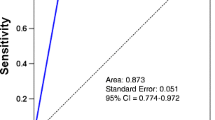
Abstract
Background
During multi-detector computed tomography (MDCT) of the chest, incidental breast lesions (IBLs) are occasionally encountered. Mammography remains the gold standard for the early detection of breast cancer. However, limitations exist in patients with dense breasts. Contrast spectral mammography (CESM) is widely available compared to MRI; it increases the sensitivity for breast cancer detection without decreasing the specificity.
Results
The study is a prospective study that included 113 female breast cancer patients for CT staging. One hundred and six of the patients had unilateral carcinoma and 7 of them had bilateral cancer with a total of 120 breasts evaluated. The CT findings were correlated with CESM findings regarding the multiplicity and bilaterality of the disease. The sensitivity, specificity, PPV, NPV, and accuracy of the CT in the detection of multiplicity were 97.44%, 100%, 100%, 95.45%, and 98.33%, respectively, and the sensitivity, specificity, PPV, NPV, and accuracy of the CT in the detection of bilateral disease were 68.18%, 97.96%, 88.24%, 93.20%, and 92.50%, respectively.
Conclusions
Breast cancer patients for MDCT chest as a part of their metastatic workup can omit the further need for CESM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Mammography remains the imaging standard for the detection of early breast cancer. However, it has limitations in dense breasts with a decrease in overall sensitivity from 85%, i.e., decreases to 68% [1].
Contrast spectral mammography (CESM) has been introduced to aid in breast cancer diagnosis, multiplicity evaluation, and deciding the type of surgery [2].
National Comprehensive Cancer Network (NCCN) guidelines recommend diagnostic contrast-enhanced chest computed tomography (CT) as part of the staging for distant metastasis in breast cancer patients classified as stage II or higher (> T2, N1) [3].
We performed a 3-year prospective study to compare contrast-enhanced MDCT chest and CESM in the detection of multifocal, multicentric, or bilateral breasts disease in already diagnosed breast cancer patients’ candidate for CT chest as a part of metastatic workup (stage II or higher) with the feasibility of MDCT chest to be a replacing diagnostic tool instead of CESM in this set of patients.
Methods
Case selection and data collection
This prospective study was carried out between June 2017 and June 2020. The study was approved by the ethical committee (ref. no. BEC202103030065), and all enrolled patients provided their informed consent. During those 3 years, we received 900 breast cancer patients, 787 patients were excluded being early breast cancer patients who did not require CT staging and lost to follow-up and those with no pathological data (flowchart). We included 113 female breast cancer patients; 7 of them had bilateral cancer, so 120 diseased breasts were included in the study. The patient's ages ranged from 25 to 75 years (mean 55 years). Our inclusion criteria were pathologically proven breast cancer patients candidates for CT staging (T3, T4, N2, or N3). We excluded patients with contraindications for contrast injection, patients with previous breast surgery, or on chemotherapy. CESM was performed then after 1-week MDCT examination was done for all patients.
Contrast-enhanced spectral mammography examination technique
Intravenous injection of an iodinated contrast agent (iohexol, 300 mg I/mL) was done at a dose of 1.5 mL/kg before the application of breast compression. This was followed by a 2-min wait before dual-energy CESM image acquisition in the two standard positions (craniocaudal and mediolateral oblique views). Low- and high-energy images were consecutively acquired in each view. Enhanced images were calculated by weighted logarithmic subtraction of the two images.
Contrast-enhanced MDCT chest examination technique
Contrast-enhanced MDCT chest was performed using an MDCT scanner (Aquilion, Toshiba Medical, Tokyo, Japan) set for 1-mm collimation and a pitch of 5.5.
We scanned our patients in the supine position from the level of the lower neck to the level of the suprarenal glands. Three breast-hold acquisitions were obtained before and after IV rapid bolus administration of non-ionic contrast material in the portal and delayed phases. We infused 100 mL of non-ionic contrast material (Omnipaque), at a rate of 3 mL/s. using a pump injector. The data were reconstructed at 0.6-mm increments in axial, coronal, and sagittal planes. Post-acquisition image processing and reconstruction were performed to achieve isotropic reconstructed volumes using a soft tissue filter and voxel size of 0.273 mm3 (standard mode). Contrast-enhanced CT images were viewed in three orthogonal orientations (sagittal, axial, and coronal) at varying slice thickness (from 0.27 to 11 mm) and 3D views to aid in interpretation.
Image analysis
MDCTs and CESM were performed within a week and were evaluated prospectively and independently by dedicated breast radiologists. Both CESM MRI and MDCT findings were classified according to the 5th edition Breast Imaging Reporting and Data System (BI-RADS®) MRI-lexicon [4].
The enhancing lesion was classified as a mass or non-mass. The mass was analyzed regarding its shape, margins, and enhancement pattern. Non-mass enhancement was analyzed regarding its distribution and enhancement pattern. The lesions were also classified as unifocal, multifocal, or multicentric. Multifocality was defined as additional sites of malignancy within the same breast quadrant of the index tumor, while multicentricity was defined as additional sites of malignancy within different quadrants of the same breast. Intraductal extension of the mass was assessed if present. The assessment also included the presence of other enhancing lesions on the contralateral side. The presence of nipple changes, skin thickening/attachment, or pathological axillary lymph nodes was also recorded.
Statistical analysis
Data were coded and entered using the SPSS (Statistical Package for the Social Sciences) version 26 (IBM Corp., Armonk, NY, USA). Data were summarized using mean, standard deviation, median, minimum, and maximum in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Standard diagnostic indices including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic efficacy were calculated [5]. For comparing categorical data, chi-square (χ2) test was performed. An exact test was used instead when the expected frequency is less than 5. A P value of less than 0.05 was considered statistically significant.
Results
This study included 113 patients diagnosed as breast cancer candidates for CT staging (stage II or higher); 7 of them had bilateral cancer, so 120 diseased breasts were evaluated. The patient’s age ranged from 25 to 75 years (mean age 55.3 ± SD). In all patients, breast cancer was diagnosed by percutaneous core needle biopsy and their histopathology results are demonstrated in Table 1. The CESM was considered as a gold standard reference to evaluate the performance of contrast-enhanced MDCT chest and the possibility of being a replacement contrast tool study for CESM.
Regarding lesion descriptors and enhancement pattern
The enhancing lesions detected in CESM and contrast-enhanced MDCT were masses, non-mass, and mass and non-mass enhancement patterns which are demonstrated in Table 2. An irregular shape, non-circumscribed margins, and heterogeneous enhancement were the most encountered descriptors for malignant masses, while segmental, regional, and clumped non-mass enhancement were the most encountered descriptors for malignant non-mass enhancement in contrast-enhanced MDCT.
Regarding the multiplicity of the lesions
The results of both CESM and contrast-enhanced MDCT chest were matched in 118 (98.3%) of the examined breasts, where 42 (35%) of the examined breasts showed single lesions, 30 (25%) showed multifocal lesions, 45 (37.5%) showed multicentric lesions (Fig. 1), and 1 (0.83%) was diffuse infiltration. Their results were mismatched in 2 (1.7%) of the examined breasts. The mismatch was in the detection of multicentricity, where CESM detected multiple breast lesions, while contrast-enhanced CT chest detected just a single lesion in one case, and in another case, contrast-enhanced CT chest detected multifocal lesions, while CESM detected only a single mass (Fig. 2).
A 40-year-old female patient complained of a right breast lump. CESM of right breast CC and MLO views (a, b) and contrast-enhanced CT chest coronal and sagittal planes (c, d) showed right breast multiple spiculated heterogeneously enhancing masses (straight arrows) (occupying UOQ and LOQ) (i.e., multicentric masses), pathologically proven invasive ductal carcinoma, grade II with multiple pathologically enlarged right axillary lymph nodes (level I, II, III) best demonstrated on coronal images (curved arrow). The histopathology result was IDC grade II
A 46-year-old female patient with positive family history complained of a left breast lump. CESM of left breast CC and MLO views (a, b) showed left UOQ single heterogeneously enhancing mass lesion. Contrast-enhanced chest CT axial and coronal images (c, d) showed left breast UOQ intensely enhancing mass lesion (arrow) along with another anteriorly located small enhancing nodule (arrowhead) representing multifocal disease. The histopathology result was IDC with tubular differentiation, grade II
Regarding the breast lesions bilaterality
Both CESM and contrast MDCT chest detected unilateral carcinoma in 106 (88.3%), while bilateral malignant lesions were detected in 14 (11.7%) of the examined breasts (Fig. 3a, b). Contralateral benign lesions were detected in 8 cases (6.7%) in CESM, and only 3 (3.5%) of them were detected in MDCT.
a A 36-year-old female patient presented with a left palpable lump with diffuse skin thickening. CESM of both breasts CC and MLO views showed right breast (a, b) UOQ clumped non-mass enhancement and scattered ill-defined enhancing masses (arrowheads). Left breast (c, d) showed retroareolar spiculated intense heterogeneous enhancing mass infiltrating the nipple. Other similar yet smaller enhancing masses were seen scattered throughout the left breast quadrants (arrows) associated with diffuse skin thickening. b Contrast-enhanced CT chest axial (a, b), sagittal (c, d) and coronal (e, f) planes showed left breast multiple irregular-shaped enhancing masses (multicentric) (arrows); one of the masses is deeply seated infiltrating the underlying pectoral muscle (a). The largest mass is seen retroareolar invading the nipple (a, e). Multiple pathologically enlarged left axillary levels I, II, and III (curved arrows). Right breast UOQ irregular-shaped speculated mass lesion along with an anterior segmental area of clumped non-mass enhancement (a–e) (arrowheads). Histopathology was ILC grade II on the right side and invasive mammary carcinoma grade III on the left side
The performance of contrast-enhanced MDCT chest in the detection of breast multifocality and multicentricity
In established breast cancer patients, contrast-enhanced MDCT chest showed 100% sensitivity (95% CI 88.4–100%), 100% specificity (95% CI 96–100%), and 100% PPV (Table 3).
The performance of MDCT chest in the detection of bilateral malignant breast lesions
The sensitivity, specificity, PPV, and NPV of MDCT in the detection of bilaterally malignant breasts lesions were 85.7% (95% CI 57.2–98.2%), 98% (95% CI 93.4–99.8%), 85.7% (95% CI 59.9–96%), and 98% (95% CI 93.5–99.5%), respectively (Table 4).
Regarding other associated imaging findings
Among the associated imaging findings, there was a statistically significant association between contrast-enhanced MDCT and CESM (P ≤ 0.001) regarding the assessment of skin attachment and demonstration of intraductal extension of the tumor (Tables 5, 6).
Discussion
Incidental breast lesions (IBLs) could be occasionally detected in routine CT chest, although this technology was not optimized for breast imaging studies and is usually performed for other indications other than breast diseases. The IBLs detected on the CT chest could be cancer [6].
Based on a systemic review, about 49% of IBLs detected on CT were malignant lesions, ranging from 28.2 to 69.6%. In addition, the frequency of primary breast carcinoma varied from 9.2 to 60.9% [7].
This study evaluates the feasibility of replacing the CESM with a contrast-enhanced MDCT chest in the assessment of breast lesions multiplicity and bilaterally in diagnosed breast cancer patients in need of a CT chest as part of their metastatic workup.
Morphological analysis of IBLs revealed that non-circumscribed margin was the highest PPV for malignancy (74%) and irregular or lobulated shape was a 5% positive predictive value [8].
In the current study, the most encountered malignant masses in contrast-enhanced MDCT were irregular in shape, of non-circumscribed margins, and showed heterogeneous contrast uptake, while segmental, regional, and clumped non-mass enhancement were the most encountered malignant non-mass enhancement.
A good correlation between MDCT and pathology in the assessment of the tumor size with an appropriate determination of adequate surgical margins was demonstrated in prior studies [9, 10]. However, the capability of MDCT to identify the intraductal component of breast cancer was a concern by different studies. While some authors have stated that MDCT is effective in the demonstration of ductal carcinoma in situ (DCIS), especially the higher grades DCIS, other authors have shown that MDCT has a lower sensitivity than MRI [11,12,13,14].
In this study, there was a statistically significant association between contrast-enhanced MDCT and CESM (P ≤ 0.001) regarding the demonstration of intraductal extension (Fig. 4), but the limited number of studied tumors with such extension needs further investigations in the future regarding the capability of the MDCT in the detection of intraductal extension. In this study, there was also a statistically significant association between contrast-enhanced MDCT and CESM (P ≤ 0.001) regarding the assessment of skin attachment.
A 53-year-old female patient presented with a palpable axillary lump. CESM of left breast CC and MLO views (a, b) and contrast-enhanced CT chest axial, sagittal, and coronal planes (c–h) showed left UOQ multiple enhancing ill-defined masses of segmental distribution (arrows), the largest mass showed anterior clumped linear non-mass enhancement representing anterior intraductal extension reaching retro areolar (arrowheads) demonstrated well in both modalities. Pathological left axillary and supraclavicular lymph nodes were demonstrated in the coronal plane (curved arrow). Histopathology was invasive mammary carcinoma grade II
Kang et al. [14] reported that MDCT could detect additional multifocal or multicentric breast lesions that were occult by mammography or ultrasound. Their reported sensitivity, specificity, and accuracy were 93.3%, 98.3%, and 97.3%, respectively.
Volterrani et al. [15] evaluated 31 breast cancer patients by dual-energy CT, and they stated that this technology demonstrates a good correlation with pathologic analysis regarding the tumor size and cancer distribution (unifocal, multifocal, or multicentric); however, there was no comparison with contrast-enhanced dedicated breast examination in that study.
Volterrani et al. [15] were capable of identification of all invasive cancer by reconstructed monochromatic images at low energies (40 keV), whereas 2 out of 10 low-grade DCIS because of their poor enhancement pattern was not detected.
In the current study, all our encountered cases were invasive cancer where contrast-enhanced MDCT chest showed 100% sensitivity, specificity, and PPV regarding cancer distribution in comparison with CESM, and it showed 85.7% sensitivity, 98% specificity, 85.7% PPV, and 98% NPV in the detection of bilaterally malignant breasts lesions compared to CESM. In this study, 3 (3.5%) out of 8 (6.7%) of contralateral benign lesions were detected by MDCT chest, and this may be explained by their poor enhancement pattern that decreases the capability of CT in their detection.
Felipe et al. [16] stated that using dedicated breast protocol in MDCT examination is feasible and shows substantial agreement with MRI in newly diagnosed breast cancer patients staged II or III.
The limitation of the present study was the evaluation of tumor size and its correlation with postoperative histopathological results, and this can be explained because not all the studied cases were a candidate for surgical management, and therefore there was no comparison between the CESM and MDCT regarding the lesion size, so we recommend further dedicated studies.
Conclusions
In contrast, MDCT chest could potentially replace CESM in the detection of invasive breast cancer distribution (unifocal, multifocal, multicentric, or bilateral) in patients’ candidates for CT staging, allowing locoregional staging with a single examination, reducing costs and excess patients’ radiation exposure.
Availability of data and materials
All are available with the authors upon request.
Abbreviations
- BIRADS:
-
Breast Imaging Reposting and Data System
- CESM:
-
Contrast-enhanced spectral mammography
- CT:
-
Computed tomography
- IBLs:
-
Incidental breast lesions
- MDCT:
-
Multi-detector computed tomography
- NCCN:
-
National Comprehensive Cancer Network
References
Chetan A, Mack J, Chan T (2016) Breast cancer screening controversies: who, when, why, and how? Clin Imaging 40(2):279–282
Fallenberg EM, Dromain C, Diekmann F, Engelken F, Krohn M, Singh JM, Ingold-Heppner B, Winzer KJ, Bick U, Renz DM (2013) Contrast-enhanced spectral mammography versus MRI: initial results in detection of breast cancer and assessment of tumor size. Eur Radiol 24(1):256–264
Lin YP, Hsu HH, Ko KH, Chu CM, Chou YC, Chang WC, Chang TH (2016) Differentiation of malignant and benign incidental breast lesions detected by chest multidetector-row computed tomography: added value of quantitative enhancement analysis. PLoS ONE 11:e0154569
National Comprehensive Cancer Network (2010) Clinical practice guidelines in oncology for breast cancer V.1. National Comprehensive Cancer Network, Fort Washington
Morris EA, Comstock CE, Lee CH (2013) ACR BI-RADS: breast imaging reporting and data system, 5th edn. American College of Radiology, Reston
Chan YH (2003) Biostatistics 103: qualitative data-tests of independence. Singap Med 44(10):498–503
Lin WC, Hsu HH, Li CS, Yu JC, Hsu GC, Yu CP, Chang TH, Huang GS (2011) Incidentally detected enhancing breast lesions on chest computed tomography. Korean J Radiol 12(1):44–51
Schramm D, Jasaabuu C, Bach AG, Tennstedt O, Spielmann RP, Surov A (2016) Costs associated with the evaluation of incidental breast lesions identified on computed tomography. Br J Radiol 89(1059):20140847
Doihara H, Fujit T, Takabatake D, Takahashi H, Ogasawara Y, Shimizu N (2006) Clinical significance of multidetector-row computed tomography in breast surgery. Breast J 12:S204–S209
Inoue T, Tamaki Y, Hamada S, Yamamoto S, Sato Y, Tamura S, Kim SJ, Tanji Y, Miyoshi Y, Taguchi T, Noguchi S (2005) Usefulness of three-dimensional multidetector-row CT images for preoperative evaluation of tumor extension in primary breast cancer patients. Breast Cancer Res Treat 89:119–125
Kimijima I, Yoshida K, Tamura R, Moriya T (2013) Effectiveness of multi-detector row computed tomography in the detection of the presence and extent of ductal carcinoma in situ. Breast Cancer 20:26–33
Shimauchi A, Yamada T, Sato A, Takase K, Usami S, Ishida T, Moriya T, Takahashi S (2006) Comparison of MDCT and MRI for evaluating the intraductal component of breast cancer. AJR Am J Roentgenol 187:322–329
Nakahara H, Namba K, Wakamatsu H, Watanabe R, Furusawa H, Shirouzu M, Matsu T, Tanaka C, Akiyama F, Ifuku H, Nakahara M, Tamura S (2002) Extension of breast cancer: comparison of CT and MRI. Radiat Med 20:17–23
Kang DK, Kim MJ, Jung YS, Yim H (2008) Clinical application of multi-detector row computed tomography in patient with breast cancer. J Comput Assist Tomogr 32:583–598
Volterrani L, Gentili F, Fausto A, Pelini V, Megha T, Sardanelli F, Mazzei MA (2020) Dual-energy CT for locoregional staging of breast cancer: preliminary results. Am J Roentgenol 214:707–714
Felipe VC, Bitencourt AGV, Souza JA, de Azevedo MM, Guatelli CS, Tyng CJ, Verza L, Makdissi FB (2020) MRI features after radiofrequency ablation in breast cancer. Breast J 26:541–542
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
RWAR and SYAA-D wrote the manuscript. EFK, RWAR, and SYAA-D collected patients data. AHR image processing and collection of patient’s images. EFK participated in the design of the study. AAM performed surgical procedure. RWAR and EFK participated in its design and coordination and helped to draft the manuscript. AHR and AAM were responsible for revision of the draft. AAM was responsible for pathological data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was reviewed and approved by the Ethics Committee of Baheya center for Early detection and Treatment of Breast Cancer.
Consent for publication
A written consent for publication was obtained for these cases.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahman, R.W.A., Al-Dhurani, S.Y.A., Radwan, A.H. et al. Multi-detector CT chest: can it omit the further need for contrast enhnaced spectral mammography in breast cancer patients candidate for CT staging?. Egypt J Radiol Nucl Med 53, 152 (2022). https://doi.org/10.1186/s43055-022-00826-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00826-9