Abstract
Objective
To assess the utility of dual-energy contrast-enhanced spectral mammography (DE-CESM) for evaluation of suspicious malignant microcalcifications.
Methods
Two hundred and fifty-six DE-CESMs were reviewed from 2012–2013, 59 cases fulfilled the following criteria and were enrolled for analysis: (1) suspicious malignant microcalcifications (BI-RADS 4) on mammogram, (2) no related mass, (3) with pathological diagnoses. The microcalcification morphology and associated enhancement were reviewed to analyse the accuracy of the diagnosis and cancer size measurements versus the results of pathology.
Results
Of the 59 microcalcifications, 22 were diagnosed as cancers, 19 were atypical lesions and 18 were benign lesions. Twenty (76.9 %) cancers, three (11.55 %) atypia and three (11.55 %) benign lesions revealed enhancement. The true-positive rate of intermediate- and high-concern microcalcifications was significantly higher than that of low-concern lesions (93.75 % vs. 50 %). Overall, the diagnostic sensitivity of enhancement was 90.9 %, with 83.78 % specificity, 76.92 % positive predictive value, 93.94 % negative predictive value and 86.4 % accuracy. Performance was good (AUC = 0.87) according to a ROC curve and cancer size correlation with a mean difference of 0.05 cm on a Bland-Altman plot.
Conclusions
DE-CESM provides additional enhancement information for diagnosing breast microcalcifications and measuring cancer sizes with high correlation to surgicohistology.
Key Points
• DE-CESM provides additional enhancement information for diagnosing suspicious breast microcalcifications.
• The enhanced cancer size closely correlates to microscopy by Bland-Altman plot.
• DE-CESM could be considered for evaluation of suspicious malignant microcalcifications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mammography remains an important technique for breast imaging, although the detection sensitivity for breast cancer is influenced by the density of superimposing breast tissue. Screening by mammography is cost effective, beneficial and has high sensitivity in detecting microcalcifications. About 20–25 % of ‘ACR BI-RADS 4’ microcalcifications (suspicious abnormality where a biopsy is recommended) are subsequently shown to be malignancies [1–3].
Breast calcifications are common mammographic findings in both screening and clinical populations. However, only certain suspicious microcalcifications (<0.5 mm) are of great concern. They may be associated with benign processes, atypia lesions or cancerous lesions. The associations of various microcalcifications with cancers according to their morphology and/or distribution have been published [4]. Using the digital mammography environment, the positive predictive valve was reported as 13 % for category 4a, 36 % for category 4b and 79 % for category 4c [5]. For managing such microcalcifications with no-mass lesions, minimizing the over- and undertreatment of biopsies is a frequent debate topic. Another issue is the measurement of tumour size due to the lack of apparent margins in cases of solitary malignant microcalcifications. Pre-operative assessment of the size or extension is thus problematic.
Rapid-processing digital imaging techniques have resulted in the recent development of tomosynthesis and dual-energy contrast-enhanced spectral mammography (DE-CESM). Both are mammographic-based techniques that provide additional images for further evaluation. Tomosynthesis solves the problem of assessing a breast lesion with superimposed breast tissues [6]. It is helpful in evaluating a suspicious soft tissue lesion but its utility for microcalcification clusters is controversial [7, 8]. Moreover, its value for microcalcification differentiation is limited. Only DE-CESM provides alternative information regarding enhancement secondary to cancer angiogenesis, which assists cancer detection and highlights tumour boundaries. The sensitivity and performance have been reported to be superior to that of conventional mammography by several interobserver blinded studies [9–12]. On the other hand, DE-CESM alone can demonstrate associated enhancement by comparing the low energy conventional mammogram (Mx) with a contrast-enhanced subtracted mammogram (CESM) during a single session and in the same position.
To our knowledge, the feasibility of DE-CESM for assessing microcalcifications had not been reported previously. In this study, we reviewed our DE-CESM cases retrospectively with regard to microcalcifications with no-mass lesions by palpation or breast ultrasound to analyse the diagnostic profiles and the feasibility of cancer size measurements using CESM enhancement. The results will facilitate the clinical management and surgical evaluation of microcalcifications without mass of concern.
Methods
Patients
With the approval of our Institutional Review Board, we reviewed retrospectively all DE-CESM examinations performed from February 2012 to December 2013. The DE-CESM cases for analysis were selected based on the following criteria: (1) patients with ACR ‘BI-RADS 4’ microcalcifications on mammograms, (2) no related mass lesion after clinical assessment with a physical examination and breast sonography, and (3) microcalcifications with pathological diagnoses, either by stereotactic mammographic-guided biopsies with a vacuum-assisted core needle or surgery. All diagnoses and post-operative cancer sizes were re-evaluated by a breast pathologist.
DE-CESM was not a routine examination for impalpable microcalcification. Indeed, all examinations were optionally chosen by the patients. Before making the decision, the advantages of the enhancement technique were explained, including its clinical value, the procedure involved, the additional radiation dose and the potential for an allergic reaction to the contrast medium. The clinical value was that the associated enhancement might assist in determining whether the microcalcification was benign or malignant, the enhancement might assist in measuring the size of the cancer or involvement, and the enhancement might be helpful in selecting a biopsy target in cases with multicentric microcalcifications. All patients were told that the result of the DE-CESM would not change the clinical follow-up course of non-cancerous lesions or the prognosis of cancerous lesions. Following exclusion of those with contraindications of renal function impairment (serum creatinine and glomerular filtration rate), pregnancy, lactation, history of an allergic reaction to contrast medium and certain systemic diseases, such as hyperthyroidism, each patient signed a written consent form agreeing to the ‘extra’ clinical examination and contrast medium injection.
DE-CESM technique
We used a commercial DE-CESM model (GE Healthcare, Senographe Essential CESM, Buc, France) providing intermittent exposure of low and high energy within 1- to 2-s intervals during a single breast-compressed position. The automatic exposure used molybdenum or rhodium with filters depending on the breast thickness to achieve the acquisition of the X-ray spectrum below and above the k-edge of iodine (33.2 keV) for successful image subtraction recombination. After eliminating noise due to non-enhanced anatomical structures, the different attenuations in the low- and high-energy images were assessed by means of a masking effect generated from a subtracted image that indicated the presence of iodine uptake. This technique allows assessment of correlations of subtle microcalcifications with enhancement at approximately the same time and position between images.
The DE-CESM procedure was standardized. Consecutive mammogram acquisitions were performed sequentially with craniocaudal (CC) and mediolateral oblique (MLO) views of the bilateral breasts within 2–5 min after the start of a single-bolus injection of non-ionic contrast medium (Omnipaque 350 mg I/mL; GE Healthcare, Dublin, Ireland) via an intravenous catheter that was inserted in the forearm prior to the examination at a rate of 3 mL/s for a total dose of 1.5 mL/kg body weight. A nurse and the mammographer were present to identify any extravasation or allergic reaction to the contrast medium. The patients were requested to hold their breath during mammography to avoid artefacts due to motion. Low- and high-energy acquisitions were immediately computerized and a subtracted mammogram was created using the low-energy mammogram as a mask. Conventional low-energy Mx and CESM were obtained in each single-study view. Eight mammography images from bilateral breasts were obtained per examination.
Data and statistical analyses
The microcalcification descriptors and associated enhancements were recorded from the clinical radiological reports. The microcalcifications of concern were subdivided into high (corresponding to BI-RADS 4c) (casting, linear, or branching), intermediate (BI-RADS 4b) (pleomorphous) or low (BI-RADS 4a) (amorphous or coarse) according to their morphologies, in accordance with the seventh edition of the BI-RADS Microcalcification Lexicon. Otherwise, the morphologies of associated enhancement were classified as a mass (>6 mm; with pushing border) or a non-mass, including a patchy focus (small patch or nodule ≤ 6 mm), regional (>6 mm, patch without pushing border), segmental (triangular converging shape), clump (cluster of foci, with or without ground-glass enhanced background) or none. We also correlated the greatest diameter of the enhanced cancer and surgiopathological findings by a Bland-Altman plot. In order to assess the improvement of size estimation, another breast radiologist was invited to measure the extent of microcalcifications independently on the mammograms.
To assess the diagnostic profile of associated enhancement, its presence over the site of a microcalcification indicated a suspicious malignancy and its absence a suspicious noncancerous lesion. With reference to the histological diagnosis, the rates of true positive (TP), true negative (TN), false positive (FP) and false negative (FN) for malignancy were determined. The sensitivity (number of TP/total number of malignant lesions), specificity (number of TN/total number of benign lesions), positive predictive value (PPV; number of TP/total number with associate enhancement), negative predictive value (NPV; number of TN/total number without associate enhancement) and accuracy (number of TP plus TN/total number of lesions) were determined. Moreover, we measured the area under the receiver operating characteristic (ROC) curve to determine the performance of DE-CESM.
Results
We reviewed 256 DE-CESM examinations from our data bank. Of them, 59 sites of microcalcification in 52 female patients (range, 30–69 years old, average 48.9 years) who fulfilled the inclusion criteria were analysed. Microcalcifications were referred from mammographic screening in 35 females, breast cancer patients with indeterminate microcalcifications on contralateral breasts in ten, and mammographic follow-up of post-operative breast cancer patients in seven. Seven patients received two biopsies, with sites of more than 2 cm apart.
All diagnoses and cancer sizes were confirmed microscopically. Of the 59 microcalcifications, 22 (37.7 %) were diagnosed as cancers (15 ductal carcinoma in situ (DCIS) and seven invasive ductal cancer (IDC)), 19 (32.2 %) as atypia lesions (six atypical ductal hyperplasia (ADH) and 13 flat epithelial atypia (FEA)) and 18 (30.5 %) as benign breast pathologies (six adenomas, four proliferative disease, three nonproliferative disease, three benign calcifications and two ductal hyperplasia).
Overall, 26 microcalcifications revealed enhancements, consisting of 20 breast cancers (13 DCIS, 7 IDC), three ADH and three benign lesions (two adenomas, one proliferative disease). Moreover, 33 microcalcifications did not enhance, including 2 DCIS, 16 atypical lesions (3 ADH, 13 FEA) and 15 benign. The false-positive and false-negative rates of enhancement were 23 % and 6 %, respectively. The sensitivity was 90.9 % (95 % CI = 70.80–98.62), specificity 83.78 % (95 % CI = 67.98–93.77), positive predictive value 76.92 % (95 % CI = 56.35–90.97), negative predictive value 93.94 % (95 % CI = 79.74–99.08), and accuracy 86.4 % (95 % CI = 77.04–95.84 %). The performance as determined by ROC was good, with an area under the curve of 0.87 (95 % CI = 0.774–0.972; Fig. 1). All IDC revealed enhancement, as did 13 of 15 (86.7 %) DCIS, three of six (50 %) ADH, 0 of 13 (0 %) FEA, and three of 18 (13 %) benign lesions (Table 1).
In total, 37 microcalcifications (amorphous microcalcifications) were classified as low concern and 22 as intermediate (20 pleomorphous microcalcifications) or high concern (two linear microcalcifications). Ten of the thirty-seven amorphous microcalcifications had associated enhancement; five were diagnosed as cancerous and five as non-cancerous. Two of the non-enhanced amorphous microcalcifications (Fig. 2) were ultimately shown to be DCIS (low and high grade in one each). The true-positive and true-negative rates were 50 % and 92.59 %, respectively. Of the 22 intermediate- and high-concern microcalcifications, 16 (15 cancers, one non-cancerous lesion) showed enhancement. The true-positive and true-negative rates were 93.75 % and 100 %, respectively. The enhancements in microcalcifications at the various levels of concern are summarized in Table 2.
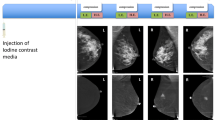
A 56-year-old female with a surgical history of breast cancer was referred for stereotactic biopsy due to the presence of newly developed suspicious malignant microcalcifications on the follow-up mammogram. The low energy conventional mammogram (CC view) (a) showed regional amorphous microcalcification in the upper outer quadrant of the left breast and the contrast-enhanced spectral mammography (b) revealed no associate enhancement. Stereotactic biopsy diagnosed it to be ductal carcinoma in situ (DCIS) and a subsequent partial mastectomy further documented it to low-grade DCIS
Among the 20 enhanced breast cancers, the appearances were regional in six, masses in five (Fig. 3), segmental in four (Fig. 4), a clump in three (Fig. 5) and foci in two. The other six non-cancerous lesions enhanced to foci in three, regional in two and a clump in one. Of the 22 breast cancers, 18 underwent mastectomies (12 partial mastectomies, five simple mastectomies and one modified radical mastectomy) in our hospital. Four of the cancers did not receive surgery in our hospital due to personal reasons. Of the two cancers without enhancement, only one undertook the surgery in our hospital. With counting the one nonenhanced operated breast cancer to 0 cm, the sizes of operated cancers were averaged to 1.77 cm on CESM and 1.72 cm by microscopy (ranges, 0–8 cm on CESM and 0.1–7.7 cm on microscopy in greatest diameter). On the other hand, the average size of microcalcification extension from mammographic interpretations was 2.14 cm (range 0.6–5.5 cm), which was significantly over-estimated from microscopic size as compared to CESM. The Bland-Altman plot showed a close correlation of CESM to microscopic size with the arithmetic mean; the difference was 0.05 cm (95 % CI = −0.078 to 0.189), with a standard deviation of 0.26. The cancer size on CESM statistically correlated with the microscopy (Fig. 6). On the other hand, the Bland-Altman plot of mammographic size to microscopic size showed a difference of 0.42 cm (95 % CI = −0.612 to 1.456), with a standard deviation of 2.07 (Fig. 7). The agreement of histological cancer size appeared to be better for CESM than for mammograms.
A 49-year-old female with suspicious malignant microcalcifications from mammographic screening was referred for further evaluation. The low energy conventional mammogram (mediolateral oblique view) (a) showed regional pleomorphous microcalcification in the right breast and the contrast-enhanced spectral mammography (b) revealed an 8-cm large lobular enhanced mass in the upper region of the right breast; however, sonography (c) did not find an associated solid mass, only a well-defined breast cyst. Finally, modified radical mastectomy subsequently proved it to be invasive ductal carcinoma
A 45-year-old female with left breast cancer underwent mammography for pre-operative evaluation. The low energy conventional mammogram (mediolateral oblique view) (a) showed two clusters of amorphous and pleomorphous microcalcifications in the lower region of right breast and the contrast-enhanced spectral mammography (b) displayed a 4-cm segmental enhancement. Partial mastectomy was finally decided on and showed it to be invasive ductal carcinoma
A 45-year-old female with discovery of suspicious microcalcifications from screening mammography was referred for stereotactic biopsy. The conventional mammogram (mediolateral oblique view) (a) showed a cluster of pleomorphous microcalcifications in the left breast and the contrast-enhanced spectral mammography (b) demonstrated a 9-mm ground glass enhanced lesion with multiple small faintly enhancing dots. Partial mastectomy finally showed it to be ductal carcinoma in situ
Discussion
DE-CESM is a recently developed breast imaging technique that provides an additional contrast-enhanced mammogram with a low energy ‘conventional’ mammography during the same examination session. The masked enhancement secondary to extravascular iodine accumulation may indicate the presence of histological changes. Technical and clinical experiences of the CESM in addition to conventional mammography have been published elsewhere [9–17]. Such a technique facilitates detection and size measurement of cancer by mammographic morphology and angiogenic enhancement.
Many blinded interobserver studies have reported that DE-CESM could improve the diagnosis with increased sensitivity, specificity, positive-predictive value, negative-predictive value and accuracy [9–12]. A study of readers with CESM experience showed a significant improvement in sensitivity compared to conventional mammography alone. Using BI-RADS scoring, the sensitivity and performance improved by 15 % (78–93 %) and 17 % (74–91 %), respectively [12]. Compared with mammography plus sonography, CESM also improved the sensitivity significantly, from 71–78 %, and clinical performance, from 83–87 % [12].
In the clinical setting, sonography and mammography are often used in breast examinations. The cancer detection depends basically on observable morphologies. For special situations, enhancement techniques with intravenous injection of contrast medium are needed occasionally, such as ‘enhanced magnetic resonance imaging (MRI)’ for detecting occult breast cancers, pre-operative planning or cancer screening in patients at high risk. The enhanced breast MRI with dedicated breast coils is currently the most accurate imaging modality for cancer detection, with a sensitivity of 79–98 % [18, 19].
Similar to enhanced breast MRI, the improvement in cancer detection is also based on angiogenesis in malignant tumours. The temporal subtraction of CESM is an approach similar to enhanced MRI [13–15]. Recently, the technique of dual energy exposure within a short time interval captured two different energy mammograms, minimizing the problem of temporal resolution and restoring the spatial resolution of microcalcifications. One study reported that the detection rate of known cancers by DE-CESM was comparable to that by enhanced MRI, with significantly improved specificity and fewer false positives of additional cancers [17].
For our analysed subpopulation, conventional mammography is an excellent method for detection of microcalcifications. Unfortunately, the range of cancer probabilities in ACR ‘BI-RADS 4’ microcalcifications is wide (2–95 %) and all are recommended for biopsy [20]. According to the morphological descriptors, the cancer probabilities were 7 % for coarse heterogeneous, 11 % for punctate, 20–26 % for amorphous, 25–41 % for fine pleomorphic and >80 % for linear/branching/casting lesions [4]. Enhanced MRI was investigated to determine whether the enhancement technique could or could not help in differentiation of the suspicious microcalcifications. The results with enhanced MRI were fair, 87 % sensitivity, 68 % specificity, 84 % PPV, 71 % NPV and 80 % accuracy [21]. Compared with our study, the specificity and NPV of CESM were better than those of enhanced MRI. The lowered oversensitivity might probably be due to the different contrast media used in these two enhancement procedures. The enhancement in CESM depends on the limited elevation of attenuation at the k-edge of iodine; however, the paramagnetic effect on enhanced MRI is assumed to be unlimited. Another concern is the difficult correlation of microcalcifications from enhanced MRI to conventional mammography, which might also influence the results. DE-CESM has the advantage of easier and more obvious interpretation of microcalcifications on conventional mammogram simultaneously to the enhancement on CESM in a single examination session and in the same position.
Our results suggest that the additional information from CESM regarding suspicious microcalcifications has a certain clinical value. The associated enhancement occurred significantly more often in malignant lesions (20/22, 90.1 %) than atypia lesions (3/19, 15.8 %) or benign lesion (3/18, 13 %). However, the presence of associated enhancement was not definitively indicative of a malignancy due to the enhancement of 10.1 % (6/59) from benign microcalcifications. One more issue is the higher predictive value of associated enhancement in intermediate- or high-concern microcalcifications than low-concern microcalcifications; however, the negative predictive value was approximate with true negative rate greater than 90 %.
In addition to the diagnosis, an imaging study should also assist further treatment. A surgical approach is almost always the first-line treatment upon detection of a malignant microcalcification. The size or extent of the cancer is important for surgical planning. In such cases of impalpable microcalcifications, the territory of cancerous involvement is often unclear. In this situation, the enhancement technique could highlight the outline of cancers secondary to neovascular formation. Many studies of enhanced MRI have reported good correlations between cancer sizes to pathology [22–25]. Enhanced MRI has been shown to be the most accurate technique for cancer size measurement, and superior to mammography and sonography [23]. Compared with enhanced MRI, DE-CESM also performs well, as it shows no significant difference in cancer size measurements and exhibits correlations with post-operative histology [25]. Among the non-mass malignant microcalcifications in our study, the cancer size was also closely correlated with the value determined by microscopy.
The preliminary results of this retrospective review were generally positive, although many limitations remain, including the relatively small number of cases, and not all patients with cancers or atypical lesions underwent surgery following biopsy of the microcalcifications. The small number of cases essentially with a gold standard of histological diagnoses might have selective bias due to the non-operated microcalcifications. However, all non-operated patients were followed for more than 6 months, during which any under-diagnosis would be detected and the long-term result would be followed up in the future. Additionally, although the enrolled patients had different referral reasons from screening, breast cancer patients for pre-operative survey or follow-up, all belonged to the subpopulation of patients with microcalcifications without associated mass.
In conclusion, DE-CESM provides additional information on the enhancement associated with breast microcalcifications without associated mass. It is not perfect for diagnosis, but has acceptable sensitivity and NPV, each over 90 %. The sizes of enhanced cancers were closely related to those determined by microscopy.
Abbreviations
- 95 % CI:
-
95 % confidence interval
- ACR:
-
America College of Radiology
- ADH:
-
Atypical ductal hyperplasia
- BI-RADS:
-
Breast Imaging Reporting and Data System
- CC:
-
Craniocaudal
- CESM:
-
Contrast-enhanced Subtracted Mammography
- DCIS:
-
Ductal carcinoma in situ
- DE-CESM:
-
Dual-energy Contrast-enhanced Spectral Mammography
- FEA:
-
Flat epithelial atypia
- FN:
-
False negative
- FP:
-
False positive
- IDC:
-
Invasive ductal carcinoma
- MLO:
-
Mediolateral oblique
- MRI:
-
Magnetic resonance imaging
- Mx:
-
Mammography
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- TN:
-
True negative
- TP:
-
True positive
References
Kettritz U, Rotter K, Schreer I et al (2004) Stereotactic vacuum-assisted breast biopsy in 2874 patients. Cancer 100:245–251
Liberman EA, Abramson AF, Squires FB, Glassman JR, Morris EA, Dershaw DD (1998) The breast imaging reporting and data system: positive predictive value of mammographic features and final assessment categories. AJR Am J Roentgenol 171:35–40
Orel SG, Kay N, Reynolds C, Sullivan DC (1999) BI-RADS categorization as a predictor of malignancy. Radiology 211:845–850
Burnside ES, Ochsner JE, Fowler KJ et al (2007) Use of microcalcification descriptors in BI-RADS 4th edition to stratify risk of malignancy. Radiology 242:388–395
Bent CK, Bassett LW, D’Orsi CJ, Sayre JW (2010) The positive predictive value of BI-RADS microcalcification descriptors and final assessment categories. AJR Am J Roentgenol 194:1378–1383
Kopans DB (2014) Digital breast tomosynthesis from concept to clinical care. AJR Am J Roentgenol 202:299–308
Michell MJ, Iqbal A, Wasan RK et al (2012) Comparison of accuracy of film-screen mammography, fill-field digital mammography, and digital breast tomosynthesis. Clin Radiol 67:976–981
Wallis MG, Moa E, Zanca F, Leifland K, Danielsson M (2012) Two-view and single-view tomosynthesis versus full-field digital mammography: high-resolution X-ray imaging observer study. Radiology 262:788–796
Dromain C, Thibault F, Diekmann F et al (2012) Dual-energy contrast-enhanced digital mammography: initial clinical results of a multireader, multicase study. Breast Cancer Res 14:R94
Diekmann F, Freyer M, Diekmann S et al (2011) Evaluation of contrast-enhanced digital mammography. Eur J Radiol 78:112–121
Cheung YC, Lin YC, Wan YL et al (2014) Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: interobserver blind-reading analysis. Eur Radiol 24:2394–2403
Dromain C, Thibault F, Muller S et al (2011) Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol 21:565–574
Jong RA, Yaffe MJ, Skarpathiotakis M et al (2003) Contrast-enhanced digital mammography: initial clinical experience. Radiology 228:842–850
Diekmann F, Diekmann S, Jeunehomme F et al (2005) Digital mammography using iodine-based contrast media: initial clinical experience with dynamic contrast medium enhancement. Investig Radiol 40:397–404
Dromain C, Balleyguier C, Muller S et al (2006) Evaluation of tumor angiogenesis of breast carcinoma using contrast enhanced digital mammography. AJR Am J Roentgenol 187:W528–W537
Lewin JM, Isaacs PK, Vance V et al (2003) Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology 229:261–268
Jochelson MS, Dershaw DD, Sung JS et al (2013) Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast cancer. Radiology 266:743–751
Morris EA, Liberman L, Ballon DJ et al (2003) MRI of occult breast carcinoma in a high- risk population. AJR Am J Roentgenol 181:619–626
Berg WA (2003) Rationale for a trial of screening breast ultrasound: American College of Radiology Imaging Network (ACRIN) 6666. AJR Am J Roentgenol 180:1225–1228
Obenauer S, Hermann KP, Grabbe E (2005) Applications and literature review of the BI-RADS classification. Eur Radiol 15:1027–1036
Bazzocchi M, Zuiani C, Panizza P et al (2006) Contrast-enhanced breast MRI in patients with suspicious microcalcifications on mammography: results of a multicenter trial. AJR Am J Roentgenol 186:1723–1732
McGhan LJ, Wasif N, Gray RJ et al (2010) Use of preoperative magnetic resonance imaging for invasive lobular cancer: good, better, but maybe not the best? Ann Surg Oncol 17:255–262
Wasif N, Garreau J, Terando A, Kirsch D, Mund DF, Giuliano AE (2009) MRI versus ultrasonography and mammography for preoperative assessment of breast cancer. Am Surg 75:970–975
Mann RM, Hoogeveen YL, Blickman JG, Boetes C (2008) MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat 107:1–14
Fallenberg EM, Dromain C, Diekmann F et al (2014) Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 24:256–264
Acknowledgments
The scientific guarantor of this publication is Dr Yun-Chung Cheung. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. A statistician did the analysis, but was not an author. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Study subjects or cohorts have not been previously reported. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheung, YC., Tsai, HP., Lo, YF. et al. Clinical utility of dual-energy contrast-enhanced spectral mammography for breast microcalcifications without associated mass: a preliminary analysis. Eur Radiol 26, 1082–1089 (2016). https://doi.org/10.1007/s00330-015-3904-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3904-z