.3, 18.0, 18.7, 19.4, 19.6, 19.9, 20.7, 22.1, 23.4, 24.1, 24.9, 27.2, and 28.5. The main 2θ peaks of SD1 were found at 8.9, 12.4, 12.7, 15.6, 17.3, 17.9, 18.7, 19.4, 19.5, 20.7, 21.9, 23.4, 24.1, 24.9, 27.2, and 28.5. In SD1, the main 2θ peaks at 8.9 (related to meglumine) differed from that of PM, and the next peaks disappeared at 19.9 (related to meglumine) and 22.1 (related to CLT). Overall, the peaks corresponding to the intensity of CLT in PM1 and SD1 decreased. In particular, the intensity of main 2θ peaks in SD1 were decreased compared to those of PM1 at 8.9, 12.4, 12.7, 17.3, 17.9, 18.7, 19.4, 19.5, 20.7, 23.4, 24.1, 24.9, 27.2, and 28.5 (Fig. 5b). The main 2θ peaks in SD1 observed at 8.9, 12.4, 17.3, 17.9, 19.4, 19.5, 24.1, and 28.5 (related to meglumine) and at 12.7, 17.9, 18.7, 19.4, 20.7, 23.4, 24.9, and 27.2 (related to CLT). In particular, SD1 showed a significant decrease at 8.9, 12.4, 17.9, 21.9, 24.1, 27.2, and 28.5. In previous studies, the crystalline peaks of piroxicam and tadalafil in SD formulation observed but the crystalline peaks were decreased compared to that of physical mixture, respectively [25,26,27,28]. The authors suggested that the crystalline and amorphous form were coexisted in SD formulation. Our results were thought to have changed the crystallinity of CLT in SD1 formulation. It is thought that the dissolution (%) of CLT in SD1 formulation was improved due to this change in CLT crystallinity.
ATR-FT-IR Spectroscopy
The interaction between CLT and meglumine in SD1 was evaluated by attenuated total reflectance-Fourier transform infrared (ATR-FT-IR) spectroscopy (Fig. 6a, b). The FT-IR spectra of CLT showed the main absorbance bands of C=O stretch (1667.7 cm−1) in tetrazole moiety at 1504.9 cm−1, N=N stretching of tetrazole moiety at 1296.9 cm−1, aromatic ether stretch at 1196.4 cm−1, and NH stretching of quinolinone moiety from 3340.6–3058.8 cm−1 [6]. The FT-IR spectra of PM1 showed the main absorbance bands of C=O stretch (1668.0 cm−1) in tetrazole moiety at 1505.2 cm−1 and N=N stretching of tetrazole moiety at 1243.0 cm−1. The FT-IR spectra of SD1 showed the main absorbance bands of C=O stretch (1668.1 cm−1) in tetrazole moiety at 1505.0 cm−1 and N=N stretching of tetrazole moiety at 1243.1 cm−1. The bands observed in PM1 and SD1 spectra at 3247.7 cm−1 and 3331.1 cm−1 were attributed to NH and OH stretching in meglumine. The peaks intensity of SD1 was decreased compared to those of PM1 (Fig. 6b). Almost all peaks in SD1 formulation did not change, possibly owing to the inter-molecular interactions as the dissolution (%) in SD1 formulation was greatly increased.
Stability Study
CB1 and CB2 (SD1) formulations were evaluated for their stability, including drug content and pre-dissolution (%) for 3 months. The reason for comparing CB1 and CB2 (SD1) was that CB1 is composed of CLT and meglumine, whereas CB2 (SD1) is composed of meglumine and Aerosil®200; therefore, the stability can be confirmed depending on the presence or absence of Aerosil®200. The drug content of CB1 and CB2 (SD1) formulations was well maintained for 3 months and was observed to be 99.5 ± 1.3% and 99.9 ± 1.1% (initial day), 101.0 ± 2.3% and 100.5 ± 2.1% (1 month), 100.2 ± 1.9% and 100.9 ± 2.3% (2 months), and 99.9 ± 2.1% and 100.2 ± 1.3% (3 months), respectively.
As shown in Fig. S2, the pre-dissolution (%) of CB1 was 87.4 ± 1.9% (initial day), 78.8 ± 3.4% (1 month), 69.9 ± 0.5% (2 months), and 70.5 ± 1.5% (3 months). The reduction in the pre-dissolution (%) of CB1 resulted in the reduction of dissolution (%) by 17% over a period of 3 months. However, the pre-dissolution (%) of CB2 (SD1) was 92.9 ± 1.5% (initial day), 92.6 ± 4.6% (1 month), 93.1 ± 1.3% (2 months), and 92.0 ± 2.2% (3 months). The pre-dissolution (%) of CB2 (SD1) did not change. These results suggest that SD1 has optimal stability. As a result, it was confirmed that Aerosil®200 significantly affects the stability as well as the dissolution (%) of CLT.
The solubility of the samples was found to be 147.8 ± 18.9 μg/mL (CB1) and 184.9 ± 14.2 μg/mL (CB2 [SD1]) at the initial day. After 3 months, the solubility of the samples were 131.5 ± 5.0 μg/mL (CB1) and 182.7 ± 11.3 μg/mL (CB2 [SD1]). The solubility of Pletal® was 142.0 ± 8.3 μg/mL. The solubility of CB1 and CB2 (SD1) decreased by 11.0% and 2.8%, respectively. The SD1 formulation still maintained high solubility. In XRD, there was no change in the crystallinity of CLT SD1 formulation after 3 months in initial SD1 formulations. Therefore, SD1 formulation was also stable for 3 months (Fig. 5a). In a previous study, the drug content of CLT-mesylate and CLT-besylate were well maintained under the conditions, 40 °C ± 2 °C/75% ± 5% RH and at 60 °C ± 2 °C for 6 weeks [14]. However, the author did not evaluate the stability including dissolution (%) and crystallinity. Moreover, other previous studies did not evaluate the stability of CLT-SD formulations at all. Therefore, our SD1 formulation is superior to other SD formulations in previous studies that it not only exhibits high dissolution (%) but also stability, including that of the drug content and pre-dissolution (%).
Conclusions
Cilostazol (CLT) is a BCS class II with low water solubility and pH-independent drug. We prepared solid dispersions using meglumine and Aerosil®200 to solubilize and stabilize CLT. The SD1 formulation is a polymer-free formulation with a dissolution (%) of 1.20-/1.13-/1.18-fold in DW/pH 1.2/pH 6.8 buffer, respectively. The physical properties of SD1 formulation in terms of thermal changes, crystallinity, and drug-excipient interactions showed slightly changed.
The inter-molecular interaction of CLT-meglumine may have improved the solubility and dissolution (%) of CLT. These results also influenced the stability of SD1 formulation for 3 months. The SD1 formulation with improved solubility and dissolution (%) of CLT has the potential to improve the oral bioavailability of CLT in animal studies.
Abbreviations
- (CLT):
-
Cilostazol
- (SD):
-
Solid dispersion
- (DW):
-
Distilled water
- (DSC):
-
Differential scanning calorimetry
- (PXRD):
-
Powder X-ray diffraction
- (ATR-FT-IR) spectroscopy:
-
Attenuated total reflectance-Fourier transform infrared spectroscopy
- (SLS):
-
Sodium lauryl sulfate
References
McDermott MM. Medical management of functional impairment in peripheral artery disease: a review. Prog Cardiovasc Dis. 2018;60:586–92.
Bedenis R, Stewart M, Cleanthis M, Robless P, Mikhailidis DP, Stansby G. Cilostazol for intermittent claudication, Cochrane. Database. Syst. Rev., (2014).
Jinno JI, Kamada N, Miyake M, Yamada K, Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K, Kimura T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J Control Release. 2006;111:56–64.
Kim MS, Lee S, Park JS, Woo JS, Hwang SJ. Micronization of cilostazol using supercritical antisolvent (SAS) process: effect of process parameters. Powder Technol. 2007;177:64–70.
Woo SK, Kang WK, Kwon KI. Pharmacokinetic and pharmacodynamic modeling of the antiplatelet and cardiovascular effects of cilostazol in healthy humans, Clin. Pharmacol. Ther., 71 (2002) 246–252.
Desai C, Prabhakar B. Development and evaluation of orally disintegrating tablets of cilostazol-β-cyclodextrin inclusion complexes. Drug Dev Ind Pharm. 2015;41:1589–607.
Kim MS, Kim JS, Hwang SJ. Enhancement of wettability and dissolution properties of cilostazol using the supercritical antisolvent process: effect of various additives. Chem Pharm Bull. 2010;58:230–3.
Park GB, Yoon H, Bae JW, Kim YU, Jeon DY, Song JE, Lee D, Khang G. Release behavior of cilostazol according to the fabrication methods and ratio of HPMC/PVP. Macromol Res. 2013;21:971–6.
Wang Y, Sun L, Jiang T, Zhang J, Zhang C, Sun C, Deng Y, Sun J, Wang S. The investigation of MCM-48-type and MCM-41-type mesoporous silica as oral solid dispersion carriers for water insoluble cilostazol. Drug Dev Ind Pharm. 2014;40:819–28.
Park JH, Choi HK. Enhancement of solubility and dissolution of cilostazol by solid dispersion technique. Arch Pharmacal Res. 2015;38:1336–44.
Baek N, Oh GH, Park C, Tran TTT, Park YJ, Oh E, Le H, Tran TT, Park JB, Lee BJ. Reprecipitation of poorly water-soluble cilostazol crystals using adsorbing carriers for enhanced dissolution and physicochemical modification. J Drug Deliv Sci Technol. 2018;43:477–86.
Shin KH, Yoon G, Yoon IS, Park JW. Preparation and evaluation of oral controlled-release cilostazol formulation: pharmacokinetics and antithrombotic efficacy in dogs and healthy male K orean participants. J Pharm Pharmacol. 2014;66:961–74.
Kim D, Park C, Meghani NM, Tran TT, Tran PH, Park JB, Lee BJ. Utilization of a fattigation platform gelatin-oleic acid sodium salt conjugate as a novel solubilizing adjuvant for poorly water-soluble drugs via self-assembly and nanonization, Int. J. Pharm., (2019) 118892.
Seo JH, Park JB, Choi WK, Park S, Sung YJ, Oh E, Bae SK. Improved oral absorption of cilostazol via sulfonate salt formation with mesylate and besylate. Drug Des Dev Ther. 2015;9:3961.
Choi JS, Design of cilostazol nanocrystals for improved solubility, J. Pharm. Innov., (2019) 1–8.
Choi JS, Ahn JB, Park JS. Amorphous multi-system of celecoxib improves its anti-inflammatory activity in vitro and oral absorption in rats. Int J Pharm. 2019;555:135–45.
Jinno JI, Kamada N, Miyake M, Yamada K, Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K, Kimura T. In vitro–in vivo correlation for wet-milled tablet of poorly water-soluble cilostazol. J Control Release. 2008;130:29–37.
Mustapha O, Kim KS, Shafique S, Kim DS, Jin SG, Seo YG, Youn YS, Oh KT, Yong CS, Kim JO. Comparison of three different types of cilostazol-loaded solid dispersion: physicochemical characterization and pharmacokinetics in rats. Colloids Surf B Biointerfaces. 2017;154:89–95.
Choi JS, Park JW, Park JS. Design of Coenzyme Q10 solid dispersion for improved solubilization and stability. Int J Pharm. 2019;572:118832.
Choi JS, Cho NH, Kim DH, Park JS. Comparison of paclitaxel solid dispersion and polymeric micelles for improved oral bioavailability and in vitro anti-cancer effects. Mater Sci Eng C. 2019;100:247–59.
Choi JS, Byeon JC, Park JS. Naftopidil-fumaric acid interaction in a solid dispersion system: improving the dissolution rate and oral absorption of naftopidil in rats. Mater Sci Eng C. 2019;95:264–74.
Choi JS, Lee SE, Jang WS, Byeon JC, Park JS. Solid dispersion of dutasteride using the solvent evaporation method: approaches to improve dissolution rate and oral bioavailability in rats. Mater Sci Eng C. 2018;90:387–96.
Liu X, Feng X, Williams RO, Zhang F. Characterization of amorphous solid dispersions. J Pharm Investig. 2018;48:19–41.
Choi JS, Park JS. Design of PVP/VA S-630 based tadalafil solid dispersion to enhance the dissolution rate. Eur J Pharm Sci. 2017;97:269–76.
Sohn JS, Kim EJ, Park JW, Choi JS. Piroxicam ternary solid dispersion system for improvement of dissolution (%) and in vitro anti-inflammation effects. Mater Sci Eng B Solid State Mater Adv Technol. 2020;261:114651.
Yu H, Chun MK, Choi HK. Preparation and characterization of piroxicam/poloxamer solid dispersion prepared by melting method and solvent method. J Pharm Investig. 2007;37:1–5.
Pereira SV, Colombo FB, de Freitas LAP. Ultrasound influence on the solubility of solid dispersions prepared for a poorly soluble drug. Ultrason Sonochem. 2016;29:461–9.
Sohn JS, Choi JS. Solubilization of tadalafil using a tartaric acid and chitosan-based multi-system, Int. J. Biol. Macromol., (2020).
Funding
This work was supported by the National Research Foundation of Korea grant, Korea government [grant number NRF-2020R1G1A1100266].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choi, D., Choi, JS. Cilostazol Solubilization and Stabilization Using a Polymer-Free Solid Dispersion System. J Pharm Innov 17, 521–533 (2022). https://doi.org/10.1007/s12247-021-09533-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09533-w