Abstract
The aim of this study was to investigate the influence of factors such as carrier type, drug/carrier ratio, binary carriers, and preparation method on the dissolution of an insoluble drug, indomethacin (IM), under supersaturation conditions. Using a solvent evaporation (SE) method, poloxamer 188 and PVP K30 showed better dissolution among the selected carriers. Furthermore, as the ratio of carriers increased (drug/carrier ratio from 1:0.5 to 1:2), the dissolution rate increased especially in almost two times poloxamer 188 solid dispersions (SDs), while the reverse results were observed for PVP K30 SDs. For the binary carrier SD, a lower dissolution was found. Under hot melt extrusion (HME), the dissolution of poloxamer 188 SD and PVP K30 SD was 0.83- and 0.94-folds lower than that using SE, respectively, while the binary carrier SD showed the best dissolution. For poloxamer 188 SDs, the drug’s crystal form changed when using SE, while no crystal form change was observed using HME. IM was amorphous in PVP K30 SDs prepared by both methods. For binary carrier systems, amorphous and crystalline drugs coexisted in SD using SE, and negligible amorphous IM was in SD using HME. This study indicated that a higher amorphous proportion in SD did not correlate with higher dissolution rate, and other factors, such as carrier type, particle size, and density, were also critical.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In recent years, combinatorial chemistry and high-throughput screening have led to the discovery of many active pharmaceutical ingredients (1). According to the literature, more than 40% of new chemical entities discovered by these techniques are compounds with poor aqueous solubility (2). Such compounds with low solubility and high permeability are categorized as class II drugs according to the Biopharmaceutics Classification System (3). These drugs are poorly soluble in water, but once they are dissolved, they are easily absorbed from the gastrointestinal tract. Their oral absorption is thus dependent upon dissolution rate (4). Thus, a great challenge for pharmaceutical technology is to create new formulations and efficient drug delivery systems to overcome these dissolution problems. Several methods have been employed to enhance the dissolution rate and bioavailability of these poorly water-soluble drugs. These methods include particle size reduction, cyclodextrin complexation, co-solvency, solid dispersion (SD), salt formation, polymorphs, solvates or hydrates, pro-drugs, and microparticulates (liposomes, microspheres, etc.) (5,6). SD is one of the first and most commonly used techniques to date for enhancing the dissolution rate of these drugs (5). Generally, SD may be defined as a pharmaceutical dosage forms in which the drug is incorporated in an inert hydrophilic carrier or matrix in the solid state. In order to prepare more stable and better dissolution SDs, combinations of various characteristic carriers have been developed in recent years (7,8). These approaches encompass a variety of formulation types because the matrix could be in the crystal or amorphous state; similarly, the drug could also be dispersed as amorphous or crystalline particles or molecularly dispersed in the carrier matrix (9). Different techniques have been used to obtain SDs, such as solvent evaporation (SE), kneading method, hot melt extrusion (HME), spray-drying, freeze-drying, and supercritical fluid technology (6,10). Often one method of preparation is arbitrarily selected, and the formulation scientist modifies the formulation until the desired product performance is achieved. However, processing of materials using different technologies may yield a product with significantly different properties and performance (11,12). In this research, SE and HME technologies were selected to prepare different SDs. In addition to the properties of drug and carriers (4,13) and the preparation method, the drug/carrier ratio also plays an important role in the performance of SD. It is has an impact not only on the dissolution rate (14,15) but also on the long-term stability of SD (16).
The drug dissolution test is proposed to be an important factor for the assessment of drug bioavailability and is considered as the most investigated topic in pharmaceutical research (17). Such a background becomes of paramount relevance in the case of insoluble drugs, where dissolution represents the most critical factor affecting the rate of systemic absorption. Currently, there are two main dissolution methods for assessing release profiles of drugs in SDs, namely dissolution under sink and superheated condition (18–20). In general, sink conditions during the dissolution test are essential for the common dosage forms in order to simulate an in vivo situation, where gastrointestinal absorption continuously reduces the concentration of the drug in the fluids. However, the sink condition, obtained by a high concentration of surfactant, may not be an appropriate approach for developing a biorelevant supersaturated dissolution method such as solid dispersion. The supersaturated condition may represent a very discriminating dissolution condition, acting like a magnifying lens for an in-depth evaluation of the dissolution phenomenology. Nucleation and crystallization of drugs in supersaturated conditions are easily observed, and this plays a key role in determining the in vivo performance of SDs. The drug dissolution test, under supersaturated conditions, can be a predictive tool during formulation development, as well as for batch-to-batch quality control. Therefore, drug dissolution tests under supersaturated conditions were selected in this research. In clinical trials, some water-insoluble drugs are needed to achieve high concentrations in gastric fluids to get immediate effective treatment; thus, a pH 2.0 medium was used as the dissolution medium. Indomethacin (IM), which was classified as a class II compound, was selected as a model drug in this study because of its poor solubility in acidic media (21).
Generally, a dissolution test will be accomplished when one SD preparation is characterized. However, a limited amount of research has been directed towards investigating the factors that influence the dissolution of SD. The objective of this study is to investigate the influence of factors such as carrier type, drug/carrier ratio, binary carriers, and preparation method on the dissolution of an insoluble drug, IM, under a supersaturation condition in pH 2.0. The SDs are thoroughly characterized by X-ray diffraction (XRD) and differential scanning calorimetry (DSC) in order to investigate their dissolution behavior.
MATERIALS AND METHODS
Materials
IM was purchased from Kangbaotai Fine-Chemicals Co., Ltd. (Hubei, China). PVP K30, PVP K90, and PVP S-630 were supplied from the China Division of ISP Chemicals Co., Ltd. (Shanghai, China). Poloxamer 188, poloxamer 407, and mannitol were obtained from BASF Co., Ltd. (Shanghai, China). d-Alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) and citric acid were gift samples from ISOCHEM (Wilmington, USA) and the China Division of Merck Co., Ltd. (Shanghai, China), respectively. Absolute ethanol (99.5%), hydrochloric acid, and acetone were supplied by Adamas-beta (Shanghai, China).
Preparation of the Physical Mixtures and SDs
Preparation of Physical Mixtures
Physical mixtures of IM and different carriers were prepared by thorough mixing in a polyethylene bag at the ratio 1:1 (w/w) or 1:0.5:0.5 (w/w/w).
Preparation of SDs by SE
Single carrier SDs with a drug/carrier ratio of 1:0.5, 1:1, and 1:2 and binary carrier SD with a drug/carrier A/carrier B ratio of 1:0.5:0.5 were prepared by dissolving specific amounts of carrier or carriers and IM in ethanol (the batch size is 10 g, and specific amounts of carrier or carriers were determined according to the drug/carrier ratio), followed by evaporation under reduced pressure at 50°C in a rotary evaporator to remove the solvent. Any residual solvent was removed by drying in a vacuum oven for 24 h at 45°C. The solid samples (not including waxy TPGS SD) were ground gently with a mortar and pestle, passed through a 60-mesh sieve, and stored at −20°C in a freezer until the next experiment.
Preparation of SDs by HME
The carrier or carriers and IM were pre-mixed (the batch size is 200 g) and then manually fed into a twin-screw extruder (Thermo Scientific Pharma 11; Thermo Fisher Scientific, Shanghai, China) at a screw speed of 20–25 rpm. In the extruder, both screws were rotated in the same direction and the eight independent barrel heaters were set depending on the characteristics of the drug and carrier or carriers. After extrusion through a 2-mm circular die, the strands were cooled to room temperature, ground gently with a mortar and pestle, passed through a 60-mesh sieve, and stored at −20°C until the next experiment.
Dissolution Test Under Supersaturated Conditions
Dissolution tests were performed with a RCZ-8M dissolution apparatus with RZQ-8D auto-sampling and Collection System (Tianda Tianfa Technology Co., Ltd., Tianjin, China) using the paddle method according to USP 40. Excess amounts of samples equivalent to 55.6 mg of IM were placed in 500 mL simulated gastric fluid without pepsin (pH 2.0). The paddle rotation speed was set at 100 rpm, and the temperature was maintained at 37 ± 0.5°C. At predetermined intervals (5, 10, 15, 30, 45, 60, and 90 min), 5 mL of the sample was withdrawn, filtered through a 0.45-μm membrane filter, and assayed by a spectrophotometer at 320 nm (UV2400PC UV-Visible Spectrophotometer; Shanghai Shunyu Hengping Scientific Instrument Co., Ltd., Shanghai, China). Fresh medium was added to maintain a constant volume after each sampling. Triplicate runs were carried out.
Effect of Carrier on the Solubility of Indomethacin
The equilibrium solubility of crystalline IM in the solution of pH 2.0 was measured at 37 ± 0.5°C in the presence and absence of the different carriers. To do this, 55.6 mg of IM was dispersed in 500 mL of test fluid at pH 2.0, in which 0.5% (w/v) of carrier or carriers had been previously dissolved and stirred at 100 rpm. After 24 h, the concentration of IM in the solutions was measured by UV. The solubility of IM in the test fluid in the absence of carrier or carriers was also evaluated. All measurements were carried out in triplicate.
Inhibitory Effect of Carriers on Recrystallization from Supersaturated Solutions
The effect of the carriers on the concentration-time profile was assessed by generating an initial supersaturated solution of IM in the test fluid. For this procedure, 0.5 g IM was dissolved in acetone of 20 mL, and then 1 mL of the solution was added to 500 mL of the test fluid, in which 25 mg of the carrier or carriers had been previously dissolved, leading to a final carrier concentration of 50 μg/mL. The temperature of the test fluid was held at 37 ± 0.5°C, and the paddle rotation speed was 100 rpm. The concentration of IM in the solution was measured at predetermined intervals (5, 10, 15, 30, 45, 60, and 90 min), using a UV spectrometer described above. Triplicate measurements were carried out.
X-ray Diffraction Analysis
XRD profiles were obtained using an X-ray diffractometer (Bruker D8 Advance, Bruker Co., Ltd., Germany) under the following conditions: target CuKα radiation, voltage 40 kV, and current 40 mA at ambient temperature. The samples were analyzed in the 2θ range 3°–40° with an increment of 0.02° and a scanning speed of 0.1 s/step.
Differential Scanning Calorimetry
DSC Q1000 from TA Universal Instruments was used to conduct DSC and modulated DSC (mDSC) tests. Approximately 3–5 mg of the powdered sample was accurately weighed, placed in an aluminum pan, and crimped with an aluminum lid. The heating rate for DSC was 10°C/min from 30 to 180°C under a nitrogen flow of 50 mL/min. The mDSC tests were used to detect the amorphous samples by a heating rate of 1°C/min with a modulation of ±0.50°C every 40 s over the range ~30–124°C. The DSC and mDSC data were analyzed by using Universal Analysis software (version 4.5A, TA Instruments).
RESULTS AND DISCUSSION
Carrier Type
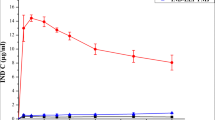
In order to investigate the effect of carrier type on the dissolution of IM from different SDs in order to further study, SDs of IM and different hydrophilic carriers, at a 1:1 (w/w) ratio, were prepared by SE, and their dissolution patterns under a supersaturation condition in the pH 2.0 medium were compared. Figure 1 illustrates the dissolution profiles of pure IM and its SDs. It was evident that the dissolution rate of pure IM was very low, exhibiting a maximum concentration of 1.17 μg/mL in 90 min. While SDs of IM with various hydrophilic carriers enhanced the dissolution rate to different degrees compared to the pure drug, poloxamer 188 SD gave the best dissolution rate of IM among the used carriers with a maximum concentration of 33.83 μg/mL in 5 min. However, after a fast release of IM within 5 min, parts of the dissolved drug gradually recrystallized from the solution, and this may be due to the low solubility of IM at pH 2.0 (Table I). Dissolution profiles of poloxamer 407 SD and citric acid SD also showed a fast release of IM within 5 min; the maximum concentrations were 22.87 and 11.31 μg/mL, respectively, but the degree of supersaturation was lower than that of poloxamer 188 SD. As shown in Fig. 1, the SDs containing mannitol, PVP K30, PVP K90, PVP S-630, and TPGS showed a relatively slower release rate of IM in the dissolution medium with maximum concentrations of 2.02, 15.43, 14.57, 11.27, and 9.04 μg/mL in 90 min, respectively.
The carrier type is known to be an important factor affecting the characteristics of SDs (10). Different carriers have various characteristics, such as viscosity, solubility, and wettability, and therefore, different dissolution behaviors appear in the dissolution test. In this study, it was evident that poloxamer 188 SD resulted in greater enhancement of dissolution rate than other SDs. Poloxamers are nonionic polyoxyethylene–polyoxypropylene copolymer nonionic surfactants used primarily in pharmaceutical formulations as emulsifying, wetting, and solubilizing agents. The polyoxyethylene segment is hydrophilic while the polyoxypropylene segment is hydrophobic. Poloxamer 188 is freely soluble in water and ethanol and has a melting point of 52–57°C; it is used in a variety of oral, parenteral, and topical pharmaceutical formulations, which is generally regarded as nontoxic and nonirritant materials (22).
In recent years, poloxamer 188 has been widely used to prepare SDs with many water-insoluble drugs (14,23), improving the dissolution rate markedly. Although poloxamer 188 was used by Chokshi et al. (24) to prepare SD with IM by HME, they only characterized its physicomechanical properties to assess suitability for HME, without carrying the dissolution of SDs. Therefore, poloxamer 188 was selected for further investigation in this study.
Currently, a major strategy used to obtain good physical stability (16), as well as enhanced dissolution and oral bioavailability, is to use glass solution SDs. In these systems, the active pharmaceutical ingredient (API) is combined with an amorphous polymer to produce a single-phase amorphous mixture of the API and the polymer. Among the amorphous polymers used in the present study, PVP K30 gave the best improvement on the dissolution rate of IM. This polymer, with a high T g value around 154°C, could prevent mobilization and recrystallization of drugs following long storage times, as well as improve the amorphous state stability of drugs (25). PVP K30 is freely soluble in water and ethanol. It has very hygroscopic, significant amounts of moisture being absorbed at low relative humidity levels. When consumed orally, PVP K30 may be regarded as essentially nontoxic since it is not absorbed from the gastrointestinal tract or mucous membranes. Therefore, PVP K30 proved to be a potential carrier and has been used in various SD formulations, such as carbamazepine, carvedilol, and loratadine SDs, improving the solubility or dissolution rate obviously (11,26,27). Although PVP K30 was used to prepare SDs with IM by some researchers, they focused on the interaction between PVP and IM and physical properties of SDs, without considering their dissolution behavior (28,29). Therefore, PVP K30 was subjected for further investigation.
The Drug/Carrier Ratio
SDs of the selected carriers with different drug/carrier ratios (w/w) were prepared by SE. Three different ratios were chosen to determine how they would affect the dissolution of poloxamer 188 SDs, interestingly; these solutions become cloudy a few seconds after the white powdery poloxamer 188 SDs were incorporated in the solution. This cloudiness existed during the dissolution test, possibly because of the rapid crystallization of the drug. The dissolution profiles are shown in Fig. 2. The physical mixture (PM) of IM and poloxamer 188 showed improvement in the dissolution rate compared with that of the pure drug, while the poloxamer 188 SDs showed marked enhancement in the dissolution characteristics relative to the pure drug and the PM. Furthermore, it was clear that the degree of supersaturation increased as the proportion of poloxamer 188 in the SDs increased. When the drug/carrier ratio was 1:2 (w/w), the maximum concentration was 45.67 μg/mL, which was 1.35-fold and 39.03-fold higher than that of poloxamer 188 SD at the ratio of 1:1 and pure drug, respectively.
Meanwhile, the same drug/carrier ratios were chosen to determine how they would affect the dissolution of PVP K30 SDs. As shown in Fig. 3, the PM of IM and PVP K30 had a slight improvement in the dissolution rate compared to the pure drug. The drug was released gradually from PVP K30 SDs, which was different from the fast release pattern of poloxamer 188 SDs. Figure 3 also shows that when the proportion of PVP K30 increased, the dissolution rate was suppressed. The maximum concentration of PVP K30 SD with the drug/carrier ratio of 1:2 (w/w) was 8.76 μg/mL in 90 min, which was only half of that of PVP K30 SD with the drug/carrier ratio of 1:0.5 (w/w).
The XRD patterns of IM, poloxamer 188, PM, and poloxamer 188 SDs are shown in Fig. 4. The diffraction spectrum of IM showed that the drug was a highly crystalline powder, which produced sharp peaks at 2θ equal to 11.6°, 17.1°, 19.6°, 21.0°, 21.9°, 26.7°, 29.5°, and 33.7°. This corresponded to the γ-crystalline form polymorph of IM (30). Poloxamer 188 showed two prominent peaks with the highest intensity at 2θ of 19.1° and 23.3°. All the characteristic peaks of poloxamer 188 and IM were present in their PM. The diffraction spectrum of poloxamer 188 SDs with different drug/carrier ratios presented several new sharp peaks (7.0°, 8.5°, 12.0°, 14.0°, 14.3°, 14.6°, 24.6°), which are not associated with IM of γ-form, indicating the formation of a new crystalline form. Furthermore, as the proportion of poloxamer 188 in the SDs increased, several peaks associated with IM of γ-form (17.1°, 19.6°, 21.0°, 21.9°, 26.7°, 29.5°) gradually disappeared. These variations in spectrums suggest that IM of γ-form gradually transformed into a new crystalline form with increased poloxamer 188 in the SDs. By searching the literature (30,31), we found that the new crystalline form was α-form, which is a high-energy metastable form of IM. It is known that the drug in the metastable form has higher apparent solubility than the drug in the stable crystalline form, which results in a higher dissolution rate. An increase in the amount of poloxamer 188 resulted in an increased dissolution rate of the drug, possibly due to the higher proportion of IM of α-form in the SDs.
Thermograms of IM, poloxamer 188, and its PM and SDs are shown in Fig. 5. The thermogram of pure IM showed a sharp endotherm at 162.05°C, which represented its standard melting point. The endothermic peak at 54.37°C was a characteristic melting peak of poloxamer 188. In thermograms of PM (1:1), poloxamer 188 SD (1:1), and poloxamer 188 SD (1:2), the endothermic peak corresponding to the melting point of the drug disappeared, indicating that IM dissolved completely into the fusional carrier. The broadening and shifting of the peak (149.65°C) of poloxamer 188 SD (1:0.5) towards the left showed that the drug partially dissolved in the carrier, possibly because of the limit of solubility of the carrier. Furthermore, the melting points of poloxamer 188 SDs were below the melting points of the drug and carrier alone. The XRD and DSC results suggest that poloxamer 188 SDs were eutectic systems.
Poloxamers were widely used as wetting agents. The presence of poloxamer 188 decreased drug surface tension and enhanced the wettability of the drug, which also contributed to the high dissolution rate of the drug. It should be noted that the high dissolution rate of poloxamer 188 SDs was not due to the micellar solubility of poloxamer 188. The CMC of poloxamer 188 was 2.4–3.2% (32), which was much higher than the maximum poloxamer 188 concentration found in our study.
PVP K30 was dominantly amorphous in nature, as indicated in the diffractogram in Fig. 6. The PM of PVP K30 and IM exhibited sharp characteristic peaks attributable to the IM of γ-form. However, PVP K30 SDs of different ratios showed the complete absence of any diffraction peaks, indicating a complete amorphization of the drug in the used carrier. Thermograms of IM, PVP K30, and its PM and SDs are shown in Fig. 7. During scanning of PVP K30, a broad endotherm was observed, indicating the loss of water due to the extremely hygroscopic nature of PVP polymers. Repeated scanning led to the disappearance of the endotherm. The thermograms of PVP K30 SDs showed a similar broad endotherm, but no endotherms were observed around the melting point of IM, suggesting that the drug was in the amorphous state. The thermogram of PM did not show a broad endotherm, but a melting endotherm corresponding to the melting point of IM, indicating the presence of crystal. The dissolution behavior of PVP K30 SDs of different drug/carrier ratios could not be explained based on XRD and DSC data, as there were no differences in the spectrums. It is well known that the property of the carrier plays an important role, as the drug in the SD is in intimate contact with the hydrophilic carrier (33,34). If a drug is molecularly dispersed into its carrier, the dissolution of the carrier becomes the dissolution rate-limiting step (35,36). Craig (36) described the mechanism of dissolution of SDs with water-soluble polymers as either carrier- or drug-controlled dissolution. For both mechanisms, the concentrated carrier layer was formed at the dissolving surface through which the drug had to pass, prior to release into the dissolution medium. As the proportion of PVP K30 increased, the viscosity of the SD systems increased (11,37), and the release of drug from SDs became more difficult. Therefore, the increased proportion of PVP K30 in the SDs led to a decrease in the dissolution rate.
Binary Carrier SD
In order to investigate the effect of binary carriers on the dissolution of IM, a binary carrier SD (IM/poloxamer 188/PVP K30 1:0.5:0.5) was prepared by SE. The ratio of 1:0.5:0.5 was selected in early stages; other ratios, such as 1:0.7:0.3 and 1:0.3:0.7, will be investigated in future research. As depicted in Fig. 8, the PM (IM/poloxamer 188/PVP K30 1:0.5:0.5) slightly improved the dissolution rate of the drug, while the binary carrier SD had better performance than the PM. The drug was rapidly released from the binary carrier SD and resulted in supersaturation in 5 min, which was similar to poloxamer 188 SD (drug/carrier 1:1). However, the presence of PVP K30 in the binary carrier SD markedly decreased the degree of supersaturation, with a maximum concentration of 12.09 μg/mL. When compared to PVP K30 SD (drug/carrier 1:1), the addition of poloxamer 188 in the SD released the drug, but a lower maximum concentration emerged in 90 min. The binary carrier SD as well as the single carrier SDs in the dissolution test did not perform.
The binary carrier SD is characterized by XRD in Fig. 9. The XRD diffraction pattern of PVP K30 SD (drug/carrier 1:1) showed a broad halo hump in the range of 7°–40° 2θ, and no sharp diffraction peaks were observed, which was indicative of complete amorphization of the drug in the SD. The diffraction spectrum of the binary carrier SD also had a broad halo hump in the range of 7°–40° 2θ, but several sharp peaks suggested that the drug remained partially crystalline in the SD. The thermogram of the binary carrier SD (Fig. 10) showed two endotherms at around 43 and 50–95°C. The melting peak, corresponding to poloxamer 188, shifted from 54.37°C to around 43°C, possibly because of the presence of PVP K30. The endotherm at 50–95°C indicated a loss of water. There was no endotherm corresponding to the drug, because the crystalline drug completely dissolved in the fusional poloxamer 188. From the XRD, DSC, and dissolution results, we found that the two carriers were mutually restrictive in the binary SD. The presence of PVP K30 in the binary carrier SD restrained the drug to transform from γ-form to α-form. Meanwhile, poloxamer 188 in the binary carrier SD expelled the drug from the PVP K30 phase and a complete amorphous SD was not achieved. These factors resulted in a decreased dissolution rate; i.e., the interaction among the drug and the two carriers resulted in the different dissolution characteristics between the binary carrier SD and the single carrier SDs. Such a case is also encountered by other researchers. Wang et al. demonstrated that although itraconazole and PVP VA64 can form a complete molecular SD, Myrj 52 can expel itraconazole from PVP VA64 phase while preparing ternary SD (38). Janssens et al. pointed out that the addition of TPGS 1000 to the PVP VA64 can lead to destabilization of the molecular dispersion of itraconazole, forming crystalline itraconazole clusters (39).
Preparation Method
In order to investigate the influence of the preparation method on the dissolution behavior of SDs, the poloxamer 188 SD (drug/carrier 1:1), PVP K30 SD (drug/carrier 1:1), and binary carrier SD (IM/poloxamer 188/PVP K30 1:0.5:0.5) were also prepared by HME, and their dissolutions were compared with SDs prepared from SE. The important processing parameters for the HME process are feed rate, screw speed, barrel temperatures, torque, and pressure. For this study, feeding rate and screw speed were kept constant for all formulations, which was a feed rate of 3–4 g/min and a screw speed of 20–25 rpm. The HME process parameters for various formulations are shown in Table II.
For poloxamer 188 SD prepared by HME, the mixture appeared uniformly cloudy after the white powder was introduced to the dissolution medium. The dissolution profiles in Fig. 11 show that the dissolution rate and supersaturation degree were lower for the HME batch compared with the corresponding SE formulation, and the maximum concentrations were 28.05 and 33.83 μg/mL, respectively. This was mainly attributed to change in crystal form during the preparation process. The XRD diffraction patterns in Fig. 12 show that the drug had a crystal form change when using the SE method, while no crystal form change was observed when using HME. The presence of a metastable form in the SE product might be attributed to the higher dissolution rate. There was no difference in the DSC thermograms (Fig. 13) of the two products. Furthermore, densification and reduction of polymeric-free volume during extrusion, compared with the porous particulate nature of SE products, might be another contribution of the higher dissolution rate. HME material was denser with less hydrophilic surface area than SE products. The carrier was subjected to high-intensity mixing and pressure during extrusion, which led to the free space present in the polymeric matrix to be reduced, resulting in a low-porosity polymeric matrix (40,41).
PVP K30 SD prepared by HME had the same appearance and dissolution phenomenon, but a different dissolution rate when compared to that prepared by SE. In Fig. 14, the SE product had a higher dissolution rate than the HME product and the maximum concentrations were 16.31 and 15.28 μg/mL, respectively. There were no sharp peaks in the XRD diffraction pattern (Fig. 15) of the HME product, which suggests that the drug was also amorphous in the SD. In the mDSC results (Fig. 16), the SE and HME products both had a single glass transition temperature (T g), indicating the drug was molecularly dispersed in the amorphous polymer. However, the HME product had a higher T g (92.14°C) than the SE product (77.43°C), which was possibly caused by the plasticizing effect of the residual solvent in the SE product. The HME material was denser with a less hydrophilic surface area than SE products, which might be the reason for the dissolution difference.
In the case of the binary carrier SD prepared by HME, the mixture was uniformly cloudy when the white powder was added to the dissolution medium, as for poloxamer 188 SDs. The dissolution rate of the HME product was improved markedly with a maximum concentration of 47.52 μg/mL (Fig. 17), which was approximately 4-fold greater than SD prepared by the SE method and 41-fold greater than pure drug. Meanwhile, the performance of the binary carrier SD was also better than the single carrier SDs prepared by HME. The XRD diffraction pattern (Fig. 18) of the HME product showed that the drug was still highly crystalline with sharp peaks corresponding to the γ-crystalline form of IM, and no amorphous halo was observed, indicating a negligible amorphous content of IM. The DSC thermogram (Fig. 19) of the HME product had an endotherm at around 142°C corresponding to the drug, while no endotherm of the drug was observed in the SE product, which indicated that there was more crystalline drug in the HME SD than in the SE SD. This means that the SE product had more amorphous drug than the HME product.
Generally, when the drug is incorporated in the amorphous state in SD, it has a higher dissolution rate than when the drug is crystalline (42). These increases arise from the lack of a highly ordered crystal with lattice energies that must be overcome to attain adequate solubility of the crystal. However, we found that the amorphous SD had a far lower dissolution rate than the crystalline SD in our study, Janssens et al. also found this phenomenon in their study (39). The prepared itraconazole ternary SD with TPGS 1000 and PVP VA64 containing both amorphous and crystalline itraconazole had a much higher dissolution rate than the amorphous itraconazole PVP VA64 SD. One possible explanation might be that after the SE process, the drug might be trapped in PVP K30 and remains in the bulk powder; therefore, the drug had to pass through the carrier layer before being dissolved in the medium (43). This would be a difficult process because of the high viscosity of PVP K30, while in the HME process, the highest operating temperature was much lower than the T g of PVP K30. Moreover, PVP K30 was not bonded tightly and would be quickly dissolved in the dissolution; therefore, the drug would not be trapped and can be rapidly released. Compared to poloxamer 188 SD prepared from HME, PVP K30 in the binary carrier SD (HME) can be quickly released into solution and inhibit the crystallization of the drug during dissolution (Fig. 20), which can further contribute to the dissolution rate. Therefore, when the binary SD was prepared by HME, PVP K30 was no longer an inhibitor but was now a promoter of the dissolution rate, and the preparation method is critical in this binary carrier SD system.
In summary, the dissolution of poorly water-soluble drugs from SD is a complicated process. Figure 21 shows the dissolution process from SD. First, the drug is released from solid dispersion to form a solution or supersaturation. If a supersaturation was formed, the drug may precipitate before it can be absorbed. Carrier properties such as viscosity, solubility, wettability, drug physical state (amorphous/crystal), granule particle size, and density can affect the dissolution. In addition, the carrier type can also affect the precipitation. To understand the dissolution of solid dispersion, all these factors must be considered.
CONCLUSION
The effect of factors such as carrier type, drug/carrier ratio, binary carrier, and preparation method on the dissolution of IM under supersaturation conditions was investigated in this study. Binary carrier SD (IM/poloxamer 188/PVP K30 1:0.5:0.5), prepared by HME, was found to have the highest dissolution rate. However, XRD and DSC results indicated that the crystal form of drug remained unchanged and negligible amounts of amorphous state drug were found in this binary carrier SD. The possible reason for its fast dissolution is that poloxamer 188 can maintain its wettability property to the drug after the HME process, and PVP K30 in binary carrier SD (HME) can be rapidly released into solution and inhibit the crystallization of drug during dissolution. The dissolution of poorly water-soluble drugs from SD is a complicated process; many factors, such as carrier type, drug physical state, granule particle size, and density, can affect the dissolution. In order to understand the dissolution of solid dispersion, it is necessary to consider all these factors.
References
Vasconcelos T, Samento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2008;39(25):1068–75.
Timpe C, Forschung L. Strategies for formulation development of poorly water-soluble drug candidates—a recent perspective. Am Pharm Rev. 2007;10:104–9.
Löbenberg R, Amidon G. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm. 2000;50(1):3–12.
Sarode AL, Wang P, Obara S, Worthen DR. Supersaturation, nucleation, and crystal growth during single- and biphasic dissolution of amorphous solid dispersions: polymer effects and implications for oral bioavailability enhancement of poorly water soluble drugs. Eur J Pharm Biopharm. 2014;86(3):351–60.
Bikiaris D. Solid dispersions, part II: new strategies in manufacturing methods for dissolution rate enhancement of poorly water-soluble drugs. Expert Opin Drug Deliv. 2011;8(12):1663–80.
Alam MA, Ali R, FI AI-J, AL-Mohizea AM. Solid dispersions: a strategy for poorly aqueous soluble drugs and technology updates. Expert Opin Drug Deliv. 2012;9(11):1419–40.
Al-Obaidi H, Ke P, Brocchini S, Buckton B. Characterization and stability of ternary solid dispersions with PVP and PHPMA. Int J Pharm. 2011;419(1–2):20–7.
Martins RM, Siqueira S, Tacon LA, Freitas LAP. Microstructured ternary solid dispersions to improve carbamazepine solubility. Powder Technol. 2012;215(1):156–65.
Mahmah O, Tabbakh R, Kelly A, Paradkar A. A comparative study of the effect of spray drying and hot-melt extrusion on the properties of amorphous solid dispersions containing felodipine. J Pharm Pharmacol. 2014;66(2):275–84.
Srinarong P, Waard HD, Frijlink HW, Hinrichs WLJ. Improved dissolution behavior of lipophilic drugs by solid dispersions: the production process as starting point for formulation considerations. Expert Opin Drug Deliv. 2011;8(9):1121–40.
Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272(1–2):1–10.
Patterson JE, James MB, Forster AH, Lancaster RW, Butler JM, Rades T. Preparation of glass solutions of three poorly water soluble drugs by spray drying, melt extrusion and ball milling. Int J Pharm. 2007;336(1):22–34.
Dalsin MC, Tale S, Reineke TM. Solution-state polymer assemblies influence BCS class II drug dissolution and supersaturation maintenance. Biomacromolecules. 2014;15(2):500–11.
Newa M, Bhandari KH, Li DX, Kwon TH, Kim JA, Yoo BK, et al. Preparation, characterization and in vivo evaluation of ibuprofen binary solid dispersions with poloxamer 188. Int J Pharm. 2007;343(1–2):228–37.
Maulvi FA, Dalwadi SJ, Thakkar VT, Soni TG, Gohel MC, Gandhi TR. Improvement of dissolution rate of aceclofenac by solid dispersion technique. Powder Technol. 2011;207(1–3):47–54.
Newman A, Knipp G, Zografi G. Assessing the performance of amorphous solid dispersions. J Pharm Sci. 2012;101(4):1355–77.
Petralito S, Zanardi I, Memoli A, Cristina M, Millucci V, Travagli V. Apparent solubility and dissolution profile at non-sink conditions as quality improvement tools. Promising Pharmaceuticals. 2012;5:83–100.
Yamashita K, Nakate T, Okimoto K, Ohike A, Tokunaga Y, Ibuki R, et al. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int J Pharm. 2003;267(1–2):79–91.
Overhoff KA, Moreno A, Miller DA, Johnston KP, Williams RO III. Solid dispersions of itraconazole and enteric polymers made by ultra-rapid freezing. Int J Pharm. 2007;336(1):122–32.
Konno H, Handa T, Alonzo DE, Taylor LS. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. Eur J Pharm Biopharm. 2008;70(2):493–9.
Valizadeh H, Nokhodchi A, Qarakhani N, Zakeri-Milani P, Azarmi S, Hassanzadeh D, et al. Physicochemical characterization of solid dispersions of indomethacin with PEG 6000, Myrj 52, lactose, sorbitol, dextrin, and Eudragit E100. Drug Dev Ind Pharm. 2004;30(3):303–17.
Rowe RC, Sheskey PJ, Quinn ME, editors. Handbook of pharmaceutical excipients. 6th ed. London: Pharmaceutical; 2009. p. 506–9.
Vippagunta SR, Maul KA, Tallavajhala S, Grant DJW. Solid state characterization of nifedipine solid dispersion. Int J Pharm. 2002;236(1–2):111–23.
Chokshi RJ, Sandhu HK, Iyer RM, Shah NH, Malick AW. Characterization of physico-mechanical properties of indomethacin and polymers to assess their suitability for hot-melt extrusion processs as a means to manufacture solid dispersion/solution. J Pharm Sci. 2005;94(11):2463–74.
Yoshioka M, Hancock BC, Zografi G. Inhibition of indomethacin crystallization in poly(vinylpyrrolidone) coprecipiatates. J Pharm Sci. 1995;84(8):983–6.
Sharma A, Jain CP. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res Pharm Sci. 2010;5(1):49–56.
Frizon F, Eloy JDO, Donaduzzi CM, Mitsui ML, Marchetti JM. Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 solid dispersions by solvent methods. Powder Technol. 2013;235:532–9.
Taylor LS, Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm Res. 1997;14(12):1691–8.
Matsumoto T, Zografi G. Physical properties of solid molecular dispersions of indomethacin with poly(vinylpyrrolidone) and poly (vinylpyrrolidone-co-vinyl-acetate) in relation to indomethacin crystallization. Pharm Res. 1999;16(11):1722–8.
Otsuka M, Kato F, Matsuda Y. Determination of indomethacin polymorphic contents by chemometric near-infrared spectroscopy and conventional powder X-ray diffractometry. Analyst. 2001;126(9):1578–82.
Pan X, Julian T, Augsburger L. Quantitative measurement of indomethacin crystallinity in indomethacin-silica gel binary system using differential scanning calorimetry and X-ray powder diffractometry. AAPS Pharm Sci Tech. 2006;7(1):E72–8.
Moghimi SM, Hunter AC, Dadswell CM, Savay S, Alving CR, Szebeni J. Causative factors behind poloxamer 188 (Pluronic F68, FlocorTM)-induced complement activation in human sera. A protective role against poloxamer-mediated complement activation by elevated serum lipoprotein levels. BBA-GEN Subjiects. 2004;1689(2):103–13.
Chutimaworapan S, Ritthidej GC, Yonemochi E, Oguchi T, Yamamoto K. Effect of water-soluble carriers on dissolution characteristics of nifedipine solid dispersions. Drug Dev Ind Pharm. 2000;26(11):1141–50.
Karavas E, Georgarakis E, Sigalas MP, Avgoustakis K, Bikiaris D. Investigation of the release mechanism of a sparingly water-soluble drug from solid dispersions in hydrophilic carriers based on physical state of drug, particle size distribution and drug-polymer interactions. Eur J Pharm Biopharm. 2007;66(3):334–47.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Sci. 2000;50(1):47–60.
Craig DQM. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm. 2002;231(2):131–44.
Paradkar A, Ambike AA, Jadhav BK, Mahadik KR. Characterization of curcumin-PVP solid dispersion obtained by spray drying. Int J Pharm. 2004;271(1–2):281–6.
Wang X, Michoel A, Mooter GV. Solid state characteristics of ternary solid dispersions composed of PVP VA64, Myrj 52 and itraconazole. Int J Pharm. 2005;303:54–61.
Janssens S, Nagels S, Armas HN, Autry WD, Schepdael AV, Mooter GV. Formulation and characterization of ternary solid dispersions made up of Itraconazole and two excipients, TPGS 1000 and PVPVA 64, that were selected based on a supersaturation screening study. Eur J Pharm Biopharm. 2008;69:158–66.
Follonier N, Doelker E, Cole ET. Various ways of modulating the release of diltiazem hydrochloride from hot-melt extruded sustained release pellets prepared using polymeric materials. J Control Release. 1995;36(3):243–50.
Andrews GP, Abu-Diak O, Kusmanto F, Homsby P, Hui Z, Jones DS. Physicochemical characterization and drug-release properties of celecoxib hot-melt extruded glass solutions. J Pharm Pharmacol. 2010;62(11):1580–90.
Hancock BC, Parks M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm Res. 2000;17(4):397–404.
Dong Z, Chatterji A, Sandhu H, Choi DS, Chokshi H, Shah N. Evaluation of solid state properties of solid dispersions prepared by hot-melt extrusion and solvent co-precipitation. Int J Pharm. 2008;355(1–2):141–9.
Acknowledgements
The authors would like to thank Professor Li Tong and Professor Wang Lihong for their helpful discussions. The Science and Technology Research and Development Project of Shiyan City in the year 2016 (No.: 16Y20) funded this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
The authors alone are responsible for the content and writing of the paper.
Rights and permissions
About this article
Cite this article
Zhang, W., Zhang, Cn., He, Y. et al. Factors Affecting the Dissolution of Indomethacin Solid Dispersions. AAPS PharmSciTech 18, 3258–3273 (2017). https://doi.org/10.1208/s12249-017-0813-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0813-2