Abstract
Purpose
In neurosurgery, ultrasound is useful in determination of the tumor location, differentiation between solid tumors and cystic components, as well as definition of the shortest and safest access to the mass. This study aims to evaluate the role of the intraoperative ultrasound in resection of pediatric brain tumors.
Methods
Intraoperative ultrasonography (conventional B-Mode) was performed in 25 pediatric patients with brain tumors pre-, during, and post-resection, in whom eight patients were supratentorial and 17 were infratentorial. Post-op Grayscale images of the brain tumors on conventional ultrasound were compared with the results of immediate postoperative magnetic resonance imaging.
Results
The border of the tumor and post-resection residual tumor were more distinguishable from healthy brain on ultrasound during the operation. Improved definition of the tumor tissue from normal brain with ultrasound was demonstrated in all cases aiding in tumor resection.
Conclusion
Intraoperative ultrasound is suggested to be a useful imaging technique in defining the border between the tumor and healthy brain tissue pre-resection, in detecting residual tumor tissues after the resection of the mass, and in guiding to the shortest and safest access to the tumor during neurosurgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brain tumors are the most common form of solid tumors in children. According to the data from the American Cancer Society, brain tumors account for 20.7% of pediatric malignancies [1]. Pediatric brain tumors are the leading cause of death from solid tumors in children [2]. Pediatric neuro-oncology has evolved into a multidisciplinary collaborative specialty that combines the expertise from radiation oncologists, neuroradiologists, neurologists, neuropathologists, and pediatric oncology. Each of these specialties provides important components of comprehensive care. Current management strategies can optimize outcomes by integrating the standard of care with novel and investigative therapies. The role of neurosurgery in pediatric brain tumors is to reduce the tumor burden, minimize symptoms, and preserve functional abilities. Recent improvements in the surgical management of brain tumors have reduced morbidity and increased the possibility for aggressive tumor resection [3]. For operable brain tumors, surgical resection remains the treatment of choice. However, maximal resection sometimes remains challenging due to lack of definite border between some malignant tumors and normal brain tissue. Therefore, defining the border of the tumor is a major concern in neurosurgery [4]. Modern imaging tools and recent advances in imaging techniques now serve as the basis of early diagnosis of brain tumors. This technology has moved into the operating room. Magnetic resonance imaging (MRI) continues to be the most sensitive and preferred diagnostic study for identifying these lesions. All imaging sequences from preoperative MRIs should be carefully reviewed. Anatomical location of a tumor, tumor size, and edema are often delineated on T1, T2, and FLAIR sequences [3]. Ultrasound offers real-time imaging; however, showing a tumor’s definite margins is controversial. Echogenic tissues such as brain edema or gliosis can interfere with the differentiation of normal from abnormal tissues [5]. One study has suggested that intraoperative sonography overestimates tumor volume, especially when used within the context of recurrent tumors [6]. In contrast, another study has suggested that sonography helps differentiate brain edema, brain tumor, and normal brain [7]. This study evaluates the role of intraoperative ultrasound resection of pediatric brain tumors: defining the shortest access to the mass as well as delineation of the solid and cystic components.
Patients and methods
Retrospective study of intraoperative ultrasound on 25 inpatients (male/female = 13:12, age range 2–17 years) with brain tumor enrolled between January 1, 2008, and July 30, 2009. All of patients (Table 1) had comprehensively physical and neurological examinations, including MRI before the operation.
Ultrasound equipment
Intraoperative ultrasound was performed with GE LOGIQ BOOK XP device (General Electric, USA) A 6.5-MHz intraoperative probe was applied. The transducer and cord were placed in a transparent plastic surgical sterile sheath secured at the scan head by a sterile rubber band. For proper transmission of the acoustic beam between the sheathed scan head and the dura mater, irrigation of the operative field—as needed—with sterile saline was done.
Intraoperative scanning
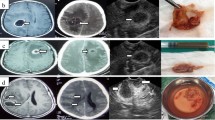
After a craniotomy was performed, the radiologist was called to the operating room to help in interpreting the data. First, the sheathed scan head was placed on the surface of the dura mater for scanning before opening the dura where we identify the site and size of the lesion before any brain shift, which could result from CSF drainage after dural opening; the distance from the tumor to the dura mater and the location and size of the tumor were measured and compared with preoperative available MRI images. Dural opening then follows, and the scan head is placed directly to the surface of the brain tissue for further scanning, during which continuous irrigation with saline is performed. Localization of the tumor, its consistency, and borders with normal brain were studied, followed by a colored mode scanning to identify any close vascular structures, then returning back to B-mode scan to look for any relation of the tumor to nearby anatomical landmarks, like: the ventricles, the tent, and the cerebral aqueduct.
After collecting all these data, we start tumor excision from the nearest safe part. As the tumor debulking proceeds, repeated scanning is performed to evaluate the tumor residue and its new relation to surrounding structures. After assuming total excision, another scan is performed after filling the tumor cavity with sterile saline to get an idea about the size of the created cavity and compare it to the original tumor size, and any tumor residue is looked for and excised.
Results
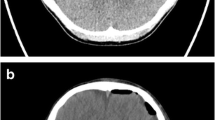
Many studies have demonstrated the reliability of intraoperative ultrasound since the 1980s explained by the high resolution and the real-time investigations [8]. In our series, we found reliable tumor localization in all 25 cases without any complications confirming the data of the literature. Tumor localization by intraoperative ultrasound reaches 100% independent of the histopathological diagnoses. Intraoperative conventional ultrasound was useful in the determination of tumor location, its most superficial portion, and in the differentiation between solid tumors and cystic components. The border of the tumor and remained tumor tissue were more distinguishable from healthy brain on ultrasound during the operation which helps in identifying residual tumors after assuming total resection under microscope. Intraoperative ultrasonography (IOUS) was very useful during resection of posterior fossa tumors extending to the lateral parts of the fourth ventricle or upwards to the aqueduct. Improving differentiation of the tumor tissue from normal brain with ultrasound was demonstrated in almost all cases. IOUS was very helpful in posterior fossa, intraventricular, and cortical tumors to ensure total and safe resection, while in craniopharyngiomas, it was helpful in the localization and excision of the cystic component in one case; in the second case, residual solid tumor was identified and excised. Comparing the results of immediate post-op MRI to those from the IOUS (Figs. 1 and 2), we were not surprised by any missed tumor residue that could have been resected.
Discussion
Intraoperative US has been used in neurosurgery for more than 20 years, and it still has many attributes that make it attractive for neurosurgical guidance [9]. Ultrasound is very useful in determination of the tumor location, its most superficial portion, and in differentiation between solid tumors and cystic components [10]. Ultrasound examination of the brain and spinal cord is hindered by the absence of an adequate acoustic window. Other important limitations of ultrasound are the necessity of a “tradeoff” between frequency range, spatial resolution, and tissue penetration of acoustic waves, not to mention that ultrasound is an operator-dependent technique. After removal of skeletal acoustic barriers, intraoperative ultrasound examination of the CNS has been suggested in the identification and characterization of brain and spinal cord lesions [11]. The precise localization provided by ultrasound imaging shortens the time of surgery and increases the safety for the patient [12].
Like intraoperative magnetic resonance imaging, intraoperative US can be used to detect any intraoperative or post-craniotomy brain shifts. IOUS can simply track a biopsy needle in real time and define cysts, ventricles, cisterns, and other fluid-filled spaces. The borders of the tumors are generally simple to define, and, although the borders of low-grade gliomas may be hard to identify, they are also frequently difficult to define by the magnetic resonance imaging. In addition, US during neurosurgery have some definite advantages over MR imaging: above all, the imaging investigations are in real time. By practice, it becomes very easy for neurosurgeons to scan the brain and look for lesions [13].
The most critical drawback in ultrasound detection of tumor borders is peritumoral edema, which appears echogenic relative to the surrounding brain [14, 15]. Surgical trauma may lead to minor hemorrhages along the cavity borders, thus making the defining of edema even more complicated. In this case, we depend on the microscopic differentiation between the brain and tumor tissue, together with comparing the size of the created cavity after resection to that before resection. In case of solid tumors, the cavity created after resection was filled with saline to compare the size of the saline-filled cavity to the pre-resection tumor size which can give us a clue about the extent of resection, and if the normal brain borders were violated during tumor resection, this was very useful especially in benign or well-circumscribed tumors or in tumors inside a ventricle as lateral and 4th ventricular tumors, but in case of malignant gliomas, it is usually difficult to differentiate between hyperechogenicity due to surrounding edema or tumor infiltration or residue which is also the case in MRI.
Furthermore, the use of Surgicel in the tumor cavity may interfere with interpretation of the IOUS. Surgicel is a gauze-like surgical hemostat composed of oxidized regenerated cellulose [16]. The air trapped between the interwoven fibers of cellulose leads to ring-down artifact, with deleterious effects on the sonographic image. This was avoided by performing the sonography before inserting the surgicel.
In all 17 cases of infratentorial tumors and three cases of supratentorial tumors, the IOUS was a very useful tool to guide us for total tumor excision and identify any tumor residue intraoperative which was confirmed by the MRI performed immediately postoperative within the following 48 h, showing that IOUS in such cases can substitute the intraoperative MRI, thus decreasing the length of the operation and making it more simple with less cost effect. In the other five cases of supratentorial gliomas, it was not easy to differentiate between hyperechogenicity due to tumor residue or that from brain edema and surgical trauma; when comparing this to the immediate postoperative MRI, it was similar difficulty that we face indicating that intraoperative MRI is not far more superior than IOUS in such cases.
IOUS was introduced in our operating room since January 2008, and we were using it since that time in certain random cases without selection to identify its value and get an idea about which cases could benefit from using IOUS; since then, it is routinely used in all our cases, as it always adds to our preoperative knowledge about the tumor without any further cost or time.
Conclusion
In contrast to intraoperative MRI, intraoperative IOUS is less time-consuming, simpler, and less expensive. It allows monitoring the progress of neurosurgical procedure and even of the brain tumor resection during surgery. Thus, intraoperative intracranial ultrasound is suggested to be a useful imaging technique in defining the border between the tumor and healthy brain tissue pre-resection and in detecting residual tumor tissues after the resection of the mass. In comparison to intraoperative MR imaging which is not affordable for most hospitals considering the primary costs including non-ferromagnetic tools and the time-consuming investigations, ultrasound presents as a practical and cost affordable alternative in most of pediatric brain tumors.
References
American Cancer Society (2009) American Cancer Society Cancer Facts & Figures. American Cancer Society, Atlanta
Orkin SH, Fisher DE, Thomas Look A, Lux S, Ginsburg D, Nathan DG (2009) Oncology of infancy and childhood. Elsevier, Amsterdam. ISBN 978-1-4160-3431-5
Baltuch GH, Bushnell C, Caplan LR, Stacy MA, Tuszynski MH (2007) Brain tumors: practical guide to diagnosis and treatment. Informa Healthcare, New York. ISBN 10-0-8493-3616-3 (Hardcover)
He W, Jiang XQ, Wang S, Zhang MZ, Zhao JZ, Liu HZ, Ma J, Xiang DY, Wang LS (2008) Intraoperative contrast-enhanced ultrasound for brain tumors. Clin Imaging 32:419–424
Shinoura N, Takahashi M, Yamada R (2006) Delineation of brain tumor margins using intraoperative sononavigation: implications for tumor resection. J Clin Ultrasound 34:177–183
LeRoux PD, Berger MS, Ojemann GA et al (1989) Correlation of intraoperative ultrasound tumor volumes and margins with preoperative computerized tomography scans: an intraoperative method to enhance tumor resection. J Neurosurg 71:691
LeRoux PD, Winter TC, Berger MS et al (1994) Comparison between preoperative magnetic resonance and intraoperative ultrasound tumor volumes and margins. J Clin Ultrasound 22:29–36
Regelsberger J, Lohmann F, Helmke K, Westphal M (2000) Ultrasound-guided surgery of deep seated brain lesions. Eur J Ultrasound 12:115–121
Rubin JM, Mirfakhraee M, Duda EE, Dohrmann GJ, Brown F (1980) Intraoperative ultrasound examination of the brain. Radiology 137:831–832
Di Rocco C (2009) Cerebellar astrocytomas. Children’s Cancer Hospital Egypt, Cairo. Accessed at www.57357.com, 1st CCHE International Pediatric Oncology Conference
Cellerini M, Innocenti P, Milazzotto M, Bartolucci M, Guizzardi G, Mennonna P (2001) Intra-operative ultrasound of brain and spinal cord lesions. Radiography 7:55–60
Chandler WF, Rubin JM (1987) The application of ultrasound during brain surgery. World J Surg 11(5):558–569
Rubin JM, Quint DJ (2000) Intraoperative US versus intraoperative MR imaging for guidance during intracranial neurosurgery. Radiology 215:3
Johnson P, Hart S, Drayer B (1989) Human cerebral gliomas: correlation of postmortem MR imaging and neuropathologic findings. Radiology 170:211–217
Rubin JM, Chandler WF (1998) Intraoperative sonography of the brain. In: Rumack CM, Wilson SR, Charboneau JW (eds) Diagnostic Ultrasound. Mosby, St. Louis, pp 631–652
Melamed JW, Paulson EK, Kliewer MA (1995) Sonographic appearance of oxidized cellulose (Surgicel): pitfall in the diagnosis of postoperative abscess. J Ultrasound Med 14:27–30
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Beltagy, M.A., Aggag, M. & Kamal, M. Role of intraoperative ultrasound in resection of pediatric brain tumors. Childs Nerv Syst 26, 1189–1193 (2010). https://doi.org/10.1007/s00381-010-1091-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-010-1091-4