Abstract
Purpose
This systematic review and meta-analysis aims to assess the impact of different doses of Ca, P, and vitamin D on bone mineralization in premature infants.
Methods
A systematic search (1990–2022) of MEDLINE (Ovid), Cochrane Library, Scopus, EMBASE, and CINAHL (EBSCO) was conducted. Randomized control trials and cohort studies, involving premature infants with birthweight ≤2.5 kg, who received supplementation and underwent bone mineral content reassessment, were included. Impact on growth indicators was also evaluated, but not systematically. Following the critical evaluation process, using PEDro scale and JBI critical appraisal checklist, high-quality studies were reviewed. Random effect meta-analyses (standardized mean difference) were performed to assess the effect of increased doses of Ca, P, and Vitamin D on bone health indicators.
Results
Eighteen studies were included, fifteen with enteral and three with parenteral nutrition regimen. The included studies’ mineral intake ranges presented high heterogeneity. The beneficial effect of higher doses of Ca and P on bone mineralization was evident in most studies, and unanimous when accompanied with higher doses of vitamin D, indicating the synergist effect of the three elements. Higher enteral nutrition doses of (a) Ca and P or (b) Ca, P, and vitamin D resulted in increased bone mineralization (standardized mean difference: 0.39; 95% CI 0.09, 0.69, and 1.72; 0.81, 2.16), respectively, while higher supplementation of only vitamin D presented no such effect (−0.01; −0.59, 0.56). Higher parenteral nutrition doses of Ca and P proved beneficial for bone mineralization (0.88; 0.34, 1.43). Higher enteral doses of all elements indicated no additional effect on growth.
Conclusions
Elevated intake of Ca (daily doses: Ca 95–135 mg/100 kcal) and P (55–95 mg/100 kcal) throughout enteral nutrition together with sufficient vitamin D intake might prove beneficial towards enhancing bone mineralization in preterm infants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prematurity can increase the risk of poor bone health and developing metabolic bone disease (MBD), by missing the last months of rapid mineral accretion [1]. World Health Organization (WHO) defines prematurity as gestational age less than completed 37 weeks [2], and classifies birthweight as low birthweight (LBW) <2500 g, very-low birthweight (VLBW) <1500 g, and extremely-low birthweight (ELBW) <1000 g [2]. MBD, a bone strength disorder, is characterized by abnormalities of phosphorus (P), calcium (Ca), and vitamin D homeostasis and reduced bone mass and structure [1], and is expected to present in 16–40% of VLBW and ELBW infants [3, 4]. Even if MBD development is avoided, the effect of low birth weight and potentially poor bone health at birth due to prematurity can impact the quality of life and bone health for several years after birth [5, 6].
Suboptimal Ca and P intake can contribute to insufficient bone mineral density (BMD), a precursor to prematurity-attributed MBD [7], while insufficient Vitamin D intake can further diminish bone density and bone mineral content (BMC) of Ca and P. Therefore, adequate intake of these elements is suggested for primary prevention of MBD, administered through intravenous route, feeding tube or orally (breastmilk, fortified human milk, and formula) [8]. Magnesium also plays a fundamental role in the bone matrix structure, but during prematurity, its role is substantially less important [9].
The recommended supplementation of Ca, P, and vitamin D in premature infants vary among health organizations. In particular, the European Society of Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) suggests an enteral nutrition (EN) intake of Ca: 120–200 mg/kg/day and P: 70–115 mg/kg/day [10]. In contrast, the American Academy of Pediatrics (AAP) recommends an intake of Ca: 180–220 mg/kg/day and P: 100–130 mg/kg/day, mainly through fortified breast milk and preterm formula [11]. Vitamin D recommendations also vary; ESPGHAN has recently changed its recommendation from 800–1600 IU/day to 400–700 IU/kg/day with a maximum dose of 1000 IU/day, while AAP recommends 400 IU/day for LBW infants and 200–400IU/day for VLBW infants [10, 11]. As for the Parenteral Nutrition (PN), the range of these nutrients recommended doses (ESPGHAN) are smaller for calcium and higher for vitamin D [12, 13].
A recent systematic review [14] examined the various formulas’ effect on growth and bone mineralization in prematurity but not the exact doses of each mineral. To cover the literature gap, we aimed to systematically define the most beneficial doses of Ca, P, and vitamin D in premature infants with non-optional birthweight, comparing different doses (EN and PN). Secondary objectives were to assess the effectiveness of those elements on weight, length, and head circumference.
Methods
Registration
The review was conducted in accordance with the PRISMA statement [15]. The review protocol was registered with the International prospective register of systematic reviews (PROSPERO); registration number: CRD42022321481 (4/14/2022).
Inclusion criteria
Participants: Infants born prematurely (<37 weeks), with at least LBW (<2.5 kg) [16]. Despite MBD being more likely to develop in VLBW infants, we included infants of LBW as well, to assess the outcome of bone health in high-risk premature infants. Studies with a sample of only MBD infants or other diseases affecting the elemental/vitamin metabolism, like kidney disease, were excluded.
Intervention: Studies that use supplementary/higher doses of Ca, P, and/or vitamin D administered through EN (preterm infant formulas or expressed breast milk) or PN.
Comparator: Control group with no supplementation or lower doses of Ca, P, and/ or Vitamin D.
Main outcomes: Bone mineralization evaluated by dual-energy X-ray absorptiometry (DXA), speed of sound (SOS), or bone transmission time (BTT), an inexpensive accurate measure of organic matrix and BMC [8]. Serum biochemical biomarkers (ALP, Ca, P) were excluded for not being specific MBD predictors and accurate measures of BMC in prematurity [8]. Measurements taking place until 24 months of life were included because bone mineralization could still be increased during this time frame [17].
Secondary outcomes: Weight gain, length accrual (measured by electronic baby scale and infant measuring, respectively), and head circumference (by flexible non-stretch tape). These outcomes were not inserted into the search strategy and were not systematically assessed.
Type of studies: Randomized control trials (RCTs) and prospective or retrospective cohort studies. Conference abstracts and expert opinions were excluded.
Search strategy
Scientific databases searched included MEDLINE (Ovid), Cochrane Library, Scopus, EMBASE, and CINAHL (EBSCO). The search terms used are presented in Fig. 1.
Publication dates were limited between January 1990 and April 2022, without language restriction. Other sources searched include ClinicalTrials.gov, theses, conference abstracts, OpenGrey, Google Scholar, and WorldWideScience.org. A full search strategy example for Ovid MEDLINE database is shown in Supplementary Table 1.
As the institutional full-access to these databases was restricted following April 2022, an update bibliographic search in COCHRANE Library and PUBMED, an interface used to search MEDLINE(Ovid), was performed (1/April/2023–1/March/2023), amounting to 314 manuscripts, of which none satisfied study selection criteria. These searches are not included in the PRISMA flowchart, but are included In Supplementary Table 2.
Study selection
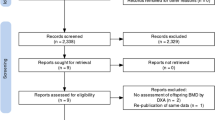
Two independent reviewers conducted the study selection to minimize the study inclusion bias. Articles retrieved were uploaded to Rayyan software for screening, and duplicates removal [18]. Following a pilot screening test, if an article was judged as relevant by the title and abstract, full-text was retrieved, unless it was retracted, unavailable, or a conference abstract. Reference lists of included studies were hand-searched. A PRISMA flow diagram provides visual mapping of the search strategy (Fig. 2) [15, 19].
Assessment of methodological quality
Methodological quality was assessed through PEDro Scale for RCTs and JBI critical appraisal checklist for cohort studies by two reviewers [20,21,22]. PEDro scores were categorized as “poor” (0-3) “fair” (4-5), “good” (6-8), and “excellent” (9-10), a classification advised but not yet validated [21]. If an RCT scored ≥7, or a cohort scored ≥8 (answer “yes”), it was included and imported into data extraction [22].
Data extraction
Both reviewers used a standardized JBI data extraction form. To ensure consistency and validity, the following details were extracted: research details, participants’ characteristics, intervention details (doses, type, and duration of administration and follow-up), outcomes assessed (bone health indicators, weight, length, and head circumference), and key findings.
Ca, P, and vitamin D content was translated to an average mg or IU per 100 kcal, whenever studies provided data on mean/exact volume intake. If not, the average mg or IU content per 100 kcal was calculated. Cross-calculations in human breast milk utilized the average content of kcal, Ca, P, and vitamin D content, as indicated by Kim and Yi [23]. Whenever median and IQR were presented, if there was some evidence of normal distribution, median was translated to mean (approximately equal) and IQR to SD, following the equation SD = IQR/1.35 [24].
Data synthesis and meta-analysis
Data synthesis followed a narrative presentation. Random effect meta-analysis was performed, categorizing the included studies into three categories: (A) higher Ca and P doses (not vitamin D); (B) higher Ca, P, and vitamin D doses; and (C) only higher vitamin D doses among groups. As there was inconsistency in the measured outcome (i.e., BMC measured in g and BMD in g/cm or g/cm2, or g/cm3) and in the method used to evaluate the same effect (i.e., BMC, BMD, SOS), a random effect standardized mean difference model was created for the included studies [24]. Additional models are presented in the supplementary material. The RevMan 5.4 software was used to perform the meta-analyses.
Results
Identified studies
The literature search yielded 1775 results (Fig. 2). After duplicate removal, screening, and hand search of citations and references, 20 full-text publications of 133 full-text screened were deemed eligible for inclusion, after methodological evaluation (Fig. 2). Faerk et al. [25] and Van de Lagemaat et al. [9] have published pertinent or duplicate results in later publications, which were excluded (the duplicates), resulting in 18 included studies (16 RCTs, one retrospective cohort, one prospective cohort study). Characteristics and key findings of included studies are presented in Table 1.
Assessment of bias
The methodological quality of the RCTs was considered either excellent (n = 9), or good (n = 8). Both cohort studies scored 8 “Yes,” and were classified as excellent or good (Table 2).
Participant characteristics
The total number of participants was 991 (263 boys, 249 girls, and 479 unidentified sex). Mean gestational age ranged from less than 28 to 34 weeks. Birthweight of premature infants varied. The vast majority of the included sample included premature infants of less than 1500 g (VLBW), as most studies included only VLBW (9/18), mostly VLBW (7/18) or mostly LBW (2/18).
Doses of minerals and vitamin
Included studies, presented at least one comparator group, with higher administration dose of minerals (HGs), compared to the control groups or LGs. Ca and P administration (varying doses) was examined in 13 studies, with the HG dose in EN regimens including an average of 73–204 mg/100 kcal Ca and 49–105 mg/100 kcal P, and the LGs including 23–99 mg/100 kcal Ca and 20–63 mg/100 kcal P. In two PN regimens, the HG dose included an average range of 73–94 mg/100 kcal Ca and 43–87 mg/100 kcal P, and the LGs including 44–69 mg/100 kcal Ca and 25.8–62 mg/100 kcal P. In two studies either milk, bank or formula average volume was not reported [25] or only the supplemented parenteral doses were reported [26].
Vitamin D administration varied in 4/12 studies, with the daily enteral supplementation in the HG ranging from 800 to 1000 IU and in the LG from 200 to 500 IU. In 7/12 studies, the intervention group received enriched formula containing approximately 215 IU/100 mL, while the control group received a standard formula containing approximately 130 IU/100 mL. Koo et al. [27] compared the effectiveness of a nutrient-enriched formula containing 80IU/kcal with a term formula containing 60 IU/kcal.
Type of administration
Only 3 studies followed PN regimens. Eleven studies administered the minerals and vitamins via enteral formulas, 2 via mother’s milk fortifier and 2 via preterm formula or breast milk fortifier.
Follow-up duration
The key outcome of bone health indicator was assessed at 1 month of life (n = 4 studies), 2 months (n = 3), 3-4 months following the start of the intervention (n = 3), following 6 months (n = 4), following 9 months (n = 1), or at 12 months (n = 3).
Main outcomes on bone mineral content
Studies combining elevated Ca, P, and vitamin D and BMC
All 7 studies on EN in which Ca, P, and vitamin D intake was higher in HG, presented significant differences in BMC. Average BMC was higher in HG after various durations of follow-up: between discharge and expected term (HG: 53.6 ± 21.7 g, LG: 38.9 ± 17.7 g, p = 0.037) [28] at hospital discharge (age adjusted: HG: 42 ± 4 mg/cm, LG: 31 ± 5 mg/cm, p = 0.002) [29] 4 months after inclusion (HG: 104.4 ± 29.2 g, LG: 87.5 ± 17.1 g) (p = 0.01) [30], 6 months after term age (p < 0.05) [9], and at 3 and 9 months after discharge (9 months mean difference: 26.8 mg/cm, 95%CI: 13.2, 40.4) [31]. In one study, BMC was reported to differ between HG and LG at 8 weeks after discharge (p < 0.001), but not at 16 weeks [32]. Only one study found significantly higher BMC in LG at 12 months of age [27].
Studies combining elevated Ca and P (non-varying vitamin D) and BMC
Among the 4 studies, 2 EN and 2 PN regimens, in which only Ca and P were higher in HG, and not vitamin D, the effect on BMC was less consistent. Only in two studies, a positive effect was evident in both boys and girls on PN (HG: 2.7 ± 0.6, LG: 2.0 ± 0.50 mg/cm/week, p = 0.025) [33] or just in boys on EN regimens (significant BMC gain: BMM, HG: 202 ± 35g, LG: 186 ± 23g, p < 0.01) [34]. In contrast, in one EN study, BMC remained similar among the two groups, 2 months after the study initiation (p > 0.05) [35]. A tendency of difference in BMC was visible among infants receiving own mothers milk and those receiving PF (p = 0.05), but after some adjustments the difference was not significant (p = 0.68) [25].
Vitamin D and BMC
Out of the three EN studies, in which only vitamin D intake differed (not Ca or P), only one study resulted in a beneficial effect in BMC. This study [36] presented a higher proportion of infants in the HG (HG: 800 IU/day, LG: 400 IU/day) having BMC measurements <10 percentile at 8 weeks (p = 0.04). The remaining two studies found no difference (p > 0.05) at 3 [37] and 6 months [38].
Main outcomes on BMD and SOS
BMD was increased in infants receiving higher doses of Ca, and/or P and/or vitamin D after 4 weeks of follow up (HG: 0.13 ± 0.01 mg/cm2, LG: 0.10 ± 0.02 mg/cm2, p < 0.001) (22), 6 months after term age (HG: 0.221 ± 0.028 g/cm2, LG: 0.198 ± 0.019 g/cm2) and after 10 weeks of life (HG: 0.198 ± 0.018, LG: 0.144 ± 0.013 g/cm2, p < 0.001) [39].
Regarding SOS measurements, two studies found contradicting results regarding EN and PN, as SOS measurements were higher in HG at 6 weeks in one study following a PN regimen (HG: 2 959 ± 123 m/s, LG: 2 853 ± 139m/s, p = 0.011) [40], but not at 6 weeks in another study following a EN regimen (HG: 3 032 ± 60 m/s, LG: 2 978 ± 83 m/s, p > 0.05) [41].
Analysis of administered dosed in light of bone mineralization improvement
Ca administration across EN studies effective at improving bone health indicators ranged between 97 and 136 mg/100 kcal. When doses of 192 against 82 mg/100 kcal were examined [32], BMC did not differ at 16 weeks. P administration in HGs across effective EN interventions displayed high variance: 49–94 mg/100 kcal. Among them, some compared doses in the higher end of this range (in HGs) with those at the lower end (in LGs), with range 57–94 mg/ 100 kcal, which were effective at improving bone health indicators [9, 28,29,30]. Studies on PN or vitamin D administration were fewer, limiting the discussion of the administered doses.
Meta-analyses
The meta-analysis model assessing the beneficial effect of (A) higher Ca and P doses (not vitamin D); (B) higher Ca, P, and vitamin D doses; and (C) higher vitamin D doses among groups can be found in Fig. 3. Four studies did not present or the exact average BMC or BMD, with the corresponding variance could not be calculated and thus were not included in the models. Higher doses of Ca and P resulted in increased bone mineralization for both EN and PN studies, with the effect being more evident for PN studies (standardized mean difference: 0.88; 0.34, 1.43 with I2 = 0%), than EN (0.39; 0.09, 0.69 with I2 = 0%). After combining the EN and PN results, a clear benefit of higher Ca and P doses was evident for bone mineralization (0.51; 95% CI 0.24, 0.77 with I2 = 0%). A similar effect on EN was higher in premature infants receiving higher doses of all three elements (1.72; 0.81, 2.16), although high heterogeneity was noted (I2 = 90%, p < 0.0001). Higher intake of vitamin D through EN (but not Ca or P) did not result in increased bone mineralization (−0.01; −0.59, 0.56 with I2 = 53%). Higher doses of Ca and P, regardless of vitamin D doses, predicted an increased bone mineralization (EN: 1.18; 0.67, 1.69 with I2 = 85%) (Supplementary Figure 1).
Forest plots of comparison: the outcome of higher compared to lower doses of Ca, P, and/or vitamin D in bone mineralization. Footnote for all figures: All included studies evaluated BMC, apart from Cooke 1999 and Alpay 1998 (BMD), and Pereira-da-Silva 2011 and Litmanovitz 2007 (Bone SOS). As outcome measures differed, but all was indicative of bone mineralization, the standardized mean difference random effect models were used. BMC, bone mineral content; BMD, bone mineral density; SOS, speed-of-sound; 95% CI, 95% confidence interval
Growth indicators
Growth indicators were measured only in EN studies. Regarding weight, only in 4/13 studies, did higher doses of Ca, P, and/or vitamin D present a positive effect on weight gain [25, 28, 30, 34], while another study provided evidence of an opposite effect [27] (Table 1). In 8/13 studies, weight values remained similar among feeding groups [9, 29, 32, 35,36,37,38, 41]. Length gain did not differ in 8/11 studies [9, 25, 28, 32, 35, 37, 38, 41], while two indicated significantly higher gain in HGs [30, 36] and one in LGs [27]. Head circumference presented similar values across 7/9 studies [25, 27, 28, 35,36,37, 41]., with only two suggesting a favorable effect in HGs [30, 38].
Discussion
Summary of key results
In a sample of mostly VLBW premature infants, we found that the overall effect of higher doses of Ca and P on bone mineralization in premature infants is likely positive, while higher dose of vitamin D on its own is unlikely to be beneficial in increasing BMC. The effect of higher doses of all three components was almost unanimous in augmenting BMC. The current meta-analysis has confirmed the aforementioned, and suggest that higher doses of Ca and P administered either through EN or PN can ameliorate the diminished bone mineralization in premature infants. The beneficial effect of higher EN doses of Ca, P, and vitamin D on weight, length, and head circumference of premature infants is improbable. Our findings suggest that premature infants receiving EN or PN may benefit from increased doses of Ca and P. According to the studies included in our review, to ensure an adequate bone mineralization, a beneficial enteral dose may be in the range of Ca: 95–135 mg/100 kcal and P: 55–95 mg/100 kcal, accompanied by sufficient doses of vitamin D
Ca and P in bone health
AAP recommends 140–160 mg/100 kcal Ca and 95–108 mg/100 kcal P [11], administered through fortified human milk or preterm formula, while ESPGHAN recommends EN doses of 70–140 mg/100 kcal Ca and 50–82 mg/100 kcal P [10, 42]. Hence, ESPGHAN recommendations are more in line our findings. However, we could not assess the effectiveness of AAP recommendations with certainty as only one study included ranges of doses supported by AAP. For PN solutions, as supported by our meta-analysis, the ideal doses of these minerals might be lower, since minerals are directly available for bone mineralization and tissue accretion and thus present better absorption [43].
Vitamin D in bone health
Apart from infants with extremely low BMC at baseline, vitamin D alone could not improve bone mineralization, even when accounting for various ranges of administration. Clinical organization presents disparities regarding the recommended doses, as the European guidance support higher doses (up to 1000 IU/day) [10] to ensure high Ca absorption [44], while AAP support lower doses (400 IU/day) [11] to prevent conditions such as hypercalcemia, hypercalciuria, and nephrocalcinosis [45].
Growth indicators
Elevated doses of Ca, P, and/or vitamin D might not influence growth indicators improvement. Significant findings across studies may be attributed to disparities in energy and protein intake or the limited capability of preterm infants to regulate their energy intake [28, 34]. Nevertheless, a systematic review has noted that 800–1000 IU/day of vitamin D may benefit length gain and head circumference [46]. As this study did not systematically search growth indicators, we believe that this review’s findings [46] present higher validity.
Limitations and strengths
High heterogeneity regarding the doses of minerals and vitamins, administration type, intervention and follow-up duration, age, birthweight, and outcomes reported was present. The administered nutrients forms were stated only in limited studies, with gluconate Ca, glycerophospate, and hydroxyl-vitamin D being the most common. Therefore, we could not identify the precise doses and forms of the examined nutrients. The total amount of administered nutrients, and in particular of vitamin D, was lower than the recommended ranges in most studies, limiting the credibility of our findings, regarding the effect of higher doses on bone mineralization. Studies following a PN regimen, although highly effective, were limited (3), and therefore, no mineral range was provided. Additionally, the meta-analysis combining the results of both EN and PN regarding Ca and P administered doses has significant limitations related to the core differences of EN and PN, in terms of administration routes and absorption rate. However, by simultaneously accounting for the effect of elevated does of Ca and P in both EN and PN (Figure 3)A3, we were also able to provide clear indications about the well-known beneficial effect of these nutrients on bone mineralization, regardless of the feeding regimen. Biochemical markers of metabolic bone disease, which can provide crucial early signs of MBD, such as serum biochemical biomarkers (ALP, Ca, P), were not assessed as primary outcomes in our study since they are not specific MBD predictors or exact measures of BMC in prematurity [8]. Analyzing the effect on bone mineralization based on these indicators as outcome measures may prove challenging due to the difficulty in incorporating these results into meta-analysis models and as they are often not considered the best option for diagnostic purposes but primarily for predicting poor bone health in the hospital setting or primary prevention. We chose to only include measurements with less variation attributed to human or device errors and focused mainly on measurements obtained from gold standard methods. Finally, growth indicators as outcomes were not systematically reviewed.
Conclusion
The identified studies suggested a possible beneficial effect of supplementary doses of Ca (EN: 95–135 mg/100 kcal) and P (EN: 55–95 mg/100 kcal) on bone mineralization among preterm infants. Vitamin D can enhance the beneficial effect of these minerals. Disparities in the recommended doses of minerals and vitamins can introduce challenges across neonatal units. This review identifies potential areas for further research, including the use of specific formulas for administration of Ca, P, and vitamin D; the monitoring of formula intake from parents or caregivers; and the establishment of the optimal duration of supplementation.
Abbreviations
- AAP:
-
American Academy of Pediatrics
- BTT:
-
Bone transmission time
- Ca:
-
Calcium
- DXA:
-
Dual-energy X-ray absorptiometry
- ELBW:
-
Extremely low birth weight
- EN:
-
Enteral nutrition
- ESPGHAN:
-
European Society of Paediatric Gastroenterology, Hepatology and Nutrition
- HG:
-
High-dose group
- LBW:
-
Low birth weight
- LG:
-
Low-dose group
- MBD:
-
Metabolic bone disease
- P:
-
Phosphorus
- PN:
-
Parenteral nutrition
- RCTs:
-
Randomized control trials
- SOS:
-
Speed of sound
- VLBW:
-
Very low birth weight
- WHO:
-
World Health Organization
References
Rustico SE, Calabria AC, Garber SJ. Metabolic bone disease of prematurity. J Clin Transl Endocrinol. 2014;1:85–91. https://doi.org/10.1016/j.jcte.2014.06.004.
World Health Organization. ICD-10: international statistical classification of diseases and related health problems: tenth revision. World Health Organization; 2004.
Backström MC, Kuusela A-L, Mäki R. Metabolic bone disease of prematurity. Ann Med. 1996;28:275–82. https://doi.org/10.3109/07853899608999080.
Takada M, Shimada M, Hosono S, et al. Trace elements and mineral requirements for very low birth weight infants in rickets of prematurity. Early Hum Dev. 1992;29:333–8. https://doi.org/10.1016/0378-3782(92)90188-m.
Tavares NHC, Coelho CG, Barreto SM, et al. Birth weight is related with bone mineral content in adulthood: results of ELSA-Brasil. Rev Saude Publica. 2022;56:103. https://doi.org/10.11606/s1518-8787.2022056004064.
Callréus M, McGuigan F, Åkesson K. Birth weight is more important for peak bone mineral content than for bone density: the PEAK-25 study of 1,061 young adult women. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2013;24:1347–55. https://doi.org/10.1007/s00198-012-2077-8.
Mihatsch W, Thome U, Saenz de Pipaon M. Update on calcium and phosphorus requirements of preterm infants and recommendations for enteral mineral intake. Nutrients. 2021;13:1470. https://doi.org/10.3390/nu13051470.
Faienza MF, D’Amato E, Natale MP, et al. Metabolic bone disease of prematurity: diagnosis and management. Front Pediatr. 2019;7:143. https://doi.org/10.3389/fped.2019.00143.
van de Lagemaat M, Rotteveel J, van Weissenbruch MM, Lafeber HN. Increased gain in bone mineral content of preterm infants fed an isocaloric, protein-, and mineral-enriched postdischarge formula. Eur J Nutr. 2013;52:1781–5. https://doi.org/10.1007/s00394-012-0481-7.
Embleton ND, Jennifer Moltu S, Lapillonne A, et al. Enteral nutrition in preterm infants (2022): a position paper from the ESPGHAN Committee on Nutrition and Invited Experts. J Pediatr Gastroenterol Nutr. 2023;76:248. https://doi.org/10.1097/MPG.0000000000003642.
Abrams SA, Committee on Nutrition (2013) Calcium and vitamin D requirements of enterally fed preterm infants. Pediatrics 131:e1676-e1683. https://doi.org/10.1542/peds.2013-0420
Mihatsch W, Fewtrell M, Goulet O, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: calcium, phosphorus and magnesium. Clin Nutr Edinb Scotl. 2018;37:2360–5. https://doi.org/10.1016/j.clnu.2018.06.950.
Bronsky J, Campoy C, Braegger C, ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition (2018) ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: vitamins. Clin Nutr Edinb Scotl 37:2366–2378. https://doi.org/10.1016/j.clnu.2018.06.951
Young L, Embleton ND, McGuire W. Nutrient-enriched formula versus standard formula for preterm infants following hospital discharge. Cochrane Database Syst Rev. 2016;12:CD004696. https://doi.org/10.1002/14651858.CD004696.pub5.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Cutland CL, Lackritz EM, Mallett-Moore T, et al. Low birth weight: case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35:6492–500. https://doi.org/10.1016/j.vaccine.2017.01.049.
Fewtrell MS, Prentice A, Jones SC, et al. Bone mineralization and turnover in preterm infants at 8–12 years of age: the effect of early diet. J Bone Miner Res Off J Am Soc Bone Miner Res. 1999;14:810–20. https://doi.org/10.1359/jbmr.1999.14.5.810.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4.
Page MJ, McKenzie J, Bossuyt P, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. 2020; https://doi.org/10.31222/osf.io/jb4dx.
Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–21. https://doi.org/10.1093/ptj/83.8.713.
Cashin AG, McAuley JH. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J Physiother. 2020;66:59. https://doi.org/10.1016/j.jphys.2019.08.005.
Tufanaru C, Munn Z, Aromataris E, et al. Chapter 3: Systematic reviews of effectiveness - JBI Manual for Evidence Synthesis - JBI Global Wiki. JBI: JBI Manual for Evidence Synthesis; 2020.
Kim SY, Yi DY. Components of human breast milk: from macronutrient to microbiome and microRNA. Clin Exp Pediatr. 2020;63:301–9. https://doi.org/10.3345/cep.2020.00059.
Higgins J, Thomas J, Chandler J, et al (2022) Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). In: Cochrane. https://training.cochrane.org/handbook. Accessed 25 Mar 2023
Faerk J, Petersen S, Peitersen B, Michaelsen KF. Diet and bone mineral content at term in premature infants. Pediatr Res. 2000;47:148–56. https://doi.org/10.1203/00006450-200001000-00025.
Mazouri A, Khosravi N, Bordbar A, et al. Does adding intravenous phosphorus to parenteral nutrition has any effects on calcium and phosphorus metabolism and bone mineral content in preterm neonates? Acta Med Iran. 2017;55:395–8.
Koo WWK, Hockman EM. Posthospital discharge feeding for preterm infants: effects of standard compared with enriched milk formula on growth, bone mass, and body composition. Am J Clin Nutr. 2006;84:1357–64. https://doi.org/10.1093/ajcn/84.6.1357.
Lapillonne A, Salle BL, Glorieux FH, Claris O. Bone mineralization and growth are enhanced in preterm infants fed an isocaloric, nutrient-enriched preterm formula through term. Am J Clin Nutr. 2004;80:1595–603. https://doi.org/10.1093/ajcn/80.6.1595.
Schanler RJ, Abrams SA. Postnatal attainment of intrauterine macromineral accretion rates in low birth weight infants fed fortified human milk. J Pediatr. 1995;126:441–7. https://doi.org/10.1016/s0022-3476(95)70465-5.
Picaud J-C, Decullier E, Plan O, et al. Growth and bone mineralization in preterm infants fed preterm formula or standard term formula after discharge. J Pediatr. 2008;153(616–621):621.e1–2. https://doi.org/10.1016/j.jpeds.2008.05.042.
Bishop NJ, King FJ, Lucas A. Increased bone mineral content of preterm infants fed with a nutrient enriched formula after discharge from hospital. Arch Dis Child. 1993;68:573–8. https://doi.org/10.1136/adc.68.5_spec_no.573.
Chan GM. Growth and bone mineral status of discharged very low birth weight infants fed different formulas or human milk. J Pediatr. 1993;123:439–43. https://doi.org/10.1016/s0022-3476(05)81754-8.
Prestridge LL, Schanler RJ, Shulman RJ, et al. Effect of parenteral calcium and phosphorus therapy on mineral retention and bone mineral content in very low birth weight infants. J Pediatr. 1993;122:761–8. https://doi.org/10.1016/s0022-3476(06)80023-5.
Cooke RJ, McCormick K, Griffin IJ, et al. Feeding preterm infants after hospital discharge: effect of diet on body composition. Pediatr Res. 1999;46:461–4. https://doi.org/10.1203/00006450-199910000-00017.
De Curtis M, Pieltain C, Rigo J. Body composition in preterm infants fed standard term or enriched formula after hospital discharge. Eur J Nutr. 2002;41:177–82. https://doi.org/10.1007/s00394-002-0374-2.
Anderson-Berry A, Thoene M, Wagner J, et al. Randomized trial of two doses of vitamin D3 in preterm infants <32 weeks: dose impact on achieving desired serum 25(OH)D3 in a NICU population. PloS One. 2017;12:e0185950. https://doi.org/10.1371/journal.pone.0185950.
Natarajan CK, Sankar MJ, Agarwal R, et al. Trial of daily vitamin D supplementation in preterm infants. Pediatrics. 2014;133:e628–34. https://doi.org/10.1542/peds.2012-3395.
Backström MC, Mäki R, Kuusela AL, et al. Randomised controlled trial of vitamin D supplementation on bone density and biochemical indices in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1999;80:F161–6. https://doi.org/10.1136/fn.80.3.f161.
Alpay F, Unay B, Narin Y, et al. Measurement of bone mineral density by dual energy X-ray absorptiometry in preterm infants fed human milk or formula. Eur J Pediatr. 1998;157:505–7. https://doi.org/10.1007/s004310050864.
Pereira-da-Silva L, Costa A, Pereira L, et al. Early high calcium and phosphorus intake by parenteral nutrition prevents short-term bone strength decline in preterm infants. J Pediatr Gastroenterol Nutr. 2011;52:203–9. https://doi.org/10.1097/MPG.0b013e3181f8b295.
Litmanovitz I, Eliakim A, Arnon S, et al. Enriched post-discharge formula versus term formula for bone strength in very low birth weight infants: a longitudinal pilot study. J Perinat Med. 2007;35:431–5. https://doi.org/10.1515/JPM.2007.095.
ESPGHAN Committee on Nutrition, Aggett PJ, Agostoni C, et al. Feeding preterm infants after hospital discharge: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2006;42:596–603. https://doi.org/10.1097/01.mpg.0000221915.73264.c7.
Shaw JCL. Evidence for defective skeletal mineralization in low-birthweight infants: the absorption of calcium and fat. Pediatrics. 1976;57:16–25. https://doi.org/10.1542/peds.57.1.16.
Golden NH, Abrams SA, Committee on Nutrition (2014) Optimizing bone health in children and adolescents. Pediatrics 134:e1229-e1243. https://doi.org/10.1542/peds.2014-2173
Adnan M, Wu S-Y, Khilfeh M, Davis V. Vitamin D status in very low birth weight infants and response to vitamin D intake during their NICU stays: a prospective cohort study. J Perinatol Off J Calif Perinat Assoc. 2022;42:209–16. https://doi.org/10.1038/s41372-021-01238-9.
Yang Y, Li Z, Yan G, et al. Effect of different doses of vitamin D supplementation on preterm infants - an updated meta-analysis. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2018;31:3065–74. https://doi.org/10.1080/14767058.2017.1363731.
Acknowledgements
We thank Professor Mary Hickson for her constructive suggestions and support of preparing this research project.
Author information
Authors and Affiliations
Contributions
A Vervesou: conceptualization, methodology, data collection, data synthesis, meta-analysis, investigation, resources data curation, writing original draft, project administration. DV Diamantis: methodology, data collection, data synthesis, meta-analysis, investigation, data curation, writing review, and editing. K Maslin, JH Carroll: conceptualization, methodology, writing review and editing, supervision.
Corresponding author
Ethics declarations
Ethics approval
An ethics statement is not applicable because this study is based exclusively on published literature.
Consent to participate
Not appliable
Consent for publication
Not appliable
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vervesou, A., Diamantis, D.V., Maslin, K. et al. Different doses of phosphorus, calcium, and vitamin D in premature infants and their effect on bone mineralization: systematic review and meta-analysis. Nutrire 48, 48 (2023). https://doi.org/10.1186/s41110-023-00235-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-023-00235-6