Abstract
Background
The cardiovascular benefits of resveratrol (RSV) have been well established by previous experimental and clinical studies. The aim of this study was to investigate the effectiveness of RSV administration on the impaired endothelial function induced by methylglyoxal (MGO), and to elucidate the role of endothelial nitric oxide synthase (eNOS) on its protective effect.
Methods
Aged Wistar rats (80 weeks old, n = 15) were used in this study. The thoracic aorta was isolated and cut into rings for organ culture. Aortic segments of rats were incubated with MGO (420 µM) in the presence or absence of RSV (30 µM) for 4 h (short-term) or 24 h (long-term). Isometric tension studies were performed by an isolated organ bath in response to acetylcholine (ACh, an endothelium-dependent vasodilator) and sodium nitroprusside (SNP, an endothelium-independent vasodilator). Beside, expressions of eNOS and phospho-eNOS (p-eNOS) (Ser 1177) in thoracic aorta rings were evaluated by immunohistochemistry.
Results
Both short-term and long-term MGO incubation significantly inhibited the relaxation response induced by ACh, while the relaxation to SNP was not significantly altered. In addition, eNOS and p-eNOS expressions decreased significantly in arteries incubated with MGO. The impaired endothelial reactivity as well as decreased expressions of eNOS and p-eNOS in MGO-incubated vessels were significantly improved by RSV treatment.
Conclusions
Endothelium-dependent vasodilatation of the thoracic aorta was significantly inhibited by MGO administration, and RSV may improve vascular endothelial function. The protective effect of RSV against MGO-induced endothelial dysfunction seems to be via increased eNOS expression and activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes in the elderly is a global public health burden, and older adults are the fastest growing segments of the diabetes population [1, 2]. Cardiovascular diseases are major complications of diabetes mellitus (DM) and constitute the leading cause of morbidity and mortality [3, 4], and this risk increases dramatically with age [5,6,7]. While there has been a reduction in diabetes-related complications in the general population, the incidence rates of macrovascular complications such as acute myocardial infarction and stroke continue to be the highest in older adults [8].
Impairment of endothelial function, known as endothelial dysfunction (ED), commonly defined as reduced endothelium-dependent vascular relaxation to a variety of vasodilatory stimuli, is a hallmark of type 2 and type 1 DM, and may be used as an important prognostic marker for cardiovascular disease [9, 10]. In recent years, aging has also been suggested as an independent predictor of ED [11]. Although aging has been clearly demonstrated to be an independent risk factor for ED, concomitant diseases such as DM may have an additional contribution to development of ED in aged individuals. However, the mechanisms underlying the age-associated increase for diabetes-related cardiovascular disease remain poorly understood.
Methylglyoxal (MGO), a highly reactive dicarbonyl compound, is an intermediate product formed during glycation of proteins by glucose and its formation involves many pathways consisting of enzymatic and non-enzymatic reactions in all mammalian cells, including vascular smooth muscle cells and endothelial cells [12,13,14]. Recent reports have shown that MGO levels are significantly increased in the blood in diabetic patients [15,16,17]. In addition to diabetic patients, increased MGO levels has been determined in aged rats and also aged people [18, 19]. Hence, older diabetic patients may be exposed to higher MGO levels than the others. Hyperglycemia and increased levels of MGO are two hallmarks of DM which can trigger the development of vascular complications in diabetes [20, 21]. MGO can cause damage and induce apoptosis in endothelial cells [22]. Recent in vitro studies clearly showed that acute exposure of isolated arteries to high levels of MGO affects endothelium-dependent relaxations in different vascular beds [20, 21, 23]. Moreover, various studies indicate a possible link between ED and functional alterations of eNOS after MGO treatment. MGO was shown to suppress eNOS phosphorylation on serine-1179 without affecting eNOS protein expression [21, 24]. In addition, the results of Sena et al. have demonstrated that MGO-induced ED seems to be mediated via a decrement in NO bioavailability [25].
Resveratrol (RSV), one of the most commonly employed dietary polyphenols, seems to be present in red wine in significant amounts, and to be partly responsible for cardiovascular benefits associated with wine consumption [26, 27]. The promising cardiovascular benefits of RSV in patients at increased cardiovascular risk have been reported by a substantial number of experimental and clinical studies [26]. According to some previous studies, RSV has been found to improve vascular NO activity and endothelial vasodilatory functions [28,29,30]. RSV prevents eNOS uncoupling and upregulates eNOS expression and activity [31,32,33,34], suggesting its protective potential against MGO-induced ED. However, determination of the effect of RSV on the MGO-induced ED remains unclear.
In light of the aforementioned studies, in the present study, we aimed to investigate the protective effect of RSV on MGO-induced diminished endothelium-dependent relaxation responses in thoracic aorta of aged rats, and also to evaluate the role of eNOS expression and/or activity in the protective effect of RSV. Our findings indicated to an important role of RSV in protecting against ED induced by MGO in aged rats.
Materials and methods
This study was registered by the Animal Ethics Committee of Akdeniz University Medical Faculty, Antalya, Turkey. Briefly, totally 15 aged male Wistar rats, at 80 weeks of age, were used in the present study. These rats did not receive any vehicle or chemical. The animals were anesthetized with intraperitoneal injection of ketamine hydrochloride (90 mg/kg) and xylazine (10 mg/kg), and decapitated. The full length of thoracic aorta was removed and cleaned of the connective tissue. Then, aortas isolated from these rats were cut into 3–4 mm width rings and incubated with MGO (420 µM) in the Dulbecco’s Modified Eagle Medium (DMEM) containing an antibiotic mixture (120 U/mL penicillin and 120 µg/mL streptomycine), in the presence or absence of RSV (30 µM) for 4 h (short-term) or 24 h (long-term). They were maintained at 37 °C in an atmosphere of 95% O2 and 5% CO2. After the incubation period, functional and histological studies were employed in these tissues.
Isolated organ bath studies
To perform functional studies, thoracic aorta rings were carefully suspended by two stainless-steel clips passed through the vessel lumen in 20 ml organ baths filled with PSS (mM: NaCl 118, KCl 5, NaHCO3 25, KH2PO4 1.0, MgSO4 1.2, CaCl2 2.5, and glucose 11.2) maintained at 37 °C gassed with 95% O2 and 5% CO2 to obtain a pH of 7.4. Isometric tension was continuously measured with an isometric force transducer (FDT10-A, Commat Ltd., Ankara, Turkey), connected to a computer-based data acquisition system (MP35, Commat Ltd., Ankara, Turkey). The rings were placed at the optimal point of length–tension relation by gradually stretching them until contraction induced by 20 mM of KCl was maximal at each level of distension. Accordingly, an optimal resting tension of 2 g, which had been obtained in a preliminary study by a construction of a passive length–tension curve, was applied to the rings of the thoracic aorta and allowed to equilibrate for 60 min. Because it was necessary to use endothelium-intact rings in our study, following a 60-min equilibration period, the presence of functional endothelium was confirmed by the ability of acetylcholine (ACh, 1 µM) to produce relaxation of tissues precontracted with phenylephrine (Phe, 1 µM). This concentration was determined from the cumulative contraction- response curves to achieve 80% of the maximum contraction. For contraction-relaxation experiments, ten thoracic aorta rings were used for each group. Relaxation responses were examined with ACh (10 nM–100 µM, an endothelium-specific vasodilator) and sodium nitroprusside (SNP, 1 pM–10 nM, an endothelium-independent dilatory agent) in all groups. Briefly, after the tissues were precontracted with Phe, concentration-relaxation responses for ACh and SNP were obtained by addition of increasing concentrations of agonist to the baths in a cumulative manner and isometric tension developed by the tissue was recorded. The tissue response was allowed to reach a stable plateau before each successive addition of the agonist.
In the first set of experiments, the effect of MGO (420 µM) on ACh- and SNP-induced relaxation responses of endothelium intact thoracic aorta rings were investigated. In another set of experiments, the effects of RSV (30 µM) incubation on MGO-induced endothelial dysfunction in thoracic aorta rings were investigated. To investigate the possible protective effect of RSV against the observed inhibitory effect of MGO in endothelium-dependent relaxations, tissues were incubated with either MGO alone or in the presence of RSV for 4 or 24 h. After the incubation period, the concentration–response curves to ACh and SNP, were obtained in these tissues.
Immunohistochemistry
Pieces of thoracic aorta were fixed in 10% formalin and embedded in paraffin. 5-m thick sections were cut and were collected onto poly-l-lysine-coated slides. The slides were deparaffinized in xylene, rehydrated in a decreasing gradient of ethanol solutions (100–70%). An antigen-retrieval procedure was performed by heating the samples with citric acid buffer (pH 6.0) (Merck, Cat #1–00244 1000) in a microwave oven at 540 W for 7 min and after cooling in this buffer for 20 min at room temperature. The slides were blocked for endogenous peroxidase activity with methanol containing 3% H2O2 for 15 min and for nonspecific binding with universal blocking reagent (Thermo; Cat #TA-125-UB) for 7 min at room temperature. Anti-rabbit eNOS (#sc-654, Santa Cruz Biotechnology) and anti-goat p-eNOS (#sc-12972, Santa Cruz Biotechnology) antibodies diluted in dilution buffer (Abcam; #ab64211) were applied for overnight at 4 °C in a humidified chamber. For negative controls, the primary antibodies were replaced by normal rabbit (Vector Lab. #I-1000) and goat IgG serum (Vector Lab. #I-5000 Burlingame, CA, USA) at the same concentration. After several washes in PBS, slides were incubated with biotinylated anti-rabbit (#BA-1000) and biotinylated anti-goat (#BA-9500) secondary antibodies (1/500 dilution) (Vector Lab. Burlingame, CA, USA) for 1 h followed by streptavidin–peroxidase complex (Invitrogen; #85–9043) incubation for 20 min and were rinsed with PBS. Antibody complexes were visualized by incubation with diaminobenzidine (DAB) chromogen (Thermo #TA-060-HD). Slides were counterstained with Mayer’s hematoxylin (Merck; #1-09240-1000) dehydrated, mounted and examined by a Zeiss-Axioplan (Oberkochen, Germany) microscope. For each group, thoracic aorta tissues from 6 animals were stained for eNOS and pe-NOS proteins.
Images of the tissue samples have been taken using Spot Imaging software version 4.6 (Diagnostic Instruments, Inc, Michigan) at 40X magnification micrographs. All of these micrographs were analyzed with Image-J Version 1.46 (National Institutes of Health, Bethesda, Maryland) for microscopy software by scanning 10 non-overlapping fields in each tissue section and expressing the positive areas as a percentage of the total area.
TUNEL staining
Thoracic aortas were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice and the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) reaction mixture (In Situ Cell Death Detection Kit, TMR, Roche) was applied for 1 h at 37 °C.
Drugs
Acetylcholine chloride, L-phenylephrine hydrochloride, sodium nitroprusside, and methylglyoxal were used. All drugs and the salts for the physiological salt solution were purchased from Sigma Chemical (St. Louis, MO, USA). All drugs were prepared fresh daily during experiments.
Statistical analysis
All values are expresses as mean ± SEM. Responses to ACh and SNP are expressed as percentages of the reversal of the tension developed in response to Phe. Statistical analysis of the results was performed by one-way analysis of variance (ANOVA). Post hoc comparisons were done using Tukey’s multiple comparison post-test. A p value lower than 0.05 was considered significant.
Results
Effects of short-term or long-term MGO incubation on endothelium-dependent and -independent relaxation responses of rat thoracic aorta
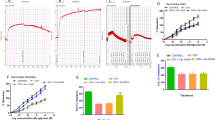
In the control artery, ACh and SNP evoked endothelium-dependent and -independent relaxation of isolated thoracic aorta rings precontracted with Phe, respectively. MGO administration for both 4 and 24 h significantly attenuated the relaxation response to ACh, which is an endothelium-dependent vasodilator agent (Figs. 1a, 2a, P < 0.05). On the other hand, the vasodilator effect of SNP, an endothelium-independent vasodilator agent, was not changed significantly by MGO incubation for both 4h and 24 h (Figs. 3a, 4a, P > 0.05).
The effect of 4 h MGO (420 µM) (a) and MGO plus RSV (30 µM) (b) incubation on NO-mediated endothelium-dependent relaxation response of thoracic aorta to acetylcholine (ACh, 1 nM–100 µM). All values are expressed as mean ± SEM. C Control, MGO methylglyoxal, RSV resveratrol. n = 8 for all groups. *P < 0.05 as compared with controls
The effect of 24 h MGO (420 µM) (a) and MGO plus RSV (30 µM) (b) incubation on NO-mediated endothelium-dependent relaxation response of thoracic aorta to acetylcholine (ACh, 1 nM–100 µM). All values are expressed as mean ± SEM. C Control, MGO methylglyoxal, RSV resveratrol. n = 8 for all groups. n = 8 for all groups. *P < 0.05 as compared with controls
Effect of RSV incubation on endothelial dysfunction induced by MGO
The inhibitory effect of MGO on ACh-induced relaxation was significantly prevented by the presence of RSV on both 4- and 24-h incubation groups (Figs. 1b, 2b, P < 0.05). Otherwise, incubation of aortic rings with RSV for both 4 and 24 h had no significant effect on SNP-induced relaxation in MGO-incubated groups (Figs. 3b, 4b, P > 0.05).
Immunohistochemistry
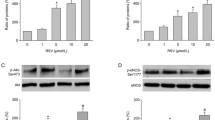
All sections in three different groups were stained synchronously to prevent inconsistent results. eNOS and p-eNOS immunostainings were clearly detected in endothelial cells in control groups. Both antibodies expressions in endothelial cells were generally decreased in MGO groups. But, the immunoreactivities were strongly observed in endothelial cells in MGO + RSV groups (Fig. 5a). Negative control immunostaining with normal rabbit IgG and normal goat IgG confirmed the specificity of eNOS and p-eNOS staining patterns in aortas. eNOS and p-eNOS Image-J analysis of immune expression levels were indicated that Fig. 5b.
Representative photomicrographs for eNOS and p-eNOS immunohistochemistry (a) in control, MGO-, and MGO + RSV-incubated thoracic aorta rings for 24 h. The graphs (b) show image analysis results after immunohistochemistry. MGO methylglyoxal, RSV resveratrol. n = 6 for all groups. *P < 0.05 as compared with controls; **P < 0.05 as compared with MGO
Evaluation of apoptosis by TUNEL
We performed TUNEL analysis to assess the incidence of apoptotic cell death in all groups. As seen in Fig. 6, TUNEL positive cells in endothelium were higher in MGO-incubated vessels when compared to controls. On the other hand, the numbers of TUNEL positive apoptotic cells in MGO plus RSV-incubated vessels were similar to the control groups (Fig. 6).
Discussion
The aim of this study was to investigate the protective effectiveness of RSV administration on endothelial function in aorta segments that were treated with MGO, and to elucidate eNOS expression and/or activity as a possible mechanism of its protective effect. This study was the first to show that RSV treatment improves endothelium-dependent relaxation in the MGO-treated thoracic aorta of aged rats primarily via an eNOS-dependent mechanism.
In patients with poorly controlled diabetes, the MGO level in blood can raise up to 400 µM [15]. It is suggested that local MGO concentration in tissues is much higher than the plasma level [35] Therefore, the use of 420 µM MGO in this study seems to be justified as these concentrations seem pathophysiologically relevant. MGO is related to diabetic vascular complications, and it triggers cellular injury and apoptosis in endothelial cells [36]. In the present study, we provide solid evidence that incubation of rat thoracic aorta with MGO for both 4 and 24 h impaired ACh-induced endothelium-dependent relaxation. Our data are in line with other studies showing a significant reduction in endothelium-dependent relaxation after MGO administration [20, 23,24,25]. The diminished endothelium-dependent relaxation response MGO-administered aged rats may be related to the changes in eNOS expression and/or activity. Indeed, Dhar et al. reported that short-term (2 h) MGO incubation impaired ACh-induced relaxation via impairment of eNOS phosphorylation [21]. Furthermore, Mukohda et al. demonstrated that long-term MGO treatment decreased protein expression of eNOS [23]. It is known that the activity of eNOS is associated with the phosphorylation of serine 1177 [37, 38]. Results of the present study indicated that both short-term and long-term MGO incubation caused a significant reduction in eNOS expression and eNOS phosphorylation of the thoracic aorta endothelium. In MGO-incubated aortic rings, inhibition of eNOS and p-eNOS immunoreactivities was also paralleled by a significant reduction in endothelial function. This indicates that a significant reduction in vascular eNOS expression and/or activity in MGO-administered aged rats may account for the impaired endothelium-dependent relaxation. On the other hand, the incubation of rat aortic rings with high level of MGO produces not only endothelial cell dysfunction, but also may cause apoptotic cell death in endothelium. Indeed, the results of TUNEL studies also indicated that number of TUNEL-positive cell increased in the endothelium of MGO-incubated aortic rings which indicates endothelial cell apoptosis. Taken together, these results suggest that incubation of thoracic aorta with MGO may lead to endothelial cell apoptosis and ED.
The protective effect of RSV against development of ED has been established by previous experimental studies [30, 32,33,34]. Here, we further extended the novel functions of RSV in the improvement of endothelial functions during high MGO levels. In the present study, we have showed that MGO-induced vascular hyporeactivity to ACh was significantly improved by RSV incubation for both 4 and 24 h. Previous studies have demonstrated that RSV attenuated hyperglycemia-induced endothelial apoptosis through inhibition of oxidative stress [39, 40]. To investigate the possible protective effect of RSV against MGO-induced endothelial cell apoptosis, we also performed TUNEL staining. Our results indicate that MGO-induced cytotoxicity is prevented by incubation with RSV in aort segments, which seems to act by regulating cell apoptotic proteins. Thus, the protective effects of RSV in MGO-induced endothelial damage may be related to many mechanisms by which RSV can promote cell survival. Activation of the eNOS potently prevents endothelial cell apoptosis, and NO either endogenously produced or exogenously applied in physiologically relevant concentrations acts as a endothelial cell survival factor in vitro [41]. On the other hand, aging of endothelial cells is associated with decreased NO synthesis and concomitantly increased sensitivity of apoptosis, which may contribute to functional impairment of the endothelial monolayer [42]. The present study demonstrates that MGO levels are associated with increased apoptosis induction of endothelial cells and a reduction of endothelial NO synthase (eNOS), which may be prevented by exogenous agents. The results of Seo et al. demonstrated that RSV is capable of protecting cells from MGO-induced mitochondrial dysfunction and apoptosis [43]. It is also known that RSV may increase eNOS enzymatic activity and NO bioavailability through inducing eNOS phosphorylation [31, 44, 45]. eNOS activity is regulated by posttranslational modification of the eNOS protein. Our results have demonstrated that the administration of RSV improved the eNOS protein expression levels of the thoracic aorta in MGO-administered aged rats. As a consequence of the increase in eNOS expression, RSV enhances NO production by endothelial cells. In addition, we have showed that eNOS serine 1177 phosphorylation was potentiated by RSV treatment, as the expression of p-eNOS was increased in RSV plus MGO-administered aged rats, when compared with MGO group. Potentiation of eNOS and p-eNOS expression with RSV also paralleled with the improvement of endothelial function. Taken together, this indicates that MGO may cause endothelial cell apoptosis and ED by decreasing eNOS expression and/or activation, and treatment with RSV prevents MGO-induced ED through increasing NO-mediated endothelium-dependent relaxation.
In conclusion, our results support the notion that RSV protects against MGO-induced ED in aortic tissues via the eNOS-dependent mechanism. Therefore, it has been suggested that RSV may be a useful tool to prevent or reverse MGO-induced vascular damage in aged population. Further studies with RSV may contribute to develop new pharmacological approaches against the MGO-induced endothelial damage in several types of cardiovascular disorders, especially in older adults.
References
Boyle JP, Thompson TJ, Gregg EW, et al (2010) Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 8:29
Narayan KM, Boyle JP, Geiss LS, et al (2006) Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care 29:2114–2116
Gu K, Cowie CC, Harris MI (1999) Diabetes and decline in heart disease mortality in US adults. JAMA 281:1291–1297
American Diabetes A (2017) cardiovascular disease and risk management. Diabetes Care 40:S75-S87
Halter JB, Musi N, Horne FMF, et al (2014) Diabetes and cardiovascular disease in older adults: current status and future directions. Diabetes 63:2578–2589
Cigolle CT, Blaum CS, Halter JB (2009) Diabetes and cardiovascular disease prevention in older adults. Clin Geriatr Med 25:607–641
Kirkman MS, Briscoe VJ, Clark N, et al (2012) Diabetes in older adults. Diabetes Care 35:2650–2664
Gregg EW, Li Y, Wang J (2014) Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med 370:1514–1523
Potenza MA, Gagliardi S, Nacci C, et al (2009) Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem 16:94–112
Barthelmes J, Nagele MP, Ludovici V, et al (2017) Endothelial dysfunction in cardiovascular disease and Flammer syndrome-similarities and differences. EPMA J 8:99–109
Versari D, Daghini E, Virdis A, et al (2009) The ageing endothelium, cardiovascular risk and disease in man. Exp Physiol 94:317–321
Yim HS, Kang SO, Hah YC, et al (1995) Free radicals generated during the glycation reaction of amino acids by methylglyoxal. A model study of protein-cross-linked free radicals. J Biol Chem 270:28228–28233
Lo CY, Li S, Tan D, et al (2006) Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol Nutr Food Res 50:1118–1128
Wu L (2005) The pro-oxidant role of methylglyoxal in mesenteric artery smooth muscle cells. Can J Physiol Pharmacol 83:63–68
Lapolla A, Flamini R, Dalla Vedova A, et al (2003) Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method. Clin Chem Lab Med 41:1166–1173
Hanssen NMJ, Scheijen J, Jorsal A (2017) Higher plasma methylglyoxal levels are associated with incident cardiovascular disease in individuals with type 1 diabetes: a 12-year follow-up study. Diabetes 66:2278–2283
Kong X, Ma MZ, Huang K, et al (2014) Increased plasma levels of the methylglyoxal in patients with newly diagnosed type 2 diabetes 2. J Diabetes 6:535–540
Srikanth V, Westcott B, Forbes J, et al (2013) Methylglyoxal, cognitive function and cerebral atrophy in older people. J Gerontol A Biol Sci Med Sci 68:68–73
Hallam KM, Li Q, Ananthakrishnan R (2010) Aldose reductase and AGE-RAGE pathways: central roles in the pathogenesis of vascular dysfunction in aging rats. Aging Cell 9:776–784
Brouwers O, Niessen PM, Haenen G, et al (2010) Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia 53:989–1000
Dhar A, Dhar I, Desai KM, et al (2010) Methylglyoxal scavengers attenuate endothelial dysfunction induced by methylglyoxal and high concentrations of glucose. Br J Pharmacol 161:1843–1856
Do MH, Kim SY (2017) Hypericin, a naphthodianthrone derivative, prevents methylglyoxal-induced human endothelial cell dysfunction. Biomol Ther (Seoul) 25:158–164
Mukohda M, Morita T, Okada M, et al (2013) Long-term methylglyoxal treatment causes endothelial dysfunction of rat isolated mesenteric artery. J Vet Med Sci 75:151–157
Turkseven S, Ertuna E, Yetik-Anacak G, et al (2014) Methylglyoxal causes endothelial dysfunction: the role of endothelial nitric oxide synthase and AMP-activated protein kinase alpha. J Basic Clin Physiol Pharmacol 25:109–115
Sena CM, Matafome P, Crisostomo J, et al (2012) Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol Res 65:497–506
Wu JM, Wang ZR, Hsieh TC, et al (2001) Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine. Int J Mol Med 8:3–17
Das DK, Maulik N (2006) Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv 6:36–47
Di Pascoli M, Divi M, Rodriguez-Vilarrupla A, et al (2013) Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J Hepatol 58:904–910
Takahashi S, Nakashima Y (2012) Repeated and long-term treatment with physiological concentrations of resveratrol promotes NO production in vascular endothelial cells. Br J Nutr 107:774–780
Yu HP, Hwang TL, Hwang TL, et al (2010) Resveratrol prevents endothelial dysfunction and aortic superoxide production after trauma hemorrhage through estrogen receptor-dependent hemeoxygenase-1 pathway. Crit Care Med 38:1147–1154
Xia N, Forstermann U, Li H (2017) Effects of resveratrol on eNOS in the endothelium and the perivascular adipose tissue. Ann NY Acad Sci 1403:132–141
Ugurel SS, Kuscu N, Ozenci CC, et al (2016) Resveratrol prevented lipopolysaccharide-induced endothelial dysfunction in rat thoracic aorta through increased eNOS expression. Balkan Med J 33:138–143
Li JY, Huang WQ, Tu RH, et al (2017) Resveratrol rescues hyperglycemia-induced endothelial dysfunction via activation of Akt. Acta Pharmacol Sin 38:182–191
Murat N, Korhan P, Kizer O, et al (2016) Resveratrol protects and restores endothelium-dependent relaxation in hypercholesterolemic rabbit corpus cavernosum. J Sex Med 13:12–21
Randell EW, Vasdev S, Gill V (2005) Measurement of methylglyoxal in rat tissues by electrospray ionization mass spectrometry and liquid chromatography. J Pharmacol Toxicol Methods 51:153–157
Figarola JL, Singhal J, Rahbar S, et al (2014) LR-90 Prevents methylglyoxal-induced oxidative stress and apoptosis in human endothelial cells. Apoptosis 19:776–788
Fleming I (2010) Molecular mechanisms underlying the activation of eNOS. Pflugers Arch 459:793–806
Fulton D, Gratton JP, McCabe TJ, et al (1999) Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597–601
Wang S, Wang J, Zhao A, et al (2017) SIRT1 Activation inhibits hyperglycemia-induced apoptosis by reducing oxidative stress and mitochondrial dysfunction in human endothelial cells. Mol Med Rep 16:3331–3338
Chen F, Qian LH, Deng B, et al (2013) Resveratrol protects vascular endothelial cells from high glucose-induced apoptosis through inhibition of NADPH oxidase activation-driven oxidative stress. CNS Neurosci Ther 19:675–681
Dimmeler S, Zeiher AM (1999) Nitric oxide-an endothelial cell survival factor. Cell Death Differ 6:964–968
Hoffmann J, Haendeler J, Aicher A, et al (2001) Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res 89:709–715
Seo K, Seo S, Han JY, et al (2014) Resveratrol attenuates methylglyoxal-induced mitochondrial dysfunction and apoptosis by Sestrin2 induction. Toxicol Appl Pharmacol 280:314–322
Klinge CM, Wickramasinghe NS, Ivanova MM, et al (2008) Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J 22:2185–2197
Zhang H, Zhang J, Ungvari Z, et al (2009) Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol 29:1164–1171
Acknowledgements
This study was partially supported by Akdeniz University Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Statement of human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The study protocol was approved by the Institutional Animal Ethics Committee of the University.
Informed consent
For this type of study informed consent is not required.
Rights and permissions
About this article
Cite this article
Tasatargil, A., Tanriover, G., Barutcigil, A. et al. Protective effect of resveratrol on methylglyoxal-induced endothelial dysfunction in aged rats. Aging Clin Exp Res 31, 331–338 (2019). https://doi.org/10.1007/s40520-018-0986-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-018-0986-x