Abstract
Quercetin can bring many benefits to skin based on its various bioactivities. However, the therapeutic effect of quercetin is limited due to the poor water solubility, pH instability, light instability, and skin permeation. The aim of the present work was applying essential oil-based microemulsions to improve the solubility, pH stability, photostability, and skin permeation of quercetin for topical application. Peppermint oil (PO-ME), clove oil (CO-ME), and rosemary oil (RMO-ME) were selected as model essential oils. Microemulsions composed of Cremophor EL/1,2-propanediol/essential oils (47:23:30, w/w) were selected as model formulations, based on the pseudo-ternary phase diagram and the characterizations. In the solubility study, the solubility of quercetin was improved dozens of times by microemulsions. Quercetin was found instable under alkaline condition, with 50% degraded in the solution of pH 13. However, PO-ME, CO-ME, and RMO-ME could protect quercetin from the hydroxide ions, with 47, 9, and 12% of quercetin degraded. In the photostability study, the essential oil-based microemulsions showed the capability of protecting quercetin from degradation under UV radiation. Where more than 67% of quercetin was degraded in aqueous solution, while less than 7% of quercetin degraded in microemulsions. At last, the in vitro skin permeation study showed that the essential oil-based microemulsions could enhance the permeation capacity of quercetin by 2.5–3 times compared to the aqueous solution. Hence, the prepared essential oil microemulsions could improve the solubility, pH stability, photostability, and skin permeation of quercetin, which will be beneficial for its topical application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Skin is the largest organ of human body, playing as a barrier to protect human body from germs, mechanical injuries, and exogenous pollutants (1). Skin is continuously exposed to the environment of ultraviolet irradiation, ionizing radiation, and toxic chemicals, which can induce oxidative stress by the increased amounts of reactive oxygen species (ROS) (2). There are some self-protection mechanisms against ROS in the skin, such as superoxide dismutase, metallothionein, and melanin (3). However, once the oxidative stress is superior to the defense mechanism of skin, skin damage can occur including lipid peroxidation, enzyme inactivation, and DNA breakage (4). Supporting skin defense mechanisms by exogenous antioxidants is a promising strategy to avoid skin damage, such as Coenzyme Q10, vitamin C, β-carotene, and polyphenols (5–7).
Quercetin, a flavonoid compound, is considered as one of the most powerful natural antioxidants (8). In vitro and in vivo experiments demonstrate that quercetin can protect keratinocytes from exogenous oxidizing agents, scavenge free radicals, prevent endogenous antioxidant depletion, and inhibit lipid peroxidation upon exposure to UV irradiation (9,10). Moreover, quercetin has various other biological activities including anti-inflammatory, antibacterial, antiviral, and anticancer properties. Quercetin can inhibit NF-κB pathway and the release of several proinflammatory cytokines, making it present broad anti-inflammatory actions (11). The combined antioxidative/anti-inflammatory actions of quercetin are also beneficial for wound healing. Furthermore, quercetin shows anti-aging activity on middle-aged keratinocyte, rejuvenating activity on terminally senescent cells, as well as whitening effect on skin (12). Hence, it is obvious that the topical application of quercetin can bring many benefits to skin. However, the therapeutic effect of quercetin is limited due to the poor water solubility, low permeation into skin, and instability under light, pH, and temperature (13,14). The disadvantages of quercetin can be overcome by formulation strategies (15). Several transdermal formulations of quercetin have been developed, including emulsions, microemulsions, liposomes, vesicles, cyclodextrin, gels, SLN, LNC, and mesoporous silica (16–21).
Microemulsions are dispersions consisting of oil phase, water phase, surfactant, and co-surfactant, which are single optically isotropic and thermodynamically stable liquid solutions with a droplet diameter usually within the range of 10–100 nm (22,23). In recent years, microemulsions have been applied to deliver many drugs for topical use, such as zaltoprofen, zidovudine, sertaconazole, resveratrol, and curcumin (24–26). They can significantly enhance their therapeutic effects, due to the advantages such as improving solubilities of poorly water-soluble drugs, improving the permeation of drugs across the skin barrier, improving the stability of unstable drugs (27,28). Moreover, the improvement of solubility, permeation, and stability of drug is highly related to the components of microemulsions.
Recently, microemulsions containing essential oil for topical use have gained much attention of researchers (29,30). Essential oils are natural, complex, multi-component systems, extracted from different parts of aromatic plants (31). Essential oils, such as peppermint oil, clove oil, and rosemary oil, have many biological activities including antioxidant, anti-inflammatory, anticancer, antibacterial, antifungal, and antiviral activities, leading them widely used in cosmetics, pharmaceutical, medicinal, and food industries (32–34). Moreover, essential oils mainly contain terpenes, which have good penetration enhancing effects on various drugs with low skin irritation at low concentrations (35). In theory, quercetin in combination with essential oils can not only improve its solubility and stability but also improve the permeation across skin. Moreover, the combination of quercetin and essential oils might result synergistic effect on protecting skin.
The objective of the present study was to prepare essential oil-based microemulsions to improve the solubility and stability of quercetin. Peppermint oil, clove oil, and rosemary oil were selected as model essential oils. The peppermint oil, clove oil, and rosemary oil-based microemulsions (PO-ME, CO-ME, and RMO-ME) were prepared and evaluated by pseudo-ternary phase diagram and particle size distribution. The solubilizing capabilities of three microemulsions on quercetin were subsequently studied. Finally, the effect of essential oil-based microemulsions on the pH stability and photostability of quercetin was studied.
MATERIALS AND METHODS
Materials
Peppermint oil, clove oil, or rosemary oil were kind gifts from Hengcheng Natural Flavor oil Co. (Jiangxi, China). Cremophor EL was purchased from Sigma Chemical Co. (St. Louis, MO, USA). 1,2-Propanediol was purchased from Damao Chemical Reagent Factory (Tianjin, China). Quercetin was obtained from Aladdin-reagent Co. (Shanghai, China). Methanol was HPLC grade and supplied from J&K Chemical Ltd. (Shanghai, China). All other chemicals and reagents were of analytical grade.
Pseudo-ternary Phase Diagram
Pseudo-ternary phase diagrams were constructed using a water titration method at room temperature. Cremophor EL and 1,2-propanediol were selected as surfactant and co-surfactant, and they were firstly mixed at the ratio of 2:1 (w/w) to form the mixed surfactant (S mix). Essential oil (peppermint oil, clove oil, or rosemary oil) and S mix were weighted into glass vials with the weight ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1, where the total weight was 0.5 g. They were mixed for 2 h on magnetic stirrers, and then the mixtures were titrated with deionized water in a drop-wise manner and mixed thoroughly by magnetic stirrers. The titration of water was stopped until the turbid mixtures converted into transparent solution. After 30 min equilibrium, the samples were clear, transparent, and homogenous, with light blue opalescence, meaning the form microemulsions. The amounts of water added in each group were recorded, and the boundary of microemulsion-forming domain was determined. Furthermore, the samples were diluted 20-fold by deionized water and stored 48 h for observation.
Preparation and Characterization of Microemulsions
Cremophor EL and 1,2-propanediol were firstly mixed at the weight ratio of 2:1 and stirred for 1 h at room temperature. Then, S mix and essential oil (peppermint oil, clove oil, or rosemary oil) were weighted into glass vials with the weight ratio of 7:3. The glass vials were immediately sealed with rubber stoppers to avoid volatilization of essential oils. The mixtures were stirred for 2 h in the dark to prepare the self-microemulsifying drug delivery systems (SMEDDS). Microemulsions (PO-ME, CO-ME, and RMO-ME) were prepared by diluting 1 g SMEDDS formulations with 19 mL deionized water. For the preparation of quercetin-loaded microemulsions, quercetin was previously mixed with S mix and essential oil before adding deionized water.
The particle sizes, polydispersity indexes (PDI), and zeta potentials of PO-ME, CO-ME, and RMO-ME were measured by Malvern ZS90 zetasizer (Malvern Instruments Corp, Malvern, UK). Briefly, 1 mL of microemulsions was put into the cuvettes for measurement. Light scattering was monitored at a fixed angle of 90° and maintained the temperature at 25°C. Three parallel samples were measured.
The electrical conductivity of PO-ME, CO-ME, and RMO-ME was measured using a DDS-307 electric conductivity meter (Shanghai Precision Scientific Instrument Corp, Shanghai, China). The electrode was dipped in the microemulsion samples at the temperature of 25°C, and electrical conductivity was recorded after equilibrium had been reached. Three parallel samples were measured.
The refractive index was determined at 25°C using a 2WAJ Abbe refractometer (Shanghai Optical Instrument Company, Shanghai, China). The transmittance of microemulsions was measured at 650 nm using the ultraviolet/visible spectrophotometer (UV2300, Techcomp, China) keeping distilled water as a blank. Three parallel samples were measured.
The viscosity of microemulsions was measured by a cone-plate viscometer (Brook-field, DV-II + Pro, USA). The electronic cone spindle CPE-40 was applied, and the gap between the cone and plate was set at 13 μm. The spindle speed was 3 rpm, and the viscosity was recorded when the number was stabilized. Three parallel samples were measured.
Solubility Study
Excess amounts of quercetin were added into test tubes containing 1 mL of water, essential oil, Cremophor EL, 1,2-propanediol, S mix, SMEDDS, diluted microemulsions, or micelles. The test tubes were sealed and shaken for 72 h in a shaking incubator (HZQ-F, Harbin Donglian Electronic Technology Development Co., LTD., Harbin, China) at 37°C, 150 rpm. After equipment, the test tubes were centrifuged at 5000g for 30 min, to remove the excess drug. The supernatant was diluted several times by ethanol, and the concentration of quercetin was measured using the ultraviolet/visible spectrophotometer at the wavelength of 370 nm. For each group, samples were performed in triplicate.
pH Stability Study
4.2 g S mix, 1.8 g essential oil (peppermint oil, clove oil, or rosemary oil), and 30 mg quercetin were added into glass vials and sealed by rubber stoppers. They were stirred for 2 h on a magnetic stirrer in dark, to obtain SMEDDS containing 5 mg/g quercetin. Then, 1 g SMEDDS (or 5 mg quercetin) was respectively diluted by 19 mL (or 20 mL) solution with the pH of 1, 4, 7, 10, or 13. The formed microemulsions or quercetin solutions were stirred for 10 h in the dark, and then the samples were diluted 10-fold by ethanol to measure the concentrations of remained resveratrol. Furthermore, the particle sizes of microemulsions in different pH solutions were measured. The solutions with different pH were respectively 0.1 M HCl solution (pH 1), 0.1 mM HCl solution (pH 4), water (pH 7), 0.1 mM NaOH solution (pH 10), and 0.1 M NaOH solution (pH 13).
Photostability Study
The photodegradation experiments were performed using a UVB lamp (Philips TL40/12 RS), emitting a continuous spectrum of 270–400 nm with a peak emission at 313 nm (36). Quercetin-loaded SMEDDS (5 mg/g) were prepared and diluted 20 times by deionized water. Quercetin aqueous solution with the concentration of 0.25 mg/mL was also prepared as control. Quercetin microemulsions and aqueous solution were kept under the light source with the distance of 15 cm. At predetermined irradiation time, the concentration of quercetin was measured by UV analysis. The photostability was evaluated by the percentage of remained quercetin at different irradiation times, compared to unexposed samples. For each group, samples were performed in triplicate.
In Vitro Skin Permeation Study
The dorsal skin for permeation study was obtained from Wistar rats. Subcutaneous fat and excess tissue were carefully removed, and the integrity was examined. The excised rat skins were washed by phosphate buffer solution (pH 6.8) and stored at 4°C before permeation experiments.
The in vitro skin permeation study was carried out by the Franz diffusion cell apparatus (HC-188, Tianjin Zhengtong Technology Co., China). The skin was fixed between the donor and the receptor chamber, with the stratum corneum side towards to the donor chamber. The receptor chamber was filed with the receptor phase (saline/ethanol = 8:2) and kept at 37°C. The skin area contacting with the receptor phase was 0.636 cm2. Quercetin-loaded SMEDDS (5 mg/g) were prepared and diluted 20 times by deionized water. Quercetin aqueous solution with the concentration of 0.25 mg/mL was also prepared as control. A 0.3 mL quercetin microemulsions or aqueous solution was added on the donor chamber. After 3, 6, 9, 12, and 24 h, 0.5 mL receptor phase was taken out and replaced with fresh solution.
The amount of quercetin was analyzed by HPLC (e2695, Waters Corporation, Milford, USA). The analysis was carried out on a SinoChrom ODS-BP column (4.6 × 250 mm, 5 μm, Dalian Elite Analytical Instruments, Dalian, China). The mixture of methanol/water/phosphoric acid (100:100:1, v/v) was used as mobile phase. The flow rate was kept at 0.8 mL/min, and the detection wavelength was set at 370 nm.
RESULTS AND DISCUSSION
Determination of Microemulsion Formulations
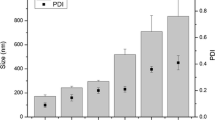
Cremophor EL and 1,2-propanediol are commonly used as the surfactant and co-surfactant of microemulsions. Here, we used the mixture of Cremophor EL/1,2-propanediol (2:1) as mixed surfactants and evaluated the feasibility of forming microemulsions with essential oil (peppermint oil, clove oil, or rosemary oil) by the pseudo-ternary phase diagrams, the appearance, and the particle size distribution. In the pseudo-ternary phase diagrams (see Fig. 1), we found that all the three types of essential oils could form microemulsions, if the S mix/oil ratio was high enough. Peppermint oil has the largest microemulsion area in the pseudo-ternary phase diagrams, where the S mix/oil ratio should be above 5:5. Similarly, microemulsions of clove oil and rosemary oil required the S mix/oil ratio higher than 7:3 and 5:5. At the beginning titration of water, the mixtures formed sticky, opaque, gel-like dispersions. As the content of water increased, they transformed into transparent, homogenous o/w microemulsions. Moreover, the formed o/w microemulsions could be diluted by bulk water. Figure 2 shows the visual appearance of the S mix/essential oil mixtures diluted 20-fold by water. We found that the mixtures with the S mix/peppermint oil ratios above 5:5 were clear, transparent, and homogenous microemulsions with light blue opalescence, while the other solutions were turbid and heterogeneous. The mixtures of S mix/clove oil and S mix/rosemary oil could also form the transparent and homogenous microemulsions at the ratios above 7:3 and 5:5. Furthermore, we measured the particle sizes of the essential oil-based microemulsions. As seen in Fig. 3, the particle sizes of microemulsions were gradually increased with the ratio of essential oil/S mix. As microemulsions could be considered as “swollen micelles,” the oil phase incorporated into the hydrophobic core of micelle leading their particle sized increased. It is worth noting that the particle sizes of microemulsions were very small, even less than 30 nm for PO-ME and RMO-ME with the oil content below 50% and less than 20 nm for CO-ME with the oil content below 30%. In summary, peppermint oil, clove oil, and rosemary oil can form o/w microemulsions with the S mix/essential oil ratio above 5:5, 7:3, and 5:5, and the particle sizes of microemulsions were smaller than 30 nm.
Characterizations of Essential Oil-Based Microemulsions
In this study, we selected the SMEDDS formulations with the S mix/essential oil ratio of 7:3 as model for further study, where the Cremophor EL/1,2-propanediol/essential oil content was 47:23:30 (w/w). The SMEDDS formulations were diluted 20 times by deionized water, and the characterizations of formed microemulsions were listed in Table I. The particle sizes, polydispersity index (PDI), and zeta potentials were measured by Malvern ZS90 zetasizer. The particle sizes of PO-ME, CO-ME, and RMO-ME were similar, which were 12.66 ± 0.54, 9.74 ± 0.04, and 11.61 ± 0.08 nm, respectively. The polydispersity index of CO-ME was smaller than PO-ME and RMO-ME, indicating that they had better homogeneity. The zeta potentials were limited from −5 to 0 mV, because there was no charged group in the compositions. The small negative charge could be attributed to the polyoxyethylene group of Cremophor EL. The electrical conductivities of PO-ME, CO-ME, and RMO-ME were measured to be 60.0 ± 3.3, 89.4 ± 4.0, and 185.6 ± 5.5 μS/cm, respectively. Moreover, their viscosities were 3.58 ± 0.36, 3.19 ± 0.16, and 3.37 ± 0.24 cP. The electrical conductivity and viscosity of microemulsions indicated that they were o/w type microemulsions. In addition, the refractive indexes of PO-ME, CO-ME, and RMO-ME were 1.3396, 1.3399, and 1.3392, very similar to the refractive index of water (1.333). At last, we found that the transmittance of the diluted microemulsions was more than 93% at 650 nm.
Solubility
The poor solubility has severely limited the application of quercetin, leading it essential to improve the solubility of quercetin by pharmaceutical method (37). The solubilizing capabilities of microemulsions as well the components are listed in Table II. The solubility of quercetin in water was just 0.013 mg/mL; however, its solubility was significantly increased in surfactant, co-surfactant, essential oils, SEMDDS, microemulsions, and micelles. Cremophor EL has the best solubilizing capability among the components, which reached to 18.17 ± 0.12 mg/mL. The quercetin solubility in essential oils varied significantly, where peppermint oil showed the best solubilizing capability of 15.73 ± 0.07 mg/mL. The solubility of quercetin in clove oil and rosemary oil was 6.84 ± 0.02 and 1.1 ± 0.03 mg/mL. The solubilities of quercetin in S mix and SMEDDS were between 8 and 21 mg/mL, among which the solubility in PO-SME was the highest, more than 1600 times higher than water solubility. Furthermore, we found that the solubilizing capabilities of S mix and SMEDDS were significantly decreased, if they were diluted. After diluted by 100-fold, the formed micelles and microemulsions have the solubility of 0.27–1.11 mg/mL, still dozens of times higher than water solubility.
pH Stability
Quercetin was chemically unstable, and the instability could be attributed to hydroxyl groups and the instable pyrone structure of quercetin (38). It is reported that quercetin was degraded under alkaline conditions (14). Moreover, the oxidation of quercetin by air oxygen could take place under moderately basic media (39). Some formulations could protect quercetin from alkaline degradation, such as lecithin-based nanoparticle, cyclodextrin, and solid dispersion (40–43). In this work, we studied the ability of essential oil-based microemulsions protecting quercetin from degradation at different pH conditions. To evaluate the stability of microemulsions in different pH solutions, the particle sizes of PO-ME, CO-ME, and RMO-ME were measured with the pH ranging from 1 to 13. As seen in Fig. 4, the particle sizes of three kinds of microemulsions were between 6 and 12 nm. Despite the particle sizes were slightly deceased if the pH increased, the changes were negligible. Hence, the essential oil-based microemulsions were stable in the wide range pH conditions. The pH stability of quercetin in aqueous solution and microemulsions is shown in Fig. 5. Quercetin was stable in aqueous solution and microemulsions under acidic and neutral conditions; unfortunately, it was degraded under alkaline conditions. The degradation of quercetin was limited in aqueous solution of pH 10, with about 94% of quercetin remained. However, the degraded quercetin reached to 50% when the pH increased to 13. Meanwhile, the remained quercetin in PO-ME, CO-ME, and RMO-ME was respectively 57, 91, and 88% at pH 13. This indicated that essential oil-based microemulsions could protect quercetin from alkaline degradation to some extent. The quercetin protective effect of clove oil and rosemary oil seemed better than peppermint oil. For drugs in microemulsions, they can be either located in the hydrophilic group of surfactant (the shell of microemulsions) or located in the hydrophobic oil region (the core of microemulsions) (44). We inferred that quercetin might be located in the core of microemulsions, where quercetin was separated from the hydroxide ions. The incorporation of quercetin has many benefits for protecting it from degradation and prolonging the shelf-life of quercetin products.
Photostability
UV exposure to the skin leads the generation of reactive oxygen species (ROS), which could damage cell membranes by peroxidation of fatty acids. Quercetin, a powerful antioxidant, can scavenge ROS to protect the skin. Moreover, quercetin could directly absorb UV radiation to prevent the formation of ROS and the consequent direct DNA damage (45). However, quercetin is instable under UV irradiation. Quercetin is prone to be degraded under light exposure, resulting in the decreased efficacy. The risk of light-induced degradation is throughout the whole process of preparation, storage, and application of quercetin formulations. Hence, it is essential to evaluate photostability of quercetin formulations. In this work, the photostability of quercetin under UV irradiation was studied. As seen in Fig. 6, the quercetin was quickly degraded in aqueous solution under UV irradiation. The remained quercetin in aqueous solution were 70.4, 50.2, 39.7, 36, and 32%, with the irradiation time of 0.5, 1.5, 3.5, 8.5, and 12.5 h. More than two-thirds of quercetin was degraded after 12.5 h irradiation. The degradation products of quercetin were complicated; products such as 2,4,6-trihydroxybenzaldehyde, 2-(3′,4′-dihydroxybenzoyloxy)-4,6-dihydroxybenzoic acid, and 3,4-dihydroxyphenylethanol had been reported (46,47). Compared to the solution, quercetin in essential oil-based microemulsions was very stable. The percentages of remained quercetin were respectively 93.7, 93.1, and 96.8% in PO-ME, CO-ME, and RMO-ME, much higher than quercetin aqueous solution. No significant difference was found among the three microemulsions. Hence, the incorporation of quercetin into essential oil-based microemulsions could significantly improve its stability. The presence of essential oil might play an important role in avoiding quercetin degradation.
In Vitro Skin Permeation
An essential requirement to evaluate the effectiveness of topical formulations is the permeation capacity through the skin. In this work, we applied the Franz diffusion cell method to evaluate the permeation capacity of essential oil-based microemulsions through rat skin. The area of skin contacting with the receptor chamber was 0.636 cm2, and the total volume of the receptor phase was 5.2 mL. SMEDDS containing 5 mg/g quercetin was diluted 20 times and the formed microemulsions were added into the donor chamber with the volume of 0.3 mL, which contained 75 μg quercetin. For PO-ME, CO-ME, RMO-ME, and the aqueous solution, the cumulative amount of quercetin permeated through rat skin is listed in Fig. 7. The amount of permeated quercetin was gradually increased with the diffusion time. The cumulative amount of quercetin reached to 2.24 ± 0.35 μg/cm2 for the aqueous solution, while it reached to 8.54 ± 0.55, 7.98 ± 0.57, and 9.12 ± 0.68 μg/cm2 for PO-ME, CO-ME, and RMO-ME. Hence, it was obvious that the essential oil-based microemulsions could significantly improve the skin permeation of quercetin, about 2.5–3 times higher than the aqueous solution. The permeation enhancement should be attributed to the combination effect of surfactant and essential oils. The enhanced skin permeation indicated that the essential oil-based microemulsions could bring more benefits to the skin, compared to the aqueous solution.
CONCLUSION
In topical application, both quercetin and essential oils have many biological activities to protect the skin. In this work, three kinds of essential oil-based microemulsions containing peppermint oil (PO-ME), clove oil (CO-ME), or rosemary oil (RMO-ME) were prepared, for topical delivery of quercetin. Pseudo-ternary phase diagram demonstrated that essential oils, Cremophor EL, and 1,2-propanediol could form the microemulsion region depending on the S mix/oil ratio (at least 5:5). Moreover, the particle sizes of microemulsions were gradually increased with the decrease of S mix/oil ratio. They formed microemulsions with similar particle sizes (9–13 nm) with the S mix/oil ratio of 7:3. Solubility study showed that SMEDDS and the components can significantly improve the solubility of quercetin. Despite the solubilizing capability decreased as SMEDDS diluted, they were still dozens of times higher than water. In pH stability study, quercetin was found instable under alkaline condition, especially with the pH of 13. However, the essential-based microemulsion could protect quercetin from degradation, and the protective effect of CO-ME and RMO-ME was better than PO-ME. In the photostability study, we found that the essential oil-based microemulsions could protect quercetin from degradation under UV radiation. Where more than 67% of quercetin was degraded in aqueous solution, while less than 7% of quercetin degraded in microemulsions. At last, the in vitro skin permeation was preformed, founding that the essential oil-based microemulsions could enhance the permeation capacity of quercetin by 2.5–3 times compared to the aqueous solution. Hence, the prepared essential oil microemulsions could improve the solubility, pH stability, photostability, and skin permeation of quercetin, which will be benefit for its topical application.
References
English JS, Dawe RS, Ferguson J. Environmental effects and skin disease. Br Med Bull. 2003;68:129–42.
Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126(12):2565–75. doi:10.1038/sj.jid.5700340.
Cap M, Vachova L, Palkova Z. Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxidative Med Cell Longev. 2012;2012:976753. doi:10.1155/2012/976753.
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1–40. doi:10.1016/j.cbi.2005.12.009.
Pardeike J, Schwabe K, Muller RH. Influence of nanostructured lipid carriers (NLC) on the physical properties of the Cutanova Nanorepair Q10 cream and the in vivo skin hydration effect. Int J Pharm. 2010;396(1–2):166–73. doi:10.1016/j.ijpharm.2010.06.007.
Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302(2):71–83. doi:10.1007/s00403-009-1001-3.
Vayalil PK, Mittal A, Hara Y, Elmets CA, Katiyar SK. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J Invest Dermatol. 2004;122(6):1480–7. doi:10.1111/j.0022-202X.2004.22622.x.
Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. doi:10.1016/j.fct.2012.09.021.
Svobodova A, Psotova J, Walterova D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. 2003;147(2):137–45.
Bustos PS, Deza-Ponzio R, Paez PL, Albesa I, Cabrera JL, Virgolini MB, et al. Protective effect of quercetin in gentamicin-induced oxidative stress in vitro and in vivo in blood cells. Effect on gentamicin antimicrobial activity. Environ Toxicol Pharmacol. 2016;48:253–64. doi:10.1016/j.etap.2016.11.004.
Hatahet T, Morille M, Hommoss A, Devoisselle JM, Mueller RH, Begu S. Quercetin topical application, from conventional dosage forms to nanodosage forms. Eur J Pharm Biopharm. 2016;108:41–53. doi:10.1016/j.ejpb.2016.08.011.
Cho S. The role of functional foods in cutaneous anti-aging. Journal of lifestyle medicine. 2014;4(1):8–16. doi:10.15280/jlm.2014.4.1.8.
Friedman M, Jurgens HS. Effect of pH on the stability of plant phenolic compounds. J Agric Food Chem. 2000;48(6):2101–10.
Zheng Y, Haworth IS, Zuo Z, Chow MSS, Chow AHL. Physicochemical and structural characterization of quercetin-beta-cyclodextrin complexes. J Pharm Sci. 2005;94(5):1079–89. doi:10.1002/jps.20325.
Vijayakumar A, Baskaran R, Jang YS, Oh SH, Yoo BK. Quercetin-loaded solid lipid nanoparticle dispersion with improved physicochemical properties and cellular uptake. AAPS PharmSciTech. 2017;18(3):875–883. doi:10.1208/s12249-016-0573-4.
Jeon S, Yoo CY, Park SN. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by layer-by-layer electrostatic deposition for quercetin delivery. Colloids and Surfaces B-Biointerfaces. 2015;129:7–14. doi:10.1016/j.colsurfb.2015.03.018.
Sapino S, Ugazio E, Gastaldi L, Miletto I, Berlier G, Zonari D, et al. Mesoporous silica as topical nanocarriers for quercetin: characterization and in vitro studies. Eur J Pharm Biopharm. 2015;89:116–25. doi:10.1016/j.ejpb.2014.11.022.
Han SB, Kwon SS, Jeong YM, Yu ER, Park SN. Physical characterization and in vitro skin permeation of solid lipid nanoparticles for transdermal delivery of quercetin. Int J Cosmet Sci. 2014;36(6):588–97. doi:10.1111/ics.12160.
Bose S, Michniak-Kohn B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur J Pharm Sci. 2013;48(3):442–52. doi:10.1016/j.ejps.2012.12.005.
Vicentini FTMC, Simi TRM, Del Ciampo JO, Wolga NO, Pitol DL, Iyomasa MM, et al. Quercetin in w/o microemulsion: in vitro and in vivo skin penetration and efficacy against UVB-induced skin damages evaluated in vivo. Eur J Pharm Biopharm. 2008;69(3):948–57. doi:10.1016/j.ejpb.2008.01.012.
Patel AR, Heussen PCM, Hazekamp J, Drost E, Velikov KP. Quercetin loaded biopolymeric colloidal particles prepared by simultaneous precipitation of quercetin with hydrophobic protein in aqueous medium. Food Chem. 2012;133(2):423–9. doi:10.1016/j.foodchem.2012.01.054.
Jain S, Jain AK, Pohekar M, Thanki K. Novel self-emulsifying formulation of quercetin for improved in vivo antioxidant potential: implications for drug-induced cardiotoxicity and nephrotoxicity. Free Radic Biol Med. 2013;65:117–30. doi:10.1016/j.freeradbiomed.2013.05.041.
Zhang J, Lv Y, Wang B, Zhao S, Tan M, Lv G, et al. Influence of Microemulsion-mucin interaction on the fate of Microemulsions diffusing through pig gastric mucin solutions. Mol Pharm. 2015;12(3):695–705. doi:10.1021/mp500475y.
Mishra R, Prabhavalkar KS, Bhatt LK. Preparation, optimization, and evaluation of Zaltoprofen-loaded microemulsion and microemulsion-based gel for transdermal delivery. J Liposome Res. 2016;26(4):297–306. doi:10.3109/08982104.2015.1120746.
Liu CH, Chang FY, Hung DK. Terpene microemulsions for transdermal curcumin delivery: effects of terpenes and cosurfactants. Colloids Surf B Biointerfaces. 2011;82(1):63–70. doi:10.1016/j.colsurfb.2010.08.018.
Yutani R, Teraoka R, Kitagawa S. Microemulsion using Polyoxyethylene Sorbitan Trioleate and its usage for skin delivery of resveratrol to protect skin against UV-induced damage. Chem Pharm Bull (Tokyo). 2015;63(9):741–5. doi:10.1248/cpb.c15-00378.
Kitagawa S, Tanaka Y, Tanaka M, Endo K, Yoshii A. Enhanced skin delivery of quercetin by microemulsion. J Pharm Pharmacol. 2009;61(7):855–60. doi:10.1211/jpp/61.07.0003.
Censi R, Martena V, Hoti E, Malaj L, Di Martino P. Permeation and skin retention of quercetin from microemulsions containing Transcutol (R) P. Drug Dev Ind Pharm. 2012;38(9):1128–33. doi:10.3109/03639045.2011.641564.
Herman A, Herman AP. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: a review. J Pharm Pharmacol. 2015;67(4):473–85. doi:10.1111/jphp.12334.
Chaiyana W, Anuchapreeda S, Leelapornpisid P, Phongpradist R, Viernstein H, Mueller M. Development of microemulsion delivery system of essential oil from Zingiber cassumunar Roxb. rhizome for improvement of stability and anti-inflammatory activity. AAPS PharmSciTech. 2017;18(4):1332–1342. doi:10.1208/s12249-016-0603-2.
Jalali-Heravi M, Parastar H. Recent trends in application of multivariate curve resolution approaches for improving gas chromatography-mass spectrometry analysis of essential oils. Talanta. 2011;85(2):835–49. doi:10.1016/j.talanta.2011.05.045.
Shen Q, Li W, Li W. The effect of clove oil on the transdermal delivery of ibuprofen in the rabbit by in vitro and in vivo methods. Drug Dev Ind Pharm. 2007;33(12):1369–74. doi:10.1080/03639040701399346.
Riachi LG, De Maria CAB. Peppermint antioxidants revisited. Food Chem. 2015;176:72–81. doi:10.1016/j.foodchem.2014.12.028.
Fu Y, Zu Y, Chen L, Shi X, Wang Z, Sun S, et al. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytotherapy research: PTR. 2007;21(10):989–94. doi:10.1002/ptr.2179.
Fox LT, Gerber M, Du Plessis J, Hamman JH. Transdermal drug delivery enhancement by compounds of natural origin. Molecules. 2011;16(12):10507–40. doi:10.3390/molecules161210507.
Vicentini FTMC, Vaz MMOLL, Fonseca YM, Bentley MVLB, Fonseca MJV. Characterization and stability study of a water-in-oil microemulsion incorporating quercetin. Drug Dev Ind Pharm. 2011;37(1):47–55. doi:10.3109/03639045.2010.491078.
Casagrande R, Georgetti SR, Verri WA Jr, Jabor JR, Santos AC, Fonseca MJ. Evaluation of functional stability of quercetin as a raw material and in different topical formulations by its antilipoperoxidative activity. AAPS PharmSciTech. 2006;7(1):E10. doi:10.1208/pt070110.
Wang J, Zhao X-H. Degradation kinetics of fisetin and quercetin in solutions affected by medium pH, temperature and co-existing proteins. J Serb Chem Soc. 2016;81(3):243–53. doi:10.2298/jsc150706092w.
Zenkevich IG, Eshchenko AY, Makarova SV, Vitenberg AG, Dobryakov YG, Utsal VA. Identification of the products of oxidation of quercetin by air oxygen at ambient temperature. Molecules. 2007;12(3):654–72. doi:10.3390/12030654.
Li B, Konecke S, Harich K, Wegiel L, Taylor LS, Edgar KJ. Solid dispersion of quercetin in cellulose derivative matrices influences both solubility and stability. Carbohydr Polym. 2013;92(2):2033–40. doi:10.1016/j.carbpol.2012.11.073.
Anandam S, Selvamuthukumar S. Fabrication of cyclodextrin nanosponges for quercetin delivery: physicochemical characterization, photostability, and antioxidant effects. J Mater Sci. 2014;49(23):8140–53. doi:10.1007/s10853-014-8523-6.
Carlotti ME, Sapino S, Ugazio E, Caron G. On the complexation of quercetin with methyl-beta-cyclodextrin: photostability and antioxidant studies. J Incl Phenom Macrocycl Chem. 2011;70(1–2):81–90. doi:10.1007/s10847-010-9864-7.
Tan Q, Liu W, Guo C, Zhai G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int J Nanomedicine. 2011;6:1621–30.
Zhang J, Lv Y, Zhao S, Wang B, Tan M, Xie H, et al. Effect of lipolysis on drug release from self-microemulsifying drug delivery systems (SMEDDS) with different Core/Shell drug location. AAPS PharmSciTech. 2014;15(3):731–40. doi:10.1208/s12249-014-0096-9.
Zvezdanovic JB, Stanojevic JS, Markovic DZ, Cvetkovic DJ. Irreversible UV-induced quercetin and rutin degradation in solution studied by UV spectrophotometry and HPLC chromatography. J Serb Chem Soc. 2012;77(3):297–312. doi:10.2298/jsc110618180z.
Dall’Acqua S, Miolo G, Innocenti G, Caffieri S. The Photodegradation of quercetin: relation to oxidation. Molecules. 2012;17(8):8898–907. doi:10.3390/molecules17088898.
Fahlman BM, Krol ES. UVA and UVB radiation-induced oxidation products of quercetin. Journal of Photochemistry and Photobiology B-Biology. 2009;97(3):123–31. doi:10.1016/j.jphotobiol.2009.08.009.
Acknowledgements
This work was supported by the National Science Foundation of China (Grant No. 81601825), Program for Science and Technology Project of Liaoning Province (Grant No. 201601237), and Educational Committee Foundation of Liaoning Province (Grant No. L2016026, L2015150).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lv, X., Liu, T., Ma, H. et al. Preparation of Essential Oil-Based Microemulsions for Improving the Solubility, pH Stability, Photostability, and Skin Permeation of Quercetin. AAPS PharmSciTech 18, 3097–3104 (2017). https://doi.org/10.1208/s12249-017-0798-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0798-x