Abstract
Purpose
Important underlying mechanisms of nitroglycerine tolerance development include oxidative stress and endothelial dysfunction, and there is paucity of information on how to reduce the tolerance during long-term administration. Taurine, a sulfonic amino acid, was reported to possess antioxidant, cardio-regulatory, neuro-modulatory, and membrane-stabilizing effect. The present study was designed to investigate the potential ability of taurine to prevent nitroglycerine-induced tolerance.
Methods
The effect of taurine on nitroglycerin-induced tolerance was investigated in endothelium intact and endothelium-denuded aortic ring preparations. In the in vivo study, male Wistar rats were pre-treated with taurine for ten days and co-treated with nitroglycerin (GTN) 50 mg/kg for 3 days. Then, the aortic ring was harvested and tested in vitro for GTN tolerance. The serum was used to test for oxidative stress parameter (malondialdehyde, reduced glutathione (GSH), catalase (CAT)).
Results
Taurine (20 mM) co-incubated with GTN significantly augmented vasodilatory response to nitroglycerin (GTN) in endothelial-denuded aortic rings. Pre-treatment with taurine (100 and 200 mg/kg) also ameliorated GTN (50 mg/kg)-induced tolerance in isolated aortic ring in rats. Also, taurine (100 and 200 mg/kg) significantly (p < 0.05) increased serum nitrite, GSH, and CAT levels while reducing malondialdehyde (MDA) concentration.
Conclusion
Taurine could prevent nitroglycerin-induced tolerance by increasing serum nitric oxide level and decreasing oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organic nitrates are a group of very effective anti-ischemic drugs. They are used for the treatment of patients with stable angina, acute myocardial infarction, and chronic congestive heart failure. A major therapeutic limitation inherent to organic nitrates is the development of tolerance which occurs during chronic treatment with these agents [1]. The mechanism for the development of nitrate tolerance is very complex since it involves neuro-hormonal counter-regulation, expansion of plasma volume (collectively classified as pseudotolerance), and intrinsic vascular processes defined as true tolerance [2]. Recent studies revealed that oxidative stress, due to overproduction of reactive oxygen species (ROS), peroxynitrate, and markers of free radical-induced lipid peroxidation play an important role in the development of nitroglycerine tolerance and endothelial dysfunction [3,4,5]. Two main sources of oxidative stress associated with nitrate tolerance are NADPH oxidases [6] and mitochondrial respiratory chain [7, 8]. This oxidative stress has been reported to cause impaired GTN bio-activation, and inhibition of NO signal transduction [9] due to oxidation of thiol groups of mitochondrial aldehyde dehydrogenase (ALDH-2) [7, 10] and soluble guanylyl cyclase (sGC) [11]. Several agents have been reported to possess strong antioxidant activities against nitrate-induced oxidative stress. These include lipoic acid [12], hydralazine [13], Qi Shen Pi dropping [14], and Shenmai injection [15]. At the moment, there is no standard agent to completely prevent nitroglycerine tolerance in clinical practice.
Taurine (2-aminoethanesulfonic acid) is a naturally occurring amino acid-like compound present in substantial amounts in many mammalian tissues [16, 17] and also present as supplement in bottled or canned drinks. It has been reported to play a role in the modulation of blood pressure, amelioration of vascular reactivity impairment, endothelial apoptosis, oxidative stress and inflammation, and increasing nitric oxide generation [18]. Various studies have reported the potential benefits of taurine in cardiovascular diseases including congestive heart failure [19], hyperlipidemia [20], hypertension, and ischemia–reperfusion injury [21]. Some of the mechanism by which taurine mediates these effects have been linked to; prevention of Ca2+ overload in cardiomyocytes [22], antioxidation [23], reduction of platelet aggregation [24], inhibition of angiotensin II [25], and direct effect on vascular function [26]. This study aimed to examine the potential effects of taurine on nitroglycerine-induced tolerance in rats. We used in vitro and in vivo methods of tolerance induction in rats to investigate the potential effects of taurine through the evaluation of vascular function in isolated aortic rings, protection of the myocardium structure by histopathology, and anti-oxidative action.
Materials and method
Reagents and drugs
Taurine was purchased from (Merck KGaA 64,271 Darmstadt, Germany), and nitroglycerin (GTN) was purchased from Neon Laboratory Ltd, (India). Carbachol, norephinephrine (NE), Ellman’s reagent {5′,5′-dithio-bis (2-nitrobenzoate) DTNB}, trichloroacetic acid (TCA), thiobarbituric acid (TBA), sulfanilamide, and N-(1-naphthyl) ethylenediamine dihydrochloride were obtained from Sigma-Aldrich (Steinheim, Germany). Sodium chloride (NaCl), potassium chloride (KCl), potassium dihydrogen phosphate (KH2PO4), calcium chloride (CaCl2), and glucose were products of British Drug House (Poole, Dorset, UK).
Animals
Adult male Wistar rats weighing 200–220 g were purchased from the University of Ibadan Animal House (Ibadan, Nigeria). The animals were housed in plastic cages in a well-ventilated vivarium at a temperature of 25 ± 2 °C, under a reversed 12-h light/dark cycle, and received a standard diet and water ad libitum. The animals were fasted for 12 h before the experiment but were allowed free access to water. All of the experiments were conducted according to the approved guidelines set by the University of Ibadan Animal Care and Use Research Ethics Committee (UI-ACUREC), which is in agreement with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Science and published by the National Institutes of Health. The ethical approval number assigned for animal use in this study was UI-ACUREC/17/0071.
Effect of taurine on nitroglycerin-induced tolerance in rat aortic rings in vitro
The thoracic aortic rings were isolated from male Wistar rats (200–220 g) and prepared as previously described by Zhu et al. [27]. The intactness of endothelium was confirmed when a significant relaxation (more than 30%) occurred in response to carbachol 3 × 10−7 M [28]. In the rings pre-contracted with NE (10−6 M) and also, in the denuded aortic rings protocol, the same process was followed except that the aorta was denuded by mechanical means using cotton pipe cleaners. The aorta was considered denuded when the carbachol-induced relaxation was less than 30%.
The protocol for induction of GTN Tolerance in rat thoracic aorta was as follows:
The aortic rings were pre-contracted first with NE (10−5 M), and then incubated with incremental doses of GTN (10−12–10−6 M) in 1-min intervals. The primary concentration–response curve to GTN was obtained. The aortic rings were washed three times in 10-min intervals to remove NE and GTN completely; the tissues were allowed to return to the baseline.
Thereafter, GTN (10−5 M) was selected as a concentration that effectively induce GTN tolerance in aortic ring [29]. After 1-h incubation with GTN, the preparation was washed 3 times in 10-min intervals to clear out GTN completely. The secondary concentration–response curve to GTN was obtained after a constant contraction to NE was achieved.
The effect of taurine (10, 20 mM) was investigated by pre-incubation with taurine before addition of GTN. The concentration–response curve of GTN was obtained in the presence of taurine (10, 20 mM).
Effect of taurine on nitroglycerin-induced tolerance in vivo and in vitro
Thirty male Wistar rats were randomly assigned into six groups of five animals per group as follows:
-
Control group (I): consisted of rats that were given normal saline 10 mL/kg, i.p. daily for 10 days.
-
GTN-induced tolerance group (II): these rats were administered normal saline 10 mL/kg, i.p. daily for 7 days, followed by GTN 50 mg/kg, s.c. daily from day 8 to 10.
-
Treatment groups (III and IV): rats were treated with taurine 100 and 200 mg/kg, i.p. daily for 10 days and GTN 50 mg/kg, s.c. daily from day 8 to 10.
-
Positive control groups (V and VI): taurine alone (100 and 200 mg/kg, i.p. daily) was administered for 10 days.
After the last drug administration, rats were lightly anesthetized with diethyl ether by inhalation, blood was collected by cardiac puncture, and the thoracic aorta was immediately isolated and transferred to petri dishes containing Krebs–Henseleit (K-H) solution (NaCl 118 mM, KCl 4.75 mM, MgSO4·7H2O, 1.2 mM, KH2PO4 1.2 mM, CaCl2 2.5 mM, NaHCO3 25 mM, and glucose 11 mM).
The surrounding fat and connective tissues of thoracic aorta were removed, and the trimmed aorta was subsequently cut into 3- to 5-mm rings cautiously to avoid any inadvertent endothelial damage. The rings were suspended horizontally between two parallel stainless hooks. One fixed to the bottom of the organ bath filled with Kreb’s solution at (37 °C) and bubbled with carbogen (95% O2 and 5% CO2), while the other end was connected to a force displacement transducer (AD Instruments Pty Ltd., Australia). A computer-assisted data acquisition system (PowerLab/4SP; AD Instruments, Australia) recorded the changes in isometric tension during the experiments [27].
The aortic ring was equilibrated at a based tension of 2.0 g for 30 min, and the K-H solution was changed twice every 15 min. The endothelial function was evaluated by norepinephrine (NE) and carbachol. First, NE (10−5 M) was added to achieve contraction, and later carbachol (3 × 10−4 M) was used to induce vaso-relaxation [28]. The aortic rings were washed 3 times in 10-min intervals to remove norepinephrine and carbachol completely. The aortic rings were again pre-contracted with NE (10−5 M), and then incubated with incremental doses of GTN (10−12–10−6 M) in 1-min intervals. The concentration–response curve to GTN was obtained in the presence of NE and carbachol.
Effect of taurine on serum nitrite and oxidative stress parameters in nitroglycerin tolerance in rats
Blood was collected from the heart into non-heparinized plain tubes, centrifuged at 3000 rpm, for 10 min and serum was stored at − 20 °C. Nitric oxide (NO) level was determined by measuring the serum nitrites (the stable end products of NO) according to the method described by Green et al. [30]. Lipid peroxidation (LPO) was quantified as malondialdehyde (MDA) according to the method described by Nagababu et al. [31]. Reduced glutathione (GSH) was determined according to the method described by Jollow et al. [32]. Catalase (CAT) activity was determined using hydrogen peroxide as a substrate according to the method described by Goth [33].
Histopathological examination of myocardial tissues
The heart tissue was removed from the chest, fixed in 10% formaldehyde solution for 48 h and embedded in paraffin. Serial sections (4 μm) were cut into slices, after standing at room temperature overnight. The sections were stained with hematoxylin–eosin as described by Alabi et al. [34]. Intracellular gap distance were measured in the photomicrographs using the Motic Plus 2000 (China) application software.
Statistical analysis
In vitro aortic ring response data were acquired using the LabChart 4 software (ADInstruments Ltd., Castle Hill, Australia). All of the data were analyzed and expressed as the means ± standard error mean (SEM) using the GraphPad Prism® software version 5.01 (GraphPad Software, Inc. La Jolla, CA 92,037 USA). Statistical significance was assessed by one-way analysis of Variance (ANOVA) followed by Tukeys post hoc test for multiple comparison. Values of P < 0.05 were considered to be statistically significant.
Results
Effect of taurine on nitroglycerine-induced tolerance in vitro in endothelium intact and denuded rat aortic rings
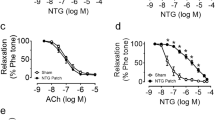
The representative tracing of the taurine co-incubation with GTN on intact and denuded aortic ring is shown in Fig. 1A–C. The concentration–effect curve of co-incubation of taurine 10 mM and 20 mM, respectively, with GTN 10−6 M for 1 h in endothelium intact aortic rings is shown in Fig. 1D. At both concentration levels, there was no significant difference in vaso-relaxant response to graded concentrations of GTN when compared with tissues incubated with nitroglycerine 10−6 M alone (p > 0.05). The AUC showed no statistically significant difference (p > 0.05) between GTN alone and GTN co-incubated with taurine (10 mM and 20 mM) in endothelium intact aortic ring (Fig. 1E).
Effect of taurine on nitroglycerin-induced tolerance in vitro in endothelium intact and denuded rat aortic rings. Representative tracings of A endothelium intact aortic rings, B endothelium-denuded aortic rings, C primary and secondary relaxation responses to graded concentrations of nitroglycerin, D concentration-effect curve of GTN in endothelium intact rat aortic ring, E area under the curve (AUC) for percentage relaxation in intact aortic ring, F concentration-effect curve of GTN in endothelium denuded aortic ring, and G AUC for percentage relaxation in denuded aortic ring. Data are means ± S.E.M (n = 4). *p < 0.05 vs control, #p < 0.05 vs GTN using 1-way ANOVA followed by Tukey’s post hoc test. GTN nitroglycerin, Tau taurine
Figure 1F revealed the effects of co-incubation of aortic tissue with taurine (10 mM and 20 mM) GTN 10−5 M for 1 h in endothelium-denuded aortic rings. There was no significant difference in the vasorelaxant response to graded concentration of nitroglycerine in tissues co-incubated with taurine 10 mM and GTN 10−5 M for 1 h when compared to rat aortic rings previously incubated with GTN 10−6 M alone. However, co-incubation with taurine 20 mM and GTN 10−6 M significantly (p < 0.05) increase the percentage relaxation response of endothelium-denuded rat aortic rings to graded concentration of GTN (Fig. 1G)
Taurine reduced nitroglycerin tolerance in vivo and in vitro aortic ring effect
As shown in Fig. 2A, compared with the control group, the concentration–response curve of GTN in aortic rings of rats (treated with GTN) caused significant relaxation (p < 0.05), indicating that the vasodilation effect of GTN was significantly attenuated in rats treated with GTN (50 mg/kg). As shown in the AUC (Fig. 2B), co-treatment with taurine (100 and 200 mg/kg) significantly (p < 0.05) reduced GTN (50 mg/kg)-induced tolerance in aortic rings, indicating that taurine enhanced the vasorelaxant effect of GTN. Also, taurine (100 mg/kg) significantly (p < 0.05) increased vaso-relaxation when compared with GTN (50 mg/kg) + Taurine (100 mg/kg).
The effect of taurine on nitroglycerine-induced tolerance in vitro in rat aortic rings. Data are means ± S.E.M (n = 5). A Percentage relaxation and B AUC for percentage relaxation. *p < 0.05 vs control, #p < 0.05 vs GTN, ap < 0.05 vs taurine (100 mg/kg) using one-way ANOVA followed by Tukey’s post hoc test. GTN nitroglycerin, Tau taurine
Effect of taurine on serum nitrite level in nitroglycerin-treated rats
As shown in Fig. 3, taurine (100 and 200 mg/kg) alone or in co-treatment with GTN (50 mg/kg) significantly (p < 0.05) increased serum nitrite level when compared with control. Also Taurine (100 and 200 mg/kg) co-administration with GTN (50 mg/kg) significantly (p < 0.05) increased serum nitrite levels when compared with GTN (50 mg/kg) alone.
Effect of taurine on serum oxidative stress parameters
Oxidative stress was measured by detecting the production of MDA, and the activity of CAT and GSH. MDA, an index of lipid peroxidation was significantly elevated in serum of GTN (50 mg/kg) alone treated animals when compared with vehicle-treated rats. Co-administration of taurine (100 and 200 mg/kg) significantly reduced serum MDA levels (Fig. 4A). As shown in Fig. 4B, GSH was significantly reduced in serum of rats treated with GTN (50 mg/kg) alone. However, co-administration with taurine (200 mg/kg) significantly prevented GSH depletion in serum of GTN-treated rats (Fig. 4B). There was no significant effect on catalase activities except in GTN (50 mg/kg) + taurine (200 mg/kg) that showed significantly increase catalase activities (Fig. 4C).
Discussion
Nitroglycerin (GTN) and other organic nitrates are widely used in the management of cardiovascular diseases. However, chronic administration of GTN easily results in reduction in hemodynamic and clinical effects. This phenomenon, known as nitrate tolerance may pose difficulties to the desired therapeutic effects of nitroglycerine [35]. One important finding of this experiment was a reduction in GTN induced tolerance when endothelium-denuded aortic rings were co-incubated with taurine (20 mM). Apart from this notable observation, taurine (10 mM) did not produce any significant reduction in GTN-induced tolerance in vitro co-incubation both in endothelium intact and endothelium-denuded aortic rings. This result suggests that taurine attenuation of GTN-induced tolerance is endothelium independent. In addition, it is consistent with reports that taurine produced vascular smooth muscle relaxation in a concentration-dependent fashion [36]. Before now, studies on isolated vascular tissues from healthy and diseased animals demonstrated that oral treatment with taurine at different concentrations exerted a powerful concentration dependent vasodilator action [19, 36,37,38]. Results obtained from our in vivo treatment with taurine in GTN-induced tolerance in rats revealed that co-treatment with taurine (200 mg/kg) improved vasorelaxant response.
A marked reduction of the vasorelaxant effect of GTN is decrease in endogenous release of NO following chronic GTN and therapy is a characteristic feature of GTN-induced tolerance. Taurine treatment augmented serum nitrites level in GTN-induced tolerance rats. Hence, the improved vasorelaxant response caused by taurine to nitroglycerine tolerance may be due to its ability to increase serum NO level. The exact mechanism of taurine’s effect is not clear, but may be due to the ability of taurine to augment sarcoplasmic reticular Ca2+ accumulation and release as reported by Baker and Berg [39]. However, this warrants further studies. Furthermore, lots of mechanisms have been proposed to be responsible for organic nitrate tolerance development ranging from neurohormonal counter-regulation, expansion of plasma volume (collectively classified as pseudotolerance), and intrinsic vascular processes defined as true tolerance which involves oxidative stress, increase in sensitivity to vasoconstrictors such as phenylephrine, angiotensin II, serotonin and thromboxane A and increased autocrine endothelin-1 [2, 40,41,42,43]. It has been demonstrated that taurine increases serum levels of NO in animal models of hypertension [44]. Fenessy et al. suggested that this ability of taurine may be linked to an up-regulation of NO-synthase expression [45].
It has also been suggested that oxidative stress induction plays a pivotal role in the development of GTN-induced tolerance and endothelial dysfunction [4, 46]. This oxidative stress has been linked to increased production of RONS (superoxide and peroxynitrate) in response to chronic GTN therapy. Oxidative stress has been associated with almost all true tolerance phenomenon resulting into decreased soluble guanylyl cyclase (sGC) responsiveness and impaired GTN biotransformation by the ALDH-2 due to oxidation of these enzymes [9, 47]. Nitrate tolerance was also associated with increased markers of free radical induced lipid peroxidation [5]. In the current study, co-treatment with taurine and nitroglycerine significantly decreased the release of MDA, and increased the activity of GSH and CAT. Taurine-reduced cardiovascular stress triggered by GTN-induced tolerance is consistent with reports of studies from various animal models [48]. The effect of taurine as an antioxidant and removal of free-oxygen radicals has been reported [49,50,51]. Various studies in diabetic mice and rats have linked the antioxidative effect of taurine to several mechanisms which include: increasing the antioxidant enzyme activity of superoxide dismutase (SOD)/ glutathione peroxidase (GSH-Px)/catalase (CAT), reducing lipid peroxidation and formation of carbonyl protein, inhibiting the activity of protein kinase C (PKC) and xanthine oxidase, reducing advanced glycation end products (AGEs) production, and repressing the expression of coenzyme II (NADPH) oxidase (p47phox)/cytochrome P450 CYP2E1 [49, 52,53,54,55,56]. Recently, Adedara et al. [49] showed that taurine markedly increased antioxidant enzymes activities and glutathione level while suppressing the increase in biomarkers of oxidative stress in the testes and epididymis of L-NAME-induced hypertensive rats [50].
Conclusion
The present study showed that taurine could prevent GTN-induced tolerance by increasing nitric oxide production and decreasing serum oxidative stress. These findings affirm the potential of taurine as a promising agent for preventing the development of GTN-induced tolerance.
Data availability
All data and material for this study will be provided upon request.
Code availability
Not Applicable.
Abbreviations
- MDA:
-
Malondialdehyde
- GSH:
-
Reduced glutathione
- CAT:
-
Catalase
- ROS:
-
Reactive oxygen species
- GTN:
-
Nitroglycerine
- ACh:
-
Acetylcholine
- NE:
-
Norepinephrine
- NO:
-
Nitric oxide
- LPO:
-
Lipid peroxidation
- NO2 − :
-
Nitrite
- GSH-Px:
-
Glutathione peroxidase
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- cyt P450:
-
Cytochrome P450
- L-NAME:
-
N-nitro-L-arginine methylester
References
Daiber A, Wenzel P, Oelze M, Schuhmacher S, Jansen T, Munzel T. Mitochondrial aldehyde dehydrogenase (ALDH-2)—maker of and marker for nitrate tolerance in response to nitroglycerin treatment. Chem Biol Interact. 2009;178:40–7.
Daiber A, Munzel T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: emphasis on redox biology and oxidative stress. Antioxid Redox Signal. 2015;23:899–942.
Sydow K, Daiber A, Oelze M, Chen Z, August M, Wendt M, et al. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Investig. 2004;113:482–9.
Cai H, Griendling KK, Harrison DG. The vascular NADPH oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–8.
Jurt U, Gori T, Ravandi A, Babaei S, Zeman P, Parker JD. Differential effects of pentaerythritol tetranitrate and nitroglycerin on the development of tolerance and evidence of lipid peroxidation: a human in vivo study. J Am Coll Cardiol. 2001;38:854–9.
Fukatsu A, Hayashi T, Miyazaki-Akita A, Matsui-Hirai H, Furutate Y, Ishitsuka A, Hattori Y, Iguchi A. Possible usefulness of apocynin, an NADPH oxidase inhibitor, for nitrate tolerance: prevention of NO donor-induced endothelial cell abnormalities. Am J Physiol Heart Circ Physiol. 2007;293:H790–7.
Daiber A, August M, Baldus S, Wendt M, Oelze M, Sydow K, Kleschyov AL, Munzel T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic Biol Med. 2004;36:101–11.
Daiber A, Oelze M, Ulyok S, Coldewey M, Schulz E, Treiber N, et al. Heterozygous deficiency of manganese superoxide dismutase in mice (Mn-SOD+/-): a novel approach to assess the role of oxidative stress for the development of nitrate tolerance. Mol Pharmacol. 2005;68:579–88.
Munzel T, Daiber A, Gori T. More answers to the still unresolved question of nitrate tolerance. Eur Heart J. 2013;34:2666–73.
Wenzel P, Hink U, Oelze M, Schuppan S, Schaeuble K, Schildknecht S, et al. Role of reduced lipoic acid in the redox regulation of mitochondrial aldehyde dehydrogenase (ALDH-2) activity. Implications formitochondrial oxidative stress and nitrate tolerance. J Biol Chem. 2007;282:792–9.
Sayed N, Kim DD, Fioramonti X, Iwahashi T, Duran WN, Beuve A. Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ Res. 2008;103:606–14.
Dudek M, Bednarski M, Bilska A, Iciek M, Sokołowska-Jeżewicz M, Filipek B, Włodek L. The role of lipoic acid in prevention of nitroglycerin tolerance. Eur J Pharmacol. 2008;591:203–10.
Munzel T, Kurz S, Rajagopalan S, Thoenes M, Berrington WR, Thompson JA, et al. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J Clin Invest. 1996;98:1465–70.
Wang T, Zhou Q, Jiang X, Li P, Tan W, Sun Y, et al. Dropping pill prevents nitroglycerin-induced tolerance in rats. Int J Clin Exp Med. 2016;9(7):13793–801.
Zhou Q, Sun Y, Tan W, Liu X, Qian Y, Ma X, et al. Effect of Shenmai injection on preventing the development of nitroglycerin- induced tolerance in rats. PLoS ONE. 2017;12(4):e0176777. https://doi.org/10.1371/journal.pone.0176777.
Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010;208:19–25.
Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–63.
Hanson SH. The role of taurine in diabetes and the development of diabetes complications. Diabetes Metab Res Rev. 2001;17:330–46.
Abebe W, Mahmood S, Mozaffari S. Role of taurine in the vasculature: an overview of experimental and human studies. Am J Cardiovasc Dis. 2011;1(3):293–311.
Azuma J, Hasegawa H, Sawamura A. Taurine for treatment of congestive heart failure. Int J Cardiol. 1982;2:303–4.
Mizushima S, Nara Y, Sawamura M, Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol. 1996;403:615–22.
Militante JD, Lombardini JB. Treatment of hypertension with oral taurine: experimental and clinical studies. Amino Acids. 2002;23:381–93.
Xu YJ, Saini HK, Zhang M, Elimban V, Dhalla NS. MAPK activation and apoptotic alterations in hearts subjected to calcium paradox are attenuated by taurine. Cardivas Res. 2006;72:163–74.
Yamauchi-Takihara K, Azuma J, Kishimoto S, Onishi S, Sperelakis N. Taurine prevention of calcium paradox-related damage in cardiac muscle. Its regulatory action on intracellular cation contents. Biochem Pharmacol. 1988;37:2651–8.
Hayes KC, Pronczuk A, Addesa AE, Stephan ZF. Taurine modulates platelet aggregation in cats and humans. Am J Clin Nutr. 1989;49:1211–6.
Mozaffari MS, Miyata N, Schaffer SW. Effects of taurine and enalapril on kidney function of the hypertensive glucose-intolerant rat. Am J Hypertens. 2003;16:673–80.
Zhu J, Kang L, Ye Q, Fan G, Liang Y, Yan C, et al. Effects of Shenfu injection and its main components on the contraction of isolated rat thoracic aortic rings. PloS one. 2013;8(10):e78026. https://doi.org/10.1371/journal.pone.0078026.
Azarmi Y, Babaei H, Alizadeh F, Gharebageri A, Fouladi DF, Nikkhah E. Allopurinol prevents nitroglycerin-induced tolerance in rat thoracic aorta. J Cardiovasc Pharmacol. 2013;63:113–9.
Zhou Q, Sun Y, Tan W, Liu X, Qian Y, Ma X, et al. Effect of Shenmai injection on preventing the development of nitroglycerin-induced tolerance in rats. PLoS ONE. 2017;12(4): e0176777. https://doi.org/10.1371/journal.pone.0176777.
Green LC, Wagner DA, Godowsky J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8.
Nagababu E, Rifkind JM, Sesikeran B, Lakshmaiah N. Assessment of antioxidant activities of eugenol by in vitro and in vivo methods. Methods Mol Biol (Clifton, NJ). 2010;610:165–80.
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4, bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–69.
Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–52.
Alabi B, Omobowale T, Badejo J, Adedapo A, Fagbemi O, Iwalewa O. Protective effects and chemical composition of Corchorus olitorius leaf fractions against isoproterenol-induced myocardial injury through p65NFkB-dependent anti-apoptotic pathway in rats. J Basic Clin Physiol Pharmacol. 2020; 31(5). https://doi.org/10.1515/jbcpp-2019-0108.
Wang X, Chen L, Wang T, Jiang X, Zhang H, Li P. Ginsenoside Rg3 antagonizes Adriamycin induced cardiotoxicity by improving endothelial dysfunction from oxidative stress via upregulating the Nrf2-ARE pathway through the activation of akt. Phytomed Int J Phytother Phytopharmacol. 2015;22(10):875–84.
Ristori MT, Verdetti J. Effects of taurine on rat aorta in vitro. Fundam Clin Pharmacol. 1991;5:245–58.
Xue W, Zhang M, Li J, Wu D, Niu L, Liang Y. Effect of taurine on aortic rings isolated from fructose-fed insulin resistance Sprague-Dawley rat are changed. Cardiovasc Drugs Ther. 2008;22:461–8.
Niu LG, Zhang MS, Liu Y, Xue WX, Liu DB, Zhang J, Liang YQ. Vasorelaxant effect of taurine is diminished by tetraethylammonium in rat isolated arteries. Eur J Pharmacol. 2008;580:169–74.
Bakker AJ, Berg HM. Effect of taurine on sarcoplasmic reticulum function and force in skinned fast-twitch skeletal muscle fibers of the rat. J Physiol. 2002;538:185–94.
Kurz S, Hink U, Nickenig G, Borthayre AB, Harrison DG, Munzel T. Evidence for a causal role of the renin-angiotensin system in nitrate tolerance. Circulation. 1999;99:3181–7.
Munzel T, Daiber A, Gori T. Nitrate therapy, new aspects concerning molecular action and tolerance. Circulation. 2011;123:2132–44.
Munzel T, Sayegh H, Freeman BA, Tarpey MM, Harrison DG. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest. 1995;95:187–94.
Oelze M, Knorr M, Kroller-Schon S, Kossmann S, Gottschlich A, Rummler R, et al. Chronic therapy with isosorbide-5-mononitrate causes endothelial dysfunction, oxidative stress, and a marked increase in vascular endothelin-1 expression. Eur Heart J. 2013;34:3206–16.
Harada H, Kitazaki K, Tsujino T, Watarai Y, Iwata S, Nonaka H, et al. Oral taurine supplementation prevents the development of ethanol induced hypertension in rats. Hypertens Res. 2000;23:277–84.
Fennessy EM, Monelet MB, Wang JH, Kelly CJ, Bouchier-Hayes DJ. Taurine and vitamin C modify monocyte and endothelial dysfunction in young smokers. Circulation. 2003;107:410–5.
Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radical Biol Med. 2011;51:978–92.
Daiber A, Wenzel P, Oelze M, Munzel T. New insights into bioactivation of organic nitrates, nitrate tolerance and cross-tolerance. Clin Res Cardiol. 2008;97:12–20.
Das J, Vasan V, Sil PC. Taurine exerts hypoglycemic effect in alloxan-induced diabetic rats, improves insulin-mediated glucose transport signaling pathway in heart and ameliorates cardiac oxidative stress and apoptosis. Toxicol Appl Pharmacol. 2012;258:296–308.
Das J, Sil PC. Taurine ameliorates alloxan-induced diabetic renal injury, oxidative stress-related signaling pathways and apoptosis in rats. Amino Acids. 2012;43:1509–23.
Adedara IA, Alake SE, Adeyemo MO, Olajide LO, Ajibade TO, Farombi EO. Taurine enhances spermatogenic function and antioxidant defense mechanisms in testes and epididymis of L-NAME-induced hypertensive rats. Biomed Pharmacother. 2018;97:181–9.
Li X, Catalina F, Grundy SM, Patel S. Method to measure apolipoprotein B-48 and B-100 secretion rates in an individual mouse: evidence for a very rapid turnover of VLDL and preferential removal of B-48-relative to B-100-containing lipoproteins. J Lipid Res. 1996;37:210–20.
Nandhini AT, Thirunavukkarasu V, Anuradha CV. Stimulation of glucose utilization and inhibition of protein glycation and AGE products by taurine. Acta Physiol Scand. 2004;181(3):297–303.
Parvez S, Tabassum H, Banerjee BD, Raisuddin S. Taurine prevents tamoxifen induced Mitochondrial oxidative damage in mice. Basic Clin Pharmacol Toxicol. 2008;102:382–7.
Schaffer SW, Jong CJ, Ramila KC, Azuma J. Physiological roles of taurine in heart and muscle. J Biomed Sci. 2010;17:2–8.
Schaffer SW, Azuma J. Taurine attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac myocytes. Eur J Pharmacol. 2000;403:181–8.
Michael AM, Ronan GC, David HO, Patricia F, Chris T, David JB. Two weeks taurine supplementation reverses endothelial dysfunction in young male type 1 diabetics. Diabetes Vasc Dis Res. 2010;7(4):300–10.
Author information
Authors and Affiliations
Contributions
This study was carried out in collaboration among all authors. The following authors OO, JAB, and OSF designed the study. OO, BAA, AAM, and JAB performed the statistical analysis, wrote the protocol and the first draft of the manuscript. EOI and OSF managed the managed literature searches and supervised the experiment. All authors read and approve the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All of the experiments were conducted according to the approved guidelines set by the University of Ibadan Animal Care and Use Research Ethics Committee (UI-ACUREC), which is in agreement with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Science and published by the National Institutes of Health. The ethical approval number assigned for animal use in this study by UI-ACUREC was UI-ACUREC/17/0071.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Rights and permissions
About this article
Cite this article
Odebiyi, O., Badejo, J., Alabi, B. et al. Evaluation of modulatory effects of taurine in the aortic and myocardial tissue of nitroglycerin-induced tolerance Wistar rats. Nutrire 46, 12 (2021). https://doi.org/10.1186/s41110-021-00141-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-021-00141-9