Abstract
In this study, non-aqueous microemulsions were developed because of the challenges associated with finding pharmaceutically acceptable solvents for topical delivery of drugs sparingly soluble in water. The formulation irritation potential and ability to modulate the penetration of lipophilic compounds (progesterone, α-tocopherol, and lycopene) of interest for topical treatment/prevention of skin disorders were evaluated and compared to solutions and aqueous microemulsions of similar composition. The microemulsions (ME) were developed with BRIJ, vitamin E-TPGS, and ethanol as surfactant-co-surfactant blend and tributyrin, isopropyl myristate, and oleic acid as oil phase. As polar phase, propylene glycol (MEPG) or water (MEW) was used (26% w/w). The microemulsions were isotropic and based on viscosity and conductivity assessment, bicontinuous. Compared to drug solutions in lipophilic vehicles, MEPG improved drug delivery into viable skin layers by 2.5–38-fold; the magnitude of penetration enhancement mediated by MEPG into viable skin increased with drug lipophilicity, even though the absolute amount of drug delivered decreased. Delivery of progesterone and tocopherol, but not lycopene (the most lipophilic compound), increased up to 2.5-fold with MEW, and higher amounts of these two drugs were released from MEW (2–2.5-fold). Both microemulsions were considered safe for topical application, but MEPG-mediated decrease in the viability of reconstructed epidermis was more pronounced, suggesting its higher potential for irritation. We conclude that MEPG is a safe and suitable nanocarrier to deliver a variety of lipophilic drugs into viable skin layers, but the use of MEW might be more advantageous for drugs in the lower range of lipophilicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Design of topical formulations for drugs considered sparingly soluble or degradable in water is challenging due to difficulties in finding pharmaceutically acceptable excipients that dissolve the drug and provide an efficient delivery without causing irritation (1,2). The influence of lipophilicity (most often expressed as logP) on skin penetration has been largely studied, and it is generally accepted that drugs with logP higher than 4 are more challenging to deliver to viable skin layers, being prone to remain retained in the vehicle and/or stratum corneum (3,4). However, a large number of compounds useful to treat skin diseases, to improve wound healing and to reduce UV-induced skin damage display logP ≥ 4. Examples of these compounds include α-tocopherol, progesterone, vitamin K, lycopene, and β-carotene. Thus, it is essential to develop formulations capable of incorporating such drugs while maximizing their delivery to viable skin layers.
Several types of nanocarriers have been used to dissolve and deliver lipophilic drugs into viable skin, and among them, microemulsions are very interesting due to their thermodynamic stability, ease of preparation, and skin penetration-enhancing ability (5,6). However, topical microemulsions generally contain significant amounts of water, as improvements in the skin penetration of lipophilic drugs are often observed as the aqueous content increases (4,7). Obviously, large contents of water may compromise the amount of a lipophilic drug that can be dissolved in the formulation, leading to drug precipitation (8–10). Based on these considerations, the first goal of this study was to obtain non-aqueous microemulsions in which propylene glycol replaces water as polar phase.
Non-aqueous dispersions were first investigated in the 1960s, when olive oil-in-polyol emulsions stabilized by various surfactants were described (11,12). Since then, other non-aqueous emulsions and microemulsions were obtained using solvents of various polarity to replace water, including other oils (oil-in-oil emulsions) (13). Solvents often used to replace water include glycerol, polyethylene glycol, propylene glycol, and ionic liquids (1,2,14–16). Even though these formulations are not strictly anhydrous since rigorous protocols of solvent dehydration are rarely used, they are nonetheless referred to as non-aqueous systems (15). This terminology was also adopted here. Although the interest in non-aqueous dispersed systems for drug delivery has increased lately, very few studies designed them for topical and transdermal delivery (1,14,17), and, to the best of our knowledge, only one attempted to assess skin penetration in comparison to aqueous systems; however, the model drug was more hydrophilic (logP ∼ −1.6) and mostly suspended in the aqueous formulation, which limit the amount capable to penetrate the skin (1,18).
In this study, non-aqueous microemulsions were obtained and characterized for their structure and rheological behavior, and subsequently evaluated for their ability to improve drug delivery into viable skin layers in comparison to simple solutions in pharmaceutically acceptable solvents. We focused on the delivery of progesterone (MW = 315 g/mol, logP = 4.04), α-tocopherol (MW = 431 g/mol, logP = 7.8), and lycopene (MW = 537, clogP = 17) as these compounds are of interest for treatment/prevention of skin diseases, and thus, should reach viable skin layers (19–24). Tocopherol and lycopene are antioxidants, helping to reduce the damage caused by reactive oxygen species formed in response to UV radiation (25–27). Topical progesterone contributes to wound healing by increasing the number of alternatively activated macrophages (28). Considering the ability of aqueous microemulsions to improve skin penetration of lipophilic drugs, the second goal of this study was to compare the penetration-enhancing effect and irritation potential of the non-aqueous formulation to aqueous microemulsions of similar composition.
MATERIAL AND METHODS
Materials
BRIJ 97 (polyoxyethylene 10 oleoyl ether), polysorbate 80 (Tween 80), sodium lauryl sulfate, propylene glycol, tributyrin, oleic acid, isopropyl myristate, progesterone, and α-tocopherol were obtained from Sigma (St. Louis, MO, USA). Lycopene was obtained from Wako Chemicals (Richmond, VA). Acetonitrile, ethanol, and methanol were purchased from J.T. Baker (Center Valley, PA, USA). Phosphate-buffered saline (PBS) was provided by MatTek Ashland, MA, USA. Vitamin E TPGS was kindly provided by Isochem (Vert Le Petit, France).
Methods
Obtainment of Ternary phase Diagrams and Microemulsion Selection
Design consideration: this study was designed with two goals in mind: (i) obtain non-aqueous microemulsions in which propylene glycol replaces water as polar phase and (ii) compare the penetration-enhancing effect and irritation potential of selected non-aqueous and aqueous microemulsions of similar composition. This is because, unlike aqueous microemulsions, their non-aqueous counterparts have not been largely studied for topical delivery, and thus, information about possible advantages are lacking. For this comparison, two requisites were set: first, the components and contents of the surfactant-co-surfactant blend, oil phase, and polar phase should be the same in aqueous and non-aqueous formulations; second, the amount of polar phase in the microemulsions should be maximized, since increases in the polar phase content generally provide higher release and skin penetration of drugs with logP > 3 (7,8,29,30). Thus, our initial challenge was to maximize both propylene glycol and water incorporation while keeping the same components/ratios. We started the study evaluating the ability of various surfactant-co-surfactant combinations to maximize the microemulsion formation area.
Non-aqueous Microemulsions
Non-aqueous microemulsions were obtained using BRIJ, polysorbate 80 (which will be referred to as Tween here), vitamin E TPGS (VitETPGS), and ethanol at various combinations: BRIJ/VitETPGS (3:1, w/w), BRIJ/VitETPGS/ethanol (2.8:1:0.2, w/w/w), and Tween/VitETPGS/ethanol (2.8:1:0.2). These mixtures will be referred to as surfactant-co-surfactant blend (S-coS blend). Because of the lack of data on the action of surfactants in non-aqueous media, BRIJ, Tween, and vitamin E TPGS were selected based on their previously demonstrated ability to form aqueous microemulsions (13,31–33). As oil phase, a mixture of isopropyl myristate, tributyrin, and oleic acid (1:1:2, w/w/w) was used. These compounds were combined to render the oil phase immiscible with propylene glycol (condition for microemulsion formation) while still allowing incorporation of water in the aqueous formulation. Our preliminary results demonstrated that tributyrin and oleic acid produced very small areas of microemulsion formation using water as polar phase; addition of isopropyl myristate improved it. Oleic acid was included due to its penetration-enhancing effect (34), while tributyrin selection was based on its ability to form large one-phase regions of non-aqueous microemulsions (35).
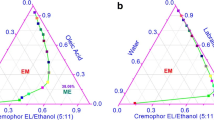
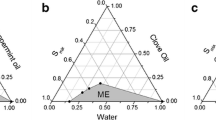
To study the relationship between composition and phase formed, pseudo-ternary phase diagrams were created. The S-coS blends and the oil phase were mixed at various ratios (1:9–9:1, w/w), which were subsequently titrated with propylene glycol (in increments of 5–10 μL) under vortexing at room temperature. The resulting systems were inspected visually to determine phase separation, fluidity, and transparency and differentiate microemulsions from other systems than can be formed; while microemulsions are fluid and transparent, emulsions are milky, and hexagonal, cubic, and lamellar phases are gels (35–40). Formulations displaying only one fluid and clear phase were classified as microemulsions and depicted in a gray-shaded area (6,7,35,41). To avoid misclassifications, the isotropy of formulations in 10% increments of propylene glycol or water was confirmed by polarized light microscopy (Leica, Wetzlar, Germany). Based on the ability to maximize the area of microemulsion formation, the S-coS blend composed of BRIJ/ViTETPGS/ethanol was selected (see Results section).
Aqueous Microemulsions
Since one of our goals was to compare the penetration-enhancing effect of non-aqueous and aqueous formulations, it was necessary to obtain a microemulsion of similar composition but containing water as polar phase. The S-coS blend selected for the non-aqueous microemulsion was also employed for the aqueous formulation, but propylene glycol was included to increase water incorporation, as amounts below 13% were incorporated in its absence. The final S-coS blend was BRIJ/VitETPGS/ethanol/PG at 2.8:1:0.2:0.25 (w/w/w/w). We acknowledge that part of propylene glycol is incorporated at the surfactant interface, while another part partitions into the aqueous compartment given propylene glycol solubility (42). Although not ideal (as we wanted less similarity between the polar phases), this was necessary to maximize water incorporation since skin penetration from microemulsions generally increases with the polar phase content (4,7). Obtainment of the phase diagram and formulation classification was performed as stated for non-aqueous systems.
Formulation Selection and Drug Incorporation
Based on the phase diagrams obtained, an S-coS/oil ratio of 6:4 (w/w) was selected, and the polar phase (water or propylene glycol) was added at 26% to obtain microemulsions. The polar phase content was limited by the maximum amount of water that could be incorporated. From now on, the aqueous and non-aqueous microemulsions will be referred to as MEW and MEPG, respectively. These formulations contain the same oil phase (29.6% of isopropyl myristate, tributyrin, and oleic acid mixed at 1:1:2, w/w/w), S-coS/oil phase ratio (6:4, w/w), content of S-coS (44.4%), and content of polar phase (26%). The main difference was the presence of water only in MEW.
Progesterone and tocopherol were dissolved in the S-CoS/oil phase mixtures prior to polar phase addition to give a final concentration of 0.8% (w/w). This concentration was at least twice smaller than the maximum amount that could be dissolved. Lycopene was also added to the surfactant-oil phase mixture, which was bath sonicated for 15 min to facilitate drug solubilization, to give a final concentration in the microemulsion of 0.06% (w/w). Lycopene solubility was smaller compared to the other two compounds, and drug crystals denoting precipitation were observed in both MEW and MEPG at drug concentrations above 0.08%.
Characterization of the Selected Microemulsions
Isotropicity of the MEPG and MEW was confirmed by polarized light microscopy (Leica, Wetzlar, Germany). Different types of microemulsions may exist depending on the components and their ratios: oil-in-water (O/W), in which oil droplets are dispersed in water, water-in-oil (W/O), in which water droplets are dispersed in oil, and bicontinuous systems, in which aqueous- and oil-continuous domains are intertwined and stabilized by sheet-like surfactant regions (6,43–47). Thus, bicontinuous microemulsions are not structured as spherical aggregates and are often observed when the content of water and oil is similar (6). Various methods can be used to evaluate the internal structure (whether O/W, bicontinuous, or W/O) of microemulsions; here, the internal structure was studied by assessing changes on the electrical conductivity and viscosity of microemulsions as a function of polar phase content (water and propylene glycol) (6,8,44,48).
Mixtures of surfactant/oil (6:4, w/w) were obtained, and conductivity was measured after addition of increasing amounts of water or propylene glycol (41); the latter contained sodium chloride at 0.1 μM to obtain stable readings as previously described (49). Higher concentrations of the salt have been used without interfering with microemulsion formation (49).
To assess changes on viscosity, four microemulsions were selected: MEPG, MEW, and two additional microemulsions obtained with the same S-coS/oil phase ratio as in MEW and MEPG (6:4 w/w) but 5% polar phase (one with water and one with propylene glycol). First, rheological behavior was assessed with a R/S Plus controlled stress rheometer with RC75-1 cone (Brookfield Engineering laboratories, Middleboro, MA), and a bath circulator for temperature control, set at 25°C. The experiments were performed in duplicate, with shear rates up to 3000 s−1. The relationship between the shear stress and the shear rate of each formulation was evaluated using the Power law equation:
where τ is the shear stress, γ is the rate of shear, K is the consistency index, and n is the flow index (36,50). All formulations displayed Newtonian behavior, and since this type of system has constant viscosity (at the same temperature), the final viscosity of each formulation was determined by averaging viscosity values at individual rates of shear.
In Vitro Skin Penetration
Skin penetration assays were conducted in Franz diffusion cells (diffusion area of 1.77 cm2; Hanson, Chatsworth, USA) using porcine ear skin as the model tissue. Skin from the outer surface of a freshly excised porcine ear was carefully dissected and stored at −80°C until the day of the experiment. The receptor phase, consisting of phosphate buffer with 20 or 30% (for lycopene) ethanol, was maintained at 37 ± 0.5°C with magnetic stirring at 350 rpm throughout the experiment.
Skin tissues were assembled in the diffusion cells and treated for 8 h with 100 mg of the aqueous and non-aqueous microemulsions loaded with the compounds. This time point was chosen because previous studies from our group demonstrated that at 8 h, significant differences among microemulsions with varying composition could be observed (31,51). As control vehicle, solutions in isopropyl myristate or myvacet oil (lycopene) were used. After treatment, skin sections were rinsed, and tape stripping was performed to separate the stratum corneum (SC) and viable skin layers (viable epidermis and dermis, ED), which were subsequently cut in small pieces. The compounds were extracted from the SC-containing tapes and from ED with methanol or acetonitrile/methanol (52:48, v/v, for lycopene). The receptor phase was concentrated four times using a vacuum concentrator. Recovery of drugs from the skin layers varied from 80 to 92%, as previously described (31,51). Samples of ED were also concentrated when the control solution of lycopene was used to treat the skin. The enhancement ratio was calculated as the ratio between amounts of each compound delivered by the microemulsions and control solution.
Transepidermal Water Loss
To investigate whether the aqueous and non-aqueous microemulsions affect the skin barrier differently, assessment of transepidermal water loss was conducted after removal of the microemulsions from the skin. Skin sections (full thickness) were mounted in Franz diffusion cells and treated with the microemulsions or water (as a control) for 5 min or 8 h. Subsequently, they were carefully wiped with tissue paper to remove the formulations, reassembled in the diffusion cells, left to equilibrate for 20 min to limit the influence of skin hydration by formulation before transepidermal water loss was measured with a closed chamber evaporimeter (Vapometer, Delfin Technologies Ltd., Kuopio, Finland) equipped with an adaptor to fit the diffusion cell (Hanson Research, Chatsworth, CA, diffusion area of 1.7 cm2) opening (52,53). This is because, when the test formulations are removed from the hydrated skin, accumulated water evaporates until steady state is reached (54). Previous studies suggest a rapid decrease in transepidermal water loss during the initial 5 min followed by an extended decrease (54); depending on the level of disturbance, equilibrium is observed after 15 min.
Considering that alterations in the skin barrier might result from tissue wiping, the measurement of transepidermal water loss at 5 min was performed to take into consideration any impact of the wiping. Results were expressed as Δ transepidermal water loss, calculated as the difference between transepidermal water loss values after treatment for 8 h and 5 min.
In Vitro Drug Release
Considering that the nature of vehicles might hinder the release and consequently, skin penetration of lipophilic drugs (30), we compared drug release differences from aqueous and non-aqueous formulations. Drug release was assessed using Franz diffusion cells as described in item 3, except that a cellulose membrane was used instead of the skin, and ethanol at 40% was used in the receptor phase for lycopene release (or no drug could be quantified due to poor solubility). Samples of the receptor phase were collected at 1, 2, 4, 6, and 8 h post-application, filtered through 0.45 μm pore membranes, and assayed for the drugs. The release rates were calculated from the slope of the linear portion of the plots of cumulative drug released against time (55).
Quantification of the Compounds
Drug quantification in the homogenates of skin layers and in the receptor phase was performed by high-performance liquid chromatography (HPLC) using an equipment consisting of a pump model LC-10AD, a controller model SIL-10ADVP and a UV detector model SPD-10AVP. Separation was performed on a Prevail C18 column (150 × 4.6 mm, Alltech, Deerfield, IL), using mobile phases composed of acetonitrile (100% at 1.2 mL/min, detection at 285 nm) for α-tocopherol, acetonitrile/water at 8:2 (v/v, flow rate of 1 mL/min, detection at 240 nm) for progesterone, and acetonitrile/methanol at 52:48 (v/v, 1.5 mL/min, detection at 470 nm) for lycopene as previously described (4,31,41,56).
Comparison of the Irritation Potential
The irritation potential of the aqueous and non-aqueous microemulsions was compared using reconstructed epidermis according to the method recommended by MatTek (57), which supplied the tissues (EpiDerm, MatTek, Ashland, MA), following The Organisation for Economic Co-operation and Development (OECD) guidelines. This model has been widely used as it mimics better the structure of the skin compared to cell monolayers and does not require formulation dilution, which can alter the formulation internal structure and consequently, its irritation potential (58,59). Tissues were topically treated with the microemulsions, a positive control (sodium lauryl sulfate at 5%), and a negative control (PBS) for 60 min; for the first 35 min, incubation was performed at 5% CO2 and 37°C followed by incubation for the remaining time at room temperature. Tissues were then thoroughly rinsed and transferred to fresh medium for 42 h. Subsequently, the MTT assay was performed by transferring the tissues to 24-well plates containing MTT solution (1 mg/mL, provided by MatTek) followed by incubation for 3 h. The blue formazan salt formed was then extracted with 2 mL of isopropanol, and the optical density of the extracted formazan was determined using a spectrophotometer at 570 nm. The formulation is considered irritant if tissue viability is below 50%.
Because the formulations contain a surfactant based on the antioxidant vitamin E, we evaluated whether the amount of formulation retained in the tissue was sufficient to reduce MTT by subjecting frozen, metabolically inactive tissues to the same treatment protocol. According to the manufacturer, if interference of the test formulation is below 30% of the negative control value, viability values can be corrected by subtracting the reading of the frozen tissues from that of viable tissues. Since interference was below 10%, tissue viability after formulation treatment was corrected.
Statistical Analyses
The results are reported as means ± SD. Data were statistically analyzed using the ANOVA test followed by Tukey post hoc test (GraphPad Prism software). Values were considered significantly different when p < 0.05.
RESULTS
Obtainment of Ternary Phase Diagrams and Microemulsion Selection
The pseudo-ternary phase diagrams of the various S-coS and polar phases used are depicted in Fig. 1. All formulations in the gray-shaded area were fluid and transparent, consistent with microemulsions. In a general manner, the area of microemulsion formation (gray-shaded area) increased from 74 (Fig. 1a) to 84% (Fig. 1b) when ethanol was added into the BRIJ-VitETPGS blend, but decreased to 68% (Fig. 1c) when BRIJ was replaced by Tween 80 as the main surfactant. Since it maximized the area of microemulsion formation, the blend composed of BRIJ/VitEPGS/ethanol (2.8:1:0.2, w/w/w) was selected. To obtain aqueous systems, propylene glycol was included in the S-coS blend as described in the “Methods” session. Even though this is not ideal, only very small amounts of water (<13%) could be incorporated if propylene glycol was not included in the S-coS blend. Using water as polar phase decreased the microemulsion formation area by approximately two-fold (Fig. 1d).
MEW and MEPG were prepared by mixing the selected S-coS and oil phase at a 6:4 (w/w) ratio, followed by addition of water or propylene glycol at 26%. This amount of polar phase was dictated by the maximum content of water that could be incorporated, since it was lower than that of propylene glycol. The maximum amount of water incorporated was actually 30%, but to avoid being too close to the borderline of microemulsion formation, 26% was used.
Characterization of the Selected Microemulsions
MEPG and MEW were isotropic, as no specific texture was visualized under the polarized light microscope. Microemulsions may exhibit percolation phenomena at certain volume fractions of water (6), and transformation from W/O to O/W systems through the emergence of bicontinuous structures may occur as the content of the polar phase increases. This transformation is accompanied by an increase in the electrical conductivity (43,60). Additionally, it is generally accepted that interlinking of droplets at percolation (and formation of bicontinuous structures) also leads to an increase in viscosity, while transformation into water-continuous systems decreases viscosity (8,61). Based on these points, the internal structure was studied by assessing changes on the electrical conductivity and viscosity of microemulsions as a function of polar phase content (water and propylene glycol).
To assess viscosity changes, microemulsions containing polar phase at 5 and 26% (four in total, as each was prepared with propylene glycol and with water) were used. Linear relationships between rate of shear and shear stress were obtained, demonstrating the Newtonian behavior of these microemulsions. To illustrate this behavior, flow curves of MEPG and MEW are depicted in Fig. 2a. This flow behavior has been previously reported for W/O, O/W and bicontinuous systems, although few studies have also observed non-Newtonian behavior for more viscous bicontinuous systems (60,62,63). The type of polar phase influenced the viscosity, with MEPG displaying values approximately 2.2-fold higher (Fig. 2a), even though it did not affect the type of rheological behavior. Since Newtonian systems have constant viscosity (at the same temperature), the final viscosity of each formulation was determined by averaging the viscosity values obtained at individual rates of shear, which is depicted in Fig. 2b. As can be observed, viscosity of formulations containing 26% of polar phase was higher compared to those containing 5% regardless of the type of polar phase. This result suggests that interlinking of droplets and structural change to a bicontinuous system occurs with polar phase increase to 26%.
Characterization of microemulsions. a Relationship between rate of sheer and sheer stress of MEW and MEPG to illustrate their Newtonian behavior. b Changes on the electrical conductivity and viscosity as a function of the content of the polar phase for characterization of the internal structure. For viscosity measurements, four microemulsions were selected: MEPG, MEW, and two additional microemulsions obtained with S-coS/oil at 6:4 (w/w, as in MEW and MEPG) and 5% water or propylene glycol. For conductivity measurements, the S-coS blend and oil phase were mixed at 6:4 (w/w), and conductivity was measured after addition of increasing amounts of water or propylene glycol
Changes on the electrical conductivity as a function of polar phase content confirmed these results. Similar to the viscosity, conductivity increased with polar phase content. The shape of the curve and the regions observed when propylene glycol was used as polar phase were very similar to that described by Krauel et al., during the development of non-aqueous microemulsions as templates for nanoparticle production, by Harrar et al., who developed microemulsions containing ionic liquid and limonene, and also to other curves titrated with water (17,49,51). Lower conductivity values were observed at water and propylene glycol contents below 14 and 17%, respectively; the values displayed for propylene glycol-containing systems were lower compared to those with water, which is in agreement with previous observations (49). More pronounced increases in conductivity were observed when the content of water or propylene glycol was increased to 15–30 or 17–44%, respectively, as demonstrated by differences in the slope at different portions of the curve. Curves in the regions of low (0–14%) and medium (15–30 or 17–44%) polar phase content could be approximated by linear fitting (yielding r 2 > 0.96), and the slope of the curve in the medium region was 2.7- (for propylene glycol) and 2.5-fold (for water) higher. This suggests the interlinking of water droplets and formation of bicontinuous structures in this region (41,64). System destabilization was observed with water content over 30%, while addition of propylene glycol over 45% led to the formation of a plateau-like region, in which little reductions in conductivity were observed, until system destabilization occurred at 63% of the solvent. Considering that the polar phase content of MEW and MEPG is 26%, their internal phase is more compatible with a bicontinuous phase. Because bicontinuous microemulsions are not structured as spherical aggregates, determination of diameter is not applicable (7,47).
In Vitro Skin Penetration
Penetration of the lipophilic compounds in the stratum corneum (SC), viable skin layers (ED), as well as permeation across the tissue is depicted in Fig. 3. The penetration of all compounds into the SC was significantly (p < 0.05) increased by 2.3- to 3.8-fold using the non-aqueous microemulsion compared to the control solutions, supporting the advantage of the nanocarrier if we consider that drug in the SC might function as a depot for delivery into viable skin (65). When it comes to delivery into viable skin layers, penetration of progesterone and tocopherol was increased by 2.5- and 26-fold (p < 0.05), respectively, while penetration of lycopene increased approximately 38-fold. These results indicate that, even though the absolute amount of drug delivered into viable skin decreased with increases in lipophilicity, the magnitude of penetration enhancement mediated by MEPG increased, most likely because it becomes more difficult to deliver drugs into viable skin without employing penetration-enhancing strategies as they become more lipophilic.
Delivery of progesterone and tocopherol was significantly enhanced (p < 0.05) by MEW both into the stratum corneum and viable skin layers compared to MEPG. Accordingly, MEW-mediated delivery of progesterone into the stratum corneum and viable skin increased by 1.5- and 2-fold, respectively. Enhancement of tocopherol penetration was slightly more pronounced, and 2- and 2.5-fold larger amounts of the compound were delivered in the stratum corneum and viable skin, respectively, by MEW. On the other hand, delivery of lycopene was not increased by MEW.
Lycopene and tocopherol were not quantified in the receptor phase most likely because their concentrations were below the quantification limit of the analytical method (which was 35 and 100 ng/mL for lycopene and tocopherol, respectively), even after the receptor phase was concentrated (4,66). This suggests very low percutaneous permeation of these compounds. As expected, progesterone permeation across the skin was significantly (p < 0.05) increased by the microemulsions compared to the propylene glycol solution, but no difference between MEW and MEPG was observed.
Transepidermal Water Loss
To evaluate whether the aqueous and non-aqueous microemulsions affected differently the barrier function of the skin, we assessed changes in transepidermal water loss. As can be observed in Fig. 4, both formulations increased the transepidermal water loss at the end of the experiment compared to water (2- and 2.3-fold for MEW and MEPG, respectively), most likely due to the presence of surfactants, co-surfactants, and other components that may act as penetration enhancers (67). MEPG displayed a slightly stronger, but non-significant effect, compared to water. Since treatment with both formulations resulted in similar increases in this parameter, it is reasonable to assume an ability of similar magnitude to disrupt the stratum corneum.
In Vitro Drug Release
We next investigated the relationship between drug skin penetration and release rates. Linear relationships in the curves of cumulative release as a function of time were observed for progesterone and tocopherol from both microemulsions (coefficient of determination superior to 0.98), suggesting that release follows zero-order kinetics and was not affected by the type of polar phase used (Fig. 5). The kinetics of lycopene release could not be determined since the drug could be quantified in the receptor phase only after 6 h.
The cumulative amount of progesterone released from MEW after 8 h was approximately 1.5-fold higher (p < 0.05) compared to MEPG. A similar difference was observed in the release rates (Table I), with MEW providing a 1.5-fold higher rate. There was also a difference in the cumulative release of α-tocopherol from the two microemulsions, with MEW releasing approximately 2-fold more drug after 8 h than MEPG. The release rate provided by MEW was approximately 1.9-fold higher (Table I). As for lycopene, no difference in the cumulative amount released at 8 h was observed comparing MEW and MEPG.
Comparison of the Irritation Potential
Evaluation of the irritation potential of the formulations was conducted using reconstructed epidermis. The method allows classification of compounds as irritant or non-irritant based on the tissue viability at the end of the incubation period. As expected, PBS-treated tissues displayed viability of 96.5 ± 8.8%, while treatment with sodium lauryl sulfate significantly (p < 0.001) reduced viability to 19.6 ± 6.1% (Fig. 6). This value is within the limits described in the acceptance criteria of the model used. Compared to the PBS-treated control, MEW and MEPG significantly reduced tissue viability to 68.4 ± 7.8 and 57.0 ± 5.8%, respectively, but this effect was less pronounced than that induced by sodium lauryl sulfate. None of the formulations were considered irritants since tissue viability was above 50%; nevertheless, the effect of MEPG was more pronounced and closer to the threshold, suggesting its stronger irritation potential.
Viability of reconstructed epidermis after exposure to the microemulsions, PBS (negative control), and sodium lauryl sulfate (SDS, positive control) for 60 min. Data shown as average ± standard deviation of 3–4 replicates. *p < 0.05, **p < 0.01, and *** p < 0.001 compared to PBS; ## p < 0.01 compared to sodium lauryl sulfate
DISCUSSION
Selection of surfactants and co-surfactants for non-aqueous systems is considered challenging due to the lack of data on their action in non-aqueous media (13). Thus, we started the study assessing the effects of adding ethanol as co-surfactant, as well as changing the main surfactant (BRIJ or Tween) on microemulsion formation. Similarly to aqueous-based systems, inclusion of ethanol in the S-coS blend increased the area of formation of microemulsions. Ethanol has been reported to increase oil solubilization in microemulsions as it penetrates into the interfacial film, affecting the spontaneous curvature of the interface and the elasticity of the amphiphilic film (8,42). Next, BRIJ was replaced by Tween, since the latter has been reported to form large areas of microemulsions in combination with lecithin as surfactant and propylene glycol as polar phase (35). However, this replacement promoted a decrease in the microemulsion area of formation, an effect previously attributed to less favored interactions between the oil phase and more hydrophilic surfactants (the HLB of Tween is higher) (42). Based on these results, the S-coS blend composed of BRIJ/VitETPGS/ethanol (2.8:1:0.2, w/w/w) was selected, but aqueous microemulsions containing water over 13% could only be obtained after addition of propylene glycol to this blend. This might be explained by the fact that part of propylene glycol partitions into the aqueous compartment, decreasing its polarity, and increasing the area of microemulsion formation (42).
Having obtained aqueous and non-aqueous microemulsions with similar compositions, we proceeded to compare their ability to alter the skin barrier and improve penetration of lipophilic compounds. The microemulsions induced similar levels of barrier disruption, as demonstrated by similar changes in transepidermal water loss. This similarity might result from the balance between the effects of individual components on the skin and formulation water activity (54). Even though propylene glycol, present in larger amounts in MEPG, is a solvent capable of entering the stratum corneum and increasing the solubility of co-administered compounds (including surfactants and penetration enhancers, which can decrease barrier function and increase transepidermal water loss), polyols have been described to decrease the water activity of formulations, which generally reduces transepidermal water loss (54,68,69).
Results from skin penetration studies clearly demonstrated the advantage of non-aqueous microemulsions over simple solutions to increase the delivery of nonpolar compounds to viable skin layers, with enhancements of a minimum of 2.5-fold. The magnitude of penetration enhancement mediated by MEPG increased with the lipophilicity of the compound, most likely because delivery of drugs into viable skin layers becomes more difficult as lipophilicity increases, and thus, the contribution of enhancement strategies becomes more valuable. Comparing the two microemulsions, the penetration enhancement promoted by the aqueous formulation was greater for progesterone and tocopherol, but not for lycopene, suggesting that MEW superiority was limited by drug lipophilicity: as logP approached ∼17, the penetration-enhancing ability of the two microemulsions became similar. These observations differ from those reported by Moniruzzaman et al., who demonstrated that a non-aqueous ionic liquid-in-oil microemulsion was more efficacious at increasing acyclovir cutaneous (2.4-fold) and transdermal (from undetected to 1.95 μg/cm2) delivery compared to a water-in-oil formulation (1). However, ours and the previous study differ in several aspects known to influence drug penetration: acyclovir is more hydrophilic (logP ∼ −1.6), was mostly suspended in the aqueous microemulsion, and the formulations contained approximately 7-fold less polar phase than ours.
A similar trend was observed for drug release; progesterone and tocopherol cumulative release from MEW was more pronounced, whereas lycopene release from MEW and MEPG was very similar. Considering the similarity of the magnitude of barrier disruption mediated by the two microemulsions and the importance of drug release for penetration, the more pronounced penetration of progesterone and tocopherol mediated by MEW might result from the higher amount released. This effect, in turn, might be explained by an increase in MEW polarity. In support of this hypothesis, an increase in paclitaxel (another lipophilic drug, logP = 3.5 (70)) release from micelles was observed after incorporation of more hydrophilic surfactants in the system (71). Increases in the water content of other microemulsions also resulted in higher release rates and penetration of drugs with logP > 3 (4,7,72). As formulation polarity increases, drug solubility decreases, resulting in less drug entrapped in the microemulsion and larger amounts released (73,74). This is consistent with the facts that progesterone solubility increased approximately 10 times (to approximately 0.17 mg/mL) with addition of only 14% propylene glycol in an aqueous-based vehicle, whereas over 200-fold more α-tocopherol could be dissolved in propylene glycol compared to water (4,75–77). As for lycopene, its solubility has been described as very low (below 10 ppm) both in water and propylene glycol (23); the microemulsion polar phase seemed to affect neither lycopene release nor its skin penetration. It is also worth mentioning that the lower viscosity of MEW might have facilitated drug diffusion (78).
In spite of the significant amount of surfactant-co-surfactant blend present in the selected microemulsions, they were considered safe as demonstrated in reconstructed skin equivalents following the OECD-recommended guidelines for assessing topical irritation. Ours results are supported by previous studies demonstrating no signs of in vivo inflammation or irritation and less pronounced reductions on cell viability compared to sodium lauryl sulfate (an irritant) caused by microemulsions containing surfactant-co-surfactant concentrations higher than 40% (62,78–84). Considering that MEW increased the penetration of tocopherol and progesterone in a more pronounced manner, its use might become more advantageous if we take into consideration the less pronounced effect on the viability of reconstructed epidermis after exposure to this formulation.
In conclusion, we were able to obtain suitable, safe non-aqueous microemulsions capable of improving skin penetration of lipophilic compounds with the components selected and demonstrated that, considering the logP range studied, the aqueous formulation was more effective at improving delivery of less lipophilic drugs while displaying a lower potential for irritation. Based on these results, the use of MEW might be more advantageous for cutaneous delivery of drugs in the lower range of lipophilicity provided that clinically relevant concentrations can be incorporated. We believe that this information could be used for selection of topical microemulsions based on drug lipophilicity.
References
Moniruzzaman M, Tamura M, Tahara Y, Kamiya N, Goto M. Ionic liquid-in-oil microemulsion as a potential carrier of sparingly soluble drug: characterization and cytotoxicity evaluation. Int J Pharm. 2010;400(1–2):243–50.
Lin CC, Yang CH, Chang NF, Wu PS, Chen YS, Lee SM, et al. Study on the stability of deoxyArbutin in an anhydrous emulsion system. Int J Mol Sci. 2011;12(9):5946–54.
Moser K, Kriwet K, Naik A, Kalia YN, Guy RH. Passive skin penetration enhancement and its quantification in vitro. Eur J Pharm Biopharm. 2001;52(2):103–12.
Cichewicz A, Pacleb C, Connors A, Hass MA, Lopes LB. Cutaneous delivery of alpha-tocopherol and lipoic acid using microemulsions: influence of composition and charge. J Pharm Pharmacol. 2013;65(6):817–26.
Lopes LB. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics. 2014;6(1):52–77.
Santos P, Watkinson AC, Hadgraft J, Lane ME. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol Physiol. 2008;21(5):246–59.
Zhang J, Michniak-Kohn B. Investigation of microemulsion microstructures and their relationship to transdermal permeation of model drugs: ketoprofen, lidocaine, and caffeine. Int J Pharm. 2011;421(1):34–44.
Pepe D, McCall M, Zheng H, Lopes LB. Protein transduction domain-containing microemulsions as cutaneous delivery systems for an anticancer agent. J Pharm Sci. 2013;102(5):1476–87.
Thomas S, Vieira CS, Hass MA, Lopes LB. Stability, cutaneous delivery, and antioxidant potential of a lipoic acid and alpha-tocopherol codrug incorporated in microemulsions. J Pharm Sci. 2014;103(8):2530–8.
Lv FF, Zheng LQ, Tung CH. Phase behavior of the microemulsions and the stability of the chloramphenicol in the microemulsion-based ocular drug delivery system. Int J Pharm. 2005;301(1–2):237–46.
Hamill RD, Petersen RV. Effect of surfactant concentration on the interfacial viscosity of a nonaqueous system. J Pharm Sci. 1966;55(11):4.
Hamill R, Olson F, Petersen R. Some interfacial properties of a nonaqueous emulsion. J Pharm Sci. 1965;54(4):4.
Suitthimeathegorn O, Jaitely V, Florence AT. Novel anhydrous emulsions: formulation as controlled release vehicles. Int J Pharm. 2005;298(2):367–71.
Atanase LI, Riess G. Block copolymer stabilized nonaqueous biocompatible sub-micron emulsions for topical applications. Int J Pharm. 2013;448(2):339–45.
Crespy D, Landfester K. Making dry fertile: a practical tour of non-aqueous emulsions and miniemulsions, their preparation and some applications. Soft Matter. 2011;7:11.
Friberg E, Podzimek M. A non-aqueous microemulsion. Colloid Polym Sci. 1984;262(3):252–3.
Harrar A, Zech O, Klaus A, Bauduin P, Kunz W. Influence of surfactant amphiphilicity on the phase behavior of IL-based microemulsions. J Colloid Interface Sci. 2011;362(2):423–9.
Charoenputtakun P, Li SK, Ngawhirunpat T. Iontophoretic delivery of lipophilic and hydrophilic drugs from lipid nanoparticles across human skin. Int J Pharm. 2015;495(1):318–28.
Magnusson BM, Cross SE, Winckle G, Roberts MS. Percutaneous absorption of steroids: determination of in vitro permeability and tissue reservoir characteristics in human skin layers. Skin Pharmacol Physiol. 2006;19(6):336–42.
Toropainen E, Ranta VP, Talvitie A, Suhonen P, Urtti A. Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest Ophthalmol Vis Sci. 2001;42(12):2942–8.
Mukai K, Tokunaga A, Itoh S, Kanesaki Y, Ohara K, Nagaoka S, et al. Structure-activity relationship of the free-radical-scavenging reaction by vitamin E (alpha-, beta-, gamma-, delta-Tocopherols) and ubiquinol-10: pH dependence of the reaction rates. J Phys Chem B. 2007;111(3):652–62.
Teichmann A, Jacobi U, Ossadnik M, Richter H, Koch S, Sterry W, et al. Differential stripping: determination of the amount of topically applied substances penetrated into the hair follicles. J Invest Dermatol. 2005;125(2):264–9.
Spernath A, Yaghmur A, Aserin A, Hoffman RE, Garti N. Food-grade microemulsions based on nonionic emulsifiers: media to enhance lycopene solubilization. J Agric Food Chem. 2002;50(23):6917–22.
Vertzoni M, Kartezini T, Reppas C, Archontaki H, Valsami G. Solubilization and quantification of lycopene in aqueous media in the form of cyclodextrin binary systems. Int J Pharm. 2006;309(1–2):115–22.
Poljsak B, Dahmane R. Free radicals and extrinsic skin aging. Dermatol Res Pract. 2012;2012:135206.
van Breemen RB, Pajkovic N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008;269(2):339–51.
Ascenso A, Pinho S, Eleuterio C, Praca FG, Bentley MV, Oliveira H, et al. Lycopene from tomatoes: vesicular nanocarrier formulations for dermal delivery. J Agric Food Chem. 2013;61(30):7284–93.
Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(1):42–50.
Kantarci G, Ozguney I, Karasulu HY, Arzik S, Guneri T. Comparison of different water/oil microemulsions containing diclofenac sodium: preparation, characterization, release rate, and skin irritation studies. AAPS PharmSciTech. 2007;8(4), E91.
Lopes LB, Murphy N, Nornoo A. Enhancement of transdermal delivery of progesterone using medium-chain mono and diglycerides as skin penetration enhancers. Pharm Dev Technol. 2009;14(5):524–9.
Hosmer J, Reed R, Bentley MV, Nornoo A, Lopes LB. Microemulsions containing medium-chain glycerides as transdermal delivery systems for hydrophilic and hydrophobic drugs. AAPS PharmSciTech. 2009;10(2):589–96.
Nornoo AO, Chow DS. Cremophor-free intravenous microemulsions for paclitaxel II. Stability, in vitro release and pharmacokinetics. Int J Pharm. 2008;349(1–2):117–23.
Jordan M, Nayel A, Brownlow B, Elbayoumi T. Development and evaluation of tocopherol-rich argan oil-based nanoemulsions as vehicles possessing anticancer activity. J Biomed Nanotechnol. 2012;8(6):944–56.
Pierre MB, Ricci Jr E, Tedesco AC, Bentley MV. Oleic acid as optimizer of the skin delivery of 5-aminolevulinic acid in photodynamic therapy. Pharm Res. 2006;23(2):360–6.
Patel N, Schmid U, Lawrence MJ. Phospholipid-based microemulsions suitable for use in foods. J Agric Food Chem. 2006;54(20):7817–24.
Hosmer JM, Steiner AA, Lopes LB. Lamellar liquid crystalline phases for cutaneous delivery of Paclitaxel: impact of the monoglyceride. Pharm Res. 2013;30(3):694–706.
Hosmer JM, Shin SH, Nornoo A, Zheng H, Lopes LB. Influence of internal structure and composition of liquid crystalline phases on topical delivery of paclitaxel. J Pharm Sci. 2011;100(4):1444–55.
Clogston J, Rathman J, Tomasko D, Walker H, Caffrey M. Phase behavior of a monoacylglycerol: (myverol 18-99K)/water system. Chem Phys Lipids. 2000;107(2):191–220.
Shah JC, Sadhale Y, Chilukuri DM. Cubic phase gels as drug delivery systems. Adv Drug Deliv Rev. 2001;47(2–3):229–50.
Wang Z, Diao Z, Liu F, Li G, Zhang G. Microstructure and rheological properties of liquid crystallines formed in Brij 97/water/IPM system. J Colloid Interface Sci. 2006;297(2):813–8.
Pepe D, Phelps J, Lewis K, Dujack J, Scarlett K, Jahan S, et al. Decylglucoside-based microemulsions for cutaneous localization of lycopene and ascorbic acid. Int J Pharm. 2012;434(1–2):420–8.
Garti N, Yaghmur A, Leser ME, Clement V, Watzke HJ. Improved oil solubilization in oil/water food grade microemulsions in the presence of polyols and ethanol. J Agric Food Chem. 2001;49(5):2552–62.
Kogan A, Aserin A, Garti N. Improved solubilization of carbamazepine and structural transitions in nonionic microemulsions upon aqueous phase dilution. J Colloid Interface Sci. 2007;315(2):637–47.
Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123–126:369–85.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45(1):89–121.
Heuschkel S, Goebel A, Neubert RH. Microemulsions--modern colloidal carrier for dermal and transdermal drug delivery. J Pharm Sci. 2008;97(2):603–31.
Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev. 2002;54 Suppl 1:S77–98.
Graf A, Ablinger E, Peters S, Zimmer A, Hook S, Rades T. Microemulsions containing lecithin and sugar-based surfactants: nanoparticle templates for delivery of proteins and peptides. Int J Pharm. 2008;350(1–2):351–60.
Krauel K, Graf A, Hook SM, Davies NM, Rades T. Preparation of poly (alkylcyanoacrylate) nanoparticles by polymerization of water-free microemulsions. J Microencapsul. 2006;23(5):499–512.
Mahdi ES, Noor AM, Sakeena MH, Abdullah GZ, Abdulkarim MF, Sattar MA. Formulation and in vitro release evaluation of newly synthesized palm kernel oil esters-based nanoemulsion delivery system for 30% ethanolic dried extract derived from local Phyllanthus urinaria for skin antiaging. Int J Nanomedicine. 2011;6:2499–512.
Lopes LB, VanDeWall H, Li HT, Venugopal V, Li HK, Naydin S, et al. Topical delivery of lycopene using microemulsions: enhanced skin penetration and tissue antioxidant activity. J Pharm Sci. 2010;99(3):1346–57.
Herwadkar A, Sachdeva V, Taylor LF, Silver H, Banga AK. Low frequency sonophoresis mediated transdermal and intradermal delivery of ketoprofen. Int J Pharm. 2012;423(2):289–96.
Mohammed D, Hirata K, Hadgraft J, Lane ME. Influence of skin penetration enhancers on skin barrier function and skin protease activity. Eur J Pharm Sci. 2014;51:118–22.
Alber C, Buraczewska-Norin I, Kocherbitov V, Saleem S, Loden M, Engblom J. Effects of water activity and low molecular weight humectants on skin permeability and hydration dynamics - a double-blind, randomized and controlled study. Int J Cosmet Sci. 2014;36(5):412–8.
Rozman B, Zvonar A, Falson F, Gasperlin M. Temperature-sensitive microemulsion gel: an effective topical delivery system for simultaneous delivery of vitamins C and E. AAPS PharmSciTech. 2009;10(1):54–61.
Rangarajan M, Zatz JL. Effect of formulation on the topical delivery of alpha-tocopherol. J Cosmet Sci. 2003;54(2):161–74.
Kandárová H, Hayden P, Klausner M, Kubilus J, Sheasgreen J. An in vitro skin irritation test (SIT) using the EpiDerm Reconstructed Human Epidermal (RHE) model. J Visual Exp. 2009;29. http://www.jove.com/index/Details.stp?ID=1366. doi: 10.3791/1366.
Brohem CA, Cardeal LB, Tiago M, Soengas MS, Barros SB, Maria-Engler SS. Artificial skin in perspective: concepts and applications. Pigment Cell Melanoma Res. 2011;24(1):35–50.
Rozman B, Gosenca M, Falson F, Gasperlin M. The influence of microemulsion structure on their skin irritation and phototoxicity potential. Int J Pharm. 2016;499(1–2):228–35.
Hathout RM, Woodman TJ, Mansour S, Mortada ND, Geneidi AS, Guy RH. Microemulsion formulations for the transdermal delivery of testosterone. Eur J Pharm Sci. 2010;40(3):188–96.
Podlogar F, Gasperlin M, Tomsic M, Jamnik A, Rogac MB. Structural characterisation of water-Tween 40/Imwitor 308-isopropyl myristate microemulsions using different experimental methods. Int J Pharm. 2004;276(1–2):115–28.
Todosijevic MN, Savic MM, Batinic BB, Markovic BD, Gasperlin M, Randelovic DV, et al. Biocompatible microemulsions of a model NSAID for skin delivery: a decisive role of surfactants in skin penetration/irritation profiles and pharmacokinetic performance. Int J Pharm. 2015;496(2):931–41.
Kogan A, Shalev DE, Raviv U, Aserin A, Garti N. Formation and characterization of ordered bicontinuous microemulsions. J Phys Chem B. 2009;113(31):10669–78.
Malheiro AR, Varanda LC, Perez J, Villullas HM. The aerosol OT+n-butanol+n-heptane+water system: phase behavior, structure characterization, and application to Pt70Fe30 nanoparticle synthesis. Langmuir. 2007;23(22):11015–20.
Gee CM, Nicolazzo JA, Watkinson AC, Finnin BC. Assessment of the lateral diffusion and penetration of topically applied drugs in humans using a novel concentric tape stripping design. Pharm Res. 2012;29(8):2035–46.
Lopes LB, Reed R. A simple and rapid method to assess lycopene in multiple layers of skin samples. Biomed Chromatogr. 2010;24(2):154–9.
Mahrhauser D, Nagelreiter C, Baierl A, Skipiol J, Valenta C. Influence of a multiple emulsion, liposomes and a microemulsion gel on sebum, skin hydration and TEWL. Int J Cosmet Sci. 2015;37(2):181–6.
Herkenne C, Naik A, Kalia YN, Hadgraft J, Guy RH. Effect of propylene glycol on ibuprofen absorption into human skin in vivo. J Pharm Sci. 2008;97(1):185–97.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56(5):603–18.
Khandavilli S, Panchagnula R. Nanoemulsions as versatile formulations for paclitaxel delivery: peroral and dermal delivery studies in rats. J Invest Dermatol. 2007;127(1):154–62.
Bernabeu E, Gonzalez L, Cagel M, Gergic EP, Moretton MA, Chiappetta DA. Novel Soluplus((R))-TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf B: Biointerfaces. 2016;140:403–11.
Wartewig S, Neubert RH. Properties of ceramides and their impact on the stratum corneum structure: a review. Part 1: ceramides. Skin Pharmacol Physiol. 2007;20(5):220–9.
Wagner H, Kostka KH, Adelhardt W, Schaefer UF. Effects of various vehicles on the penetration of flufenamic acid into human skin. Eur J Pharm Biopharm. 2004;58(1):121–9.
Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Med. 2006;72(4):311–6.
Avdeef A, Bendels S, Tsinman O, Tsinman K, Kansy M. Solubility-excipient classification gradient maps. Pharm Res. 2007;24(3):530–45.
El Maghraby GM. Occlusive and non-occlusive application of microemulsion for transdermal delivery of progesterone: mechanistic studies. Sci Pharm. 2012;80(3):765–78.
Dubbs MD, Gupta RB, 590. Solubility of vitamin E (a-Tocopherol) and vitamin K3 (Menadione) in ethanol-water mixture. J Chem Eng Data. 1998;43(590):2.
Djekic L, Martinovic M, Stepanovic-Petrovic R, Tomic M, Micov A, Primorac M. Design of block copolymer costabilized nonionic microemulsions and their in vitro and in vivo assessment as carriers for sustained regional delivery of ibuprofen via topical administration. J Pharm Sci. 2015;104(8):2501–12.
Cavalcanti AL, Reis MY, Silva GC, Ramalho IM, Guimaraes GP, Silva JA, et al. Microemulsion for topical application of pentoxifylline: in vitro release and in vivo evaluation. Int J Pharm. 2016;506(1–2):351–60.
Erdal MS, Ozhan G, Mat MC, Ozsoy Y, Gungor S. Colloidal nanocarriers for the enhanced cutaneous delivery of naftifine: characterization studies and in vitro and in vivo evaluations. Int J Nanomedicine. 2016;11:1027–37.
Mishra R, Prabhavalkar KS, Bhatt LK. Preparation, optimization, and evaluation of Zaltoprofen-loaded microemulsion and microemulsion-based gel for transdermal delivery. J Liposome Res. 2016:1–10.
Sharma G, Dhankar G, Thakur K, Raza K, Katare OP. Benzyl benzoate-loaded microemulsion for topical applications: enhanced dermatokinetic profile and better delivery promises. AAPS PharmSciTech; 2015.
Nasr M, Abdel-Hamid S. Optimizing the dermal accumulation of a tazarotene microemulsion using skin deposition modeling. Drug Dev Ind Pharm. 2016;42(4):636–43.
Zhao L, Wang Y, Zhai Y, Wang Z, Liu J, Zhai G. Ropivacaine loaded microemulsion and microemulsion-based gel for transdermal delivery: preparation, optimization, and evaluation. Int J Pharm. 2014;477(1–2):47–56.
Acknowledgments
This study was supported by São Paulo Research Foundation (FAPESP grant#2013/16617-7) and PhRMA Foundation (Research Starter Grant in Pharmaceutics). Fellowships from CAPES (V. Carvalho) and FAPESP (D. de Lemos and A. Migotto) are greatly appreciated. We would like to thank Dr. A. Steiner for critical comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vanessa F. Carvalho and Debora P. de Lemos contributed equally to this work.
Rights and permissions
About this article
Cite this article
Carvalho, V.F., de Lemos, D.P., Vieira, C.S. et al. Potential of Non-aqueous Microemulsions to Improve the Delivery of Lipophilic Drugs to the Skin. AAPS PharmSciTech 18, 1739–1749 (2017). https://doi.org/10.1208/s12249-016-0643-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0643-7