Abstract
Pulses are an affordable source of proteins, starch, lipids, minerals and high value nutritional sources. This study was conducted to evaluate relationship between protein functional properties and their preparation methods. Therefore, the functional properties of Grass pea protein concentrates (GPPC) prepared using isoelectric precipitation (IE), salt extraction (SE) and ultrafiltration-diafiltration methods (UF/D) were determined. The GPP processed by those three precipitation methods contained all of the amino acids which aspartic acid and glutamic acid were dominate amino acids followed by arginine and leucine. However, methionine and tryptophan were limited amino acids. Water binding capacity was in following order: UF/D-GPPC > SE-GPPC > IE-GPPC. Meanwhile, highest value of oil binding capacity belonged to UF/D-GPPC. GPPC prepared using UF/D method had highest solubility. In term of interfacial tension, it was revealed that the interfacial tension of all isolates did not significantly reduced (P > 0/05). Net negative zeta potential with values was observed which IE-GPPC had highest surface charge followed by UF/D-GPPC and SE-GPPC, respectively. In terms of surface hydrophobicity, it was altered in the following order: IE-GPPC > SE-GPPC > UF/D-GPPC. It was observed that foaming capacity ranged between 85.06 and 89.78% and foaming stability ranged between 77.34 and 84.35%. Emulsifying capacity, emulsifying activity index and emulsifying stability index ranged between 105.06–109.78%, 31.09–36.29 m2/g and 12.90–18.86 min respectively. Evaluation of least gelling concentration showed that UF/D-GPPC were capable to form firm gel at low concentration (10% W/V). The functional properties of proteins are influenced by their extraction technique and can be achieve maximum functional characteristics by selecting appropriate extraction method. The results indicated the technological potential of GPP for health-promoting food formulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulses are an affordable source of proteins, starch, lipids, minerals and high value nutritional sources. Pulse proteins (20–50%) are at high interest because of providing essential amino acids and possessing functional properties such as solubility, water/oil binding capacities, foaming and emulsifying properties and gel formation which could improve the quality of food products. (Boye et al. 2010b) the utilization of plant protein in food products is limited to soybean and wheat. (Stone et al. 2015a) However, increasing worldwide demand and wide variety of food products encourage us to find new sources of protein. Grass pea (Lathyrussativus L.) known as a Kholar in Iran is a novel source of protein (20–30%) appertains to Fabaceae family which is an annual pulse crop mostly harvests and consumes as a human consumption in Asian countries. Functional properties of proteins impress through parameters such as molecular weight, amino acid composition and surface characteristics. On the other hand, these properties can be influenced by extraction methods (Boye et al. 2010a; Stone et al. 2015a, b). Therefore, applying the appropriate extraction methods is necessary. Numerous methods for fractionation of pulses such as milling, air classification and wet extraction (alkaline extraction-isoelectric precipitation, salt extraction-dialysis, ultrafiltration-diafiltration and micellar precipitation) have been reported (Boye et al. 2010a; Karaca et al. 2011; Mondor et al. 2012; Papalamprou et al. 2009; Stone et al. 2015a, b; Tian et al. 1999).
Unlike wet extraction methods, proteins processed by air classification and pin milling methods have low purity (38–65%) and further processing is often required (Tian et al. 1999). Among wet extraction methods, alkaline extraction-isoelectric precipitationis the most practical extraction method, provides high protein purity (> 70%) and has a significant effect on protein functional properties through affecting on globulin/albumin or legumin/vicilin ratio and the physicochemical characteristics of the protein (Karaca et al. 2011; Makri et al. 2005; Papalamprou et al. 2009).
Globulins and albumins are the major proteins in pulses (Karaca et al. 2011; Liu et al. 2008b).The globulin consists most of the proteins prepared by the isoelectric method. Whereas, a mixture of both globulins and albumins proteins achieve as a result of salt extraction (Karaca et al. 2011; Liu et al. 2008b; Makri et al. 2005). Ultrafiltration as an alternative protein extraction method for isoelectric precipitation is a mild operating that yield higher protein concentration (Mondor et al. 2012).
The effect of isoelectric precipitation, salt extraction and ultrafiltration methods, alone or at the same time, on structure and functional properties of various legumes such as chickpea, faba bean, lentil and pea proteins have been studied (Boye et al. 2010a; Karaca et al. 2011; Mondor et al. 2012; Papalamprou et al. 2009; Stone et al. 2015a, b). However, to the best of our knowledge, no study has been conducted on the effect of different extraction methods (isoelectric precipitation, salt extraction and ultrafiltration-diafiltration) on the functional properties of GPPI as a novel plant protein source. Therefore, this research was conducted in order to characterize the functional properties of Grass pea protein concentrates (GPPC) prepared by aforementioned methods. Amino acid composition, water/oil binding capacity, emulsifying capacity, emulsifying capacity/stability indices, protein solubility, surface charge, surface hydrophobicity (H0), interfacial tension and least gelling concentration properties were used for this purpose.
Materials and methods
Materials
Grass pea was purchased from local market in Shirvan, Iran (6 months of ripening time, harvesting as soon as the leaves begin to turn yellow and 3 months of aging period). Briefly, Grass pea flour prepared by removing foreign matter, cracking, dehulling and grinding into a fine flour followed by defatting of oil using hexane (1:5(w/v) flour:hexane ratio). The flour was dried using cross-ventilation (Soroush Medical Company, Khorasan Razavi province, Iran) and kindly ground by laboratory mill (IKA, Model A11, Germany). Proximate composition (moisture, protein, fat, ash, and carbohydrate) of Grass pea flour (GPF) and Grass pea protein (GPP) were determined according to AOAC Official Methods (AOAC 2000; Feyzi et al. 2018). Carbohydrate content was calculated based on the difference from 100%. All chemicals used were of analytical grade and were prepared from Sigma Chemical Co. (St Louis, MO, USA), and Merck KGaA (Darmstadt, Germany).
Protein extraction
Grass pea protein (GPP) was prepared using three precipitation methods based on preliminary experiments in the lab. Prior to isolates extraction, defatting process was carried out to improve protein extraction as a function of reducing protein–lipid interactions (Karaca et al. 2011).
Isoelectric precipitation (IE)
15 g defatted GPF (with 8.3% moisture, 28.09% protein, 0.54% fat, 4.4% ash, and 58.67% carbohydrate based on dry weight) was mixed with water at the ratio of 1/15(w/v) with pH = 9.7 at 30 °C. The mixture was stirred at room temperature for 1 h, then adjusted to pH = 4.5 (the isoelectric pH) with 1.0 M HCl followed by centrifuging at 4500 × g for 30 min at 25 °C. After removing supernatant, the precipitate was washed twice with deionized water (re-suspending in water at the ratio of 1/15 and stirring for 1 h at 25 °C in order to clear acid), then centrifuged at 4500 × g for 20 min. After that, precipitate was resolubilized by adjusting the pH to 7.2 using 1 M NaOH. Eventually, the precipitate was dried using a freeze dryer (XO-12N model Top press Freezing Dryer, China). The dry GPPC powder was stored at 4 °C for further analysis.
Ultrafiltration/diafiltration (UF/DF) extraction
The alkaline protein solution was diluted with one volume of deionized water and the pH was lowered from 9.7 to the value of 6.0. The solution was then subjected to ultrafiltration to concentrate the proteins using molecular weight cut-off of 50 kDa MWCO Millipore membranes. Prior to the diafiltration, the volume was concentrated fivefold at ultrafiltration step. Finally, resulting concentrated protein solution was freeze dried (XO-12 N model Top press Freezing Dryer, China) (Boye et al. 2010a).
Salt extraction (SE)
100 g of defatted GPF was mixed with 0.1 M sodium phosphate buffer (pH = 8.00) containing 6.4% KCl at 1:10 ratio (w/v) and stirred at 500 rpm for 24 h at room temperature. After centrifuging at 4500 × g for 20 min at 4 °C, the supernatant was dialyzed at 4 °C for 72 h (6–8 kDa cut off; Spectrum Laboratories, Inc., Rancho Dominguez, CA, USA) against Milli-Q™ water at 4 °C refreshing three times until the conductivity of the dialysis water reached ~ 20 μs/cm. Finally, the extract was stored at − 30 °C until freeze-dried (Boye et al. 2010b).
Amino acid composition
Amino acid composition of GPPC was identified by digestion of 50 mg of GPPC under nitrogenatmosphere in 4 mL of 6 M HCl at 110 °C for 25 h. Knauer high-performance liquid chromatography (HPLC) (fluorescence detector (Knauer, RF-20 Axs), wavelengthsof at 330 nm for excitation and 450 nm for emission and a Dikma C18 column (250 mm × 4.6 mm, with 5 μm particle size, Berlin, Germany) was carried out at 30 °C after pre-column derivatization with o-phthaldialdehyde (OPA). Mobile phase A (pH = 7) was a solution of 50 mM sodium acetate, water and tetrahydrofurane, while mobile phase B (pH = 5) was a solution of 300 mM sodium acetate buffer, methanol and tetrahydrofuran (Yuan et al. 2009; Feyzi et al. 2018).
Functional properties
Water binding capacity (WBC) and oil binding capacity (OBC) of GPPC were measured according to the procedure of Aydemir and Yemenicioglu (2013). Foaming capacity (FC), foam stability (FS), and also emulsifying capacity (EC) were determined according to the method described by Cano-Medina et al. (2011).
Emulsifying activity (EAI) and stability (ESI) indices
Protein solutions with concentration of 0.25% (w/w) dispersed in a 10 mM sodium phosphate buffer (pH = 7.0) followed by stirring (500 rpm) overnight at 40 °C. 3 g of protein solution and 3 g of corn oil were mixed and homogenized in 15 mL plastic centrifuge tubes. 50 μL emulsion sample was immediately taken from the bottom of the tube and diluted in 7.5 mL of 10 mM sodium phosphate buffer (pH = 7.00) containing 0.1% sodium dodecyl sulphate (SDS) and this solution was vortexed for 10 s. An aliquot of this suspension was taken at 10 min, and the absorbance of the diluted emulsion was measured at 600 nm using a spectrophotometer (S2000 UV/VIS Spectrophotometr, China) using plastic cuvettes (1 cm path length) (Pearce and Kinsella 1978; Boye et al. 2010a). EAI and ESI were calculated by using the following equations:
where A0 is the absorbance of the diluted emulsion immediately after homogenization, N is the dilution factor (× 150), C is the weight of protein per volume (g/mL), φ is the oil volume fraction of the emulsion, ∆A is the change in absorbance between 0 and 10 min (A0–A10) and t is the time interval (10 min).
Protein solubility
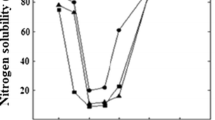
In order to determine protein solubility, the pH of aqueous solution (approximately 1.0%, w/v) of the GPPC in deionized water was adjusted in rang 2–10 using either 0.5 M HCl or 0.5 M NaOH with stirring for 30 min. After that, it was centrifuged at 10,000 × g for 20 min. Protein concentration in each supernatant was then determined by kjeldahl method (AOAC 2000) using the conversion factor of 6.25. Percent solubility was expressed as the ratio of weight of amount of protein in the supernatant to the weight of amount of total protein in the initial sample (× 100%) (Boye et al. 2010a). All measurement was carried out in duplicates and the plot of protein solubility (%) versus pH was considered as a solubility profile.
Interfacial tension
Interfacial tension between corn oil and distilled water containing 0.25%, w/w GPPC was measured as a compare of its interfacial tension with interfacial tension of protein-free solution (corn oil and distilled water mixture) according to the Du Noüy ring method using a semi-automatic tensiometer (tensiometer model K100, KRUSS Co., Germany) (Karaca et al. 2011). Maximum force (Fmax) of Eq. (3) was considered as an interfacial tension.
where γ is the interfacial tension, R is the radius of the ring, β is a correction factor that depends on the dimensions of the ring and the density of the liquid involved.
Surface charge (zeta potential)
The surface charge (or zeta potential) of each GPPC was measured based on change in the electrophoretic mobility (UE) at pH = 7.0 using a Zetasizer (CordouanTechnol, France). 0.05% w/w of GPPC in 10 mM sodium phosphate buffer (pH = 7.0) which had been stirred overnight at 4 °C adjusted to pH = 7.0 with 0.05 M HCl or NaOH and then 1 ml of solution was poured into a folded capillary cell and the electrophoretic mobility was measured (Karaca et al. 2011). Electrophoretic mobility is a measure of the velocity of a particle within an electric field, which can be related to the zeta potential (ζ) using the Henry equation (Eq. 4), where η is the dispersion viscosity, ε is the permittivity, and f(κα) is a function related to the ratio of particle radius(α) and the Debye length (κ). Using the Smoluchowski approximation, f(κα) equaled 1.5.
Surface hydrophobicity (H0)
Surface hydrophobicity was determined according to the procedure of Horax et al. (2011) using the aromatic fluorescent probe, 8-anilino-1-naphthalene sulfonic acid (ANS). All fluorescence measurements were recorded by using a spectrofluorophotometer (Shimadzu Crop., Kyoto, Japan, PC, RF-540) with the excitation and emission wavelengths at 390 and 470 nm, respectively. Protein solutions (0.01% w/v) prepared in 0.01 M sodium phosphate buffer (pH = 7.0) were diluted in the 0.01 M sodium phosphate buffer to prepare series of protein solutions with concentrations of 0.002, 0.004, 0.006, 0.008 and 0.01% (w/v). 20 µL of 8 mM ANS solution immersed in 0.01 M sodium phosphate buffer (pH = 7.00) was added to 4 mL of each of protein solutions and mixed well by vortexing for 10 s. The initial slope of the plot of the fluorescence intensities versus protein concentration was calculated using linear regression analysis and used as an index of H0.
Least gelation concentration (LGC)
The determination of LGC was carried out by preparing dispersions (10 ml) with 1–20% (w/v) solids concentration. All samples were entirely mixed on a vortex mixer for 5 min and then heated in sealed tubes (to avoid evaporation) at 95 °C in a water bath for 1 h. The mixture was cooled in a cold room at 4 °C for 2 h and then the tubes were inverted and the sample with the lowest concentration that did not fall down was considered as a LGC (Corredig 2006; Adebiyi and Aluko 2011).
Statistical analysis
The experimental data was subjected to analysis of variance (ANOVA) using Statistical Analysis System software, release 9.1 (SAS Inst, Cary, N.C. USA) and the means were compared using Duncan’s multiple comparison tests. Significance differences were considered equal to P < 0.05. Experiments data were an average of three replicates.
Results and discussion
Chemical composition
The proximate composition of the GPF, IE-GPPC, SE-GPPC and UF/D-GPPC were shown in Table 1. The protein content of GPF was in range reported by Boye et al. (2010a) and Karaca et al. (2011) for chickpea, faba bean, lentil and pea flours with concentrations of 16.7–23.9%. Processing of GPF by IE, SE and UF/D methods resulted in protein concentration to 80.03, 77.87 and 90.49, respectively. The protein content of IE-GPPC, SE-GPPC and UF/D-GPPC were similar to that reported by others (Feyzi et al. 2018; Karaca et al. 2011). UF/D-GPPC had highest protein content and extraction yield (%). Whereas, the highest moisture and carbohydrate content were obtained by SE and the highest ash content was belonged to IE-GPPC. Compare to GPF, the lipid and carbohydrate contents of GPP prepared by those three extraction methods decreased significantly (P < 0.05) which might attribute toalkali condition usedduring protein extraction. The lipid content for all IE-GPPC, SE-GPPC and UF/D-GPPC were lower that 1% and not significantly changed as a function of preparation methods (P > 0.05). IE-GPPC had higher ash content compare to GPF which was confirmed by Sosulski and McCurdy (1987) who demonstrated that strong acid and alkali used at protein extraction procedure may participate in salt formation and hence the ash content increases.
The amino acid compositions of IE-GPPC, SE-GPPC and UF/D-GPPC were presented in Table 2. It was found that there were slight differences between our results and Feyzi et al. (2018) who studied amino acids profile of GPP. The GPP processed by those three methods contained all of the amino acids which aspartic acid and glutamic acid were dominate amino acids (typical amino acid of pulses) followed by arginine and leucine. Meanwhile, methionine and tryptophan were limited amino acids. In this regard, Yuan et al. (2009) observed similar glutamic and aspartic contents for soy glycinin polypeptides. High amount of glutamic and aspartic acids in GPP confirmed their acidic isoelectric pH. All proteins had similar amino acids profile, indicate that precipitation methods did not have considerable effect on amino acids profiles. Stone et al. (2015a) demonstrated that protein isolates prepared from three pea cultivars by IE, SE and micellar precipitation did not show considerable differences about amino acids profile. GPPC prepared by UF/D method had higher total, charged hydrophilic, uncharged hydrophilic and hydrophilic amino acids relative to others. Likewise, GPPC processed by SE method had lowest values. Overall, all GPPC can be used as appropriate amino acid supplier, especially for feeding infants and children.
Water binding/oil binding capacity (WBC/OBC)
WBC and OBC values of GPPC extracted with various precipitation methods were presented in Table 3. The results revealed that WBC ranged between 2.24 and 2.83 (g g−1) and were in following order: UF/D-GPPC > SE-GPPC > IE-GPPC. This values were in ranges reported for chickpea and lentil isolates(Boye et al. 2010a). Furthermore, Boye et al. (2010a) Comparison IE and UF extraction methods showed that the red lentil protein processed by UF had higher WBC than IE. However, yellow pea, green lentil, Desi and Kabuli chickpeas proteins prepared by IE method had higher WBC than UF. Stone et al. (2015b) also reported that yellow, green, and dun market classes protein isolates extracted by SE procedure showed higher WBC compare IE method. Those authors supposed that SE procedure exposed more side chains and polar groups to form hydrogen bonding. Different results in studies refer to amino acid composition, protein conformation, ratio of surface charge to hydrophobicity, charged groups, amide groups and hydroxyl groups (Lam et al. 2018). Low WBC of IE-GPPC could be related to relatively high hydrophobic-hydrophilic balance (Table 4). Meanwhile, high amino acid content and charged hydrophilic amino acid level of UF/D-GPPC might impact on its higher WBC (Table 2).
OBC values ranged between 0.67 and 2.21 (g g−1) which highest value belonged to UF/D-GPPC. However, no significant differences were observed between UF/D-GPPC and SE-GPPC (Table 3). Nonpolar side chains of amino acids is one of the most important parameters that impact on OBC (Withana-Gamage et al. 2011). Therefore, high OBC of UF/D-GPPC might be refer to its high hydrophobic amino acids content (Table 2). These results confirmed with other studies (Boye et al. 2010a; Withana-Gamage et al. 2011). Similar to our results, Boye et al. (2010a) revealed that red lentil and yellow pea protein isolated by UF had higher OBC compared to IE preparation. Stone et al. (2015b) also showed OBC of yellow, green, and dun market classes pea protein prepared by SE was higher that IE method. In contrast to our results, some other studies (Du et al. 2018; Yu et al. 2015) revealed that OBC decreased as protein concentration raised, like UF/D-GPPC (Table 2), which was attributed to increase steric effect at high concentration (Lam et al. 2018; Yu et al. 2015).
Protein solubility and interfacial properties
Protein solubility affects texture, sensory and nutritional properties of products owning functional properties like emulsification, foaming and gel forming. Protein solubility is impressed as a function of properties such as protein content, the structure of the protein, protein–protein interactions, protein–solvent interactions and surface hydrophobicity. Protein–solvent interactions improve protein hydration and solubility. On the contrary, high hydrophobic interactions between proteins lead to precipitation and lower solubility (Damodaran 2017; Krause et al. 2002). The results showed that at a pH range of 2–10, the protein solubility altered as following order: UF/D-GPPC > IE-GPPC > SE-GPPC. Protein solubility, first, decreased until increasing pH to 4–5 and then it increased by increasing pH (Fig. 1). These results were in accordance with Kaur and Singh (2007) and Papalamprou et al. (2009) for chickpea flours and protein isolates. Contrary to our results, Karaca et al. (2011) showed that the SE isolates had significantly higher solubility relative to those prepared by IE method. The high solubility of UF/D-GPPC is referring to relatively high surface charge and low surface hydrophobicity. Ulloa et al. (2011) also stated that the isolated produced by UF had better protein solubility than that of isolates obtained by IE precipitation. In the case of SE-GPPC, the solubility was low despite having relatively high surface charge, attributed to its high surface hydrophobicity. On the other hand, the solubility of IE-GPPC was high despite having high surface hydrophobicity, attributed to its high surface charge. Previous studies also revealed that in some cases such as chickpea and pea protein isolates, there were no direct relationship between protein surface characteristics and solubility which was attributed to total parameters that impact on protein-water interactions such as number of exposed hydrophilic and hydrophobic groups and folding and aggregation properties of proteins (Karaca et al. 2011). Unfolding of polypeptide chains of proteins by increasing short-range repulsive forces upon isolation enhance solubility (Wu et al. 2009).
In term of interfacial tension, it was revealed that the interfacial tension of all isolates did not significantly reduced (P > 0/05) (Table 4). Interfacial tension value of isolates ranged between 37.39 and 37.44 mN/m which was found all isolates were capable to reduce interfacial tension relative to water (49.27 mN/m). Karaca et al. (2011) reported that various pea protein (chickpea, faba bean, lentil and pea protein) solutions of 0.25% (w/w) produced by IE and SE methods lowered the interfacial tension (~ 42.3 mN/m) compare to water (48.4 mN/m).
Surface characteristics
Net surface charge and surface hydrophobicity of molecules are the most important features that affect on functional properties such as emulsifying activity. The presence of hydrophobic patches on protein surface and also appropriate solubility of protein are necessary to its adsorption at the oil–water interface. Meanwhile, enough net charge is required to afford electrostatic repulsion between oil droplets to prevent aggregation (McClements 2004). The surface charge of GPPC extracted by UF/D, IE and SE methods were determined at pH = 7.0 (Table 4). Net negative zeta potential with values ranging between − 21.73 and − 24.96 mV was observed. IE-GPPC had highest surface charge followed by UF/D-GPPC and SE-GPPC, respectively. These results confirmed by Karaca et al. (2011) and Stone et al. (2015b) who studied different cultivar pea protein. In general, the amino acid content, conformation of proteins, solvent conditions (ionic strength and temperature) and protein concentration are important parameters that impact on the surface charge of proteins (Damodaran 2017; Lam et al. 2018; Stone et al. 2015b).
In terms of surface hydrophobicity, it was altered in the following order: IE-GPPC > SE-GPPC > UF/D-GPPC; ranged between 158.59 and 166.56 (Table 4) which were higher relative to pea cultivars have been reported in the previous studies (Karaca et al. 2011; Stone et al. 2015a, b). However, these values were in accordance with Feyzi et al. (2018) study who conducted optimization of GPPC extraction and various drying methods. Karaca et al. (2011) demonstrated that proteins of different legumes (chickpea, lentil, and pea) processed by IE precipitation had greater surface hydrophobicity relative to the salt extracted proteins. It might be related to protein denaturation during acid precipitation in IE precipitation method which cause interactions between protein and non-protein components, lead to lower solubility (Krause et al. 2002). Surface hydrophobicity of globulin fraction was higher than the albumin fraction (Karaca et al. 2011). Therefore, high surface hydrophobicity of proteins prepared by IE precipitation method was attributed to globulin fractions which are demining fraction in those proteins prepared by IE precipitation. On the other hand, the presence of both globulin and albumin fractions in proteins processed by SE and UF/D is a reason why their surface charges were low (Karaca et al. 2011; Makri et al. 2005).
Foaming properties
The foaming properties (FC and FS) of GPPC prepared by various extraction methods were presented in Table 3. The FC (the ability of a protein reducing interfacial tension between
an aqueous and an oil phase) ranged between 105.06 and 109.78% and FS (the ability of protein to preventing air bubbles against their collapse, stresses, drainage and mechanical shock) ranged between 118.34 and 121.35% after a 30-min waiting period. These values were near to the range have been reported for other legumes (Boye et al. 2010a; Shevkani et al. 2015; Stone et al. 2015a). In order to obtain superior FC, proteins must be adsorbed rapidly at the air/water interface followed by rapid conformational change and rearrangement of protein at the interface. Whereas, intermolecular interactions participate in the creating a cohesive viscoelastic film which enhance FS (Makri et al. 2005). FC values altered in following order: IE-GPPC > SE-GPPC > UF/D-GPPC. These results might be related to the ratio of globular/albumin fractions in extracted proteins. Globulin molecules (demine fraction of IE precipitated proteins) adsorbed at the interface are more effective in reduction of interfacial tension value due to greater number of hydrophobic patches relative to albumin molecules. As a result, FC promoted in those proteins prepared by IE precipitation (Makri et al. 2005; Papalamprou et al. 2009; Wierenga and Gruppen 2010). Similar to our findings, Boye et al. (2010a) revealed that FC of green lentil protein concentrate was low when processed by UF compared to IE. However, Desi chickpea protein concentrate provided by UF had higher FC compared to IE. Likewise, Linares et al. (2000) studies supported our findings that FC enhanced by SE method owning salting-out phenomena in salt solution.
The highest value of FS was belonged to IE-GPPC followed by UF/D-GPPC and SE-GPPC, respectively. High value of GPPC processed by IE attributed to this fact that at the isoelectric pH of a protein, minimum electrostatic repulsion and maximum adsorption of protein to the interface occur. Consequently, FS promotes through formation of viscous film and steric stabilization (Kinsella 1981; Wierenga and Gruppen 2010).Likewise, high FS value of isolates prepared by UF/D compare to SE might be related to its high protein content which was confirmed by Kinsella (1981) who reported FS usually improves at higher protein concentrations.
Emulsifying properties
The emulsifying properties (EC, EAI and ESI) of GPPC extracted by various methods were presented in Table 3. EC defines the maximum amount of oil that can be emulsified per unit weight of protein. Meanwhile, EAI refer to the capacity of protein to create emulsion following interfacial area that can be stabilized per unit weight of protein (Pearce and Kinsella 1978). Furthermore, ESI defines the ability of the protein to form strength emulsion to resist changes over a prescribed time period (Liu et al. 2008a). The results indicate that EC altered as following order: UF/D-GPPC > IE-GPPC > SE-GPPC. Karaca et al. (2011) reported high surface charge lowers EC because of increasing electrostatic repulsion which attenuates a stable film around oil droplets. Whilst, Yoshie-Stark et al. (2008) have been showed a direct relation between EC and solubility. As mentioned before, IE -GPPC had highest surface charge followed by UF/D-GPPC and SE-GPPC. However, solubility altered as following order: UF/D-GPPC > IE-GPPC > SE-GPPC. Therefore, it can be deduced that in this study the EC mostly followed solubility changes.
The EAI (ranged between 31.09 and 36.29 m2/g) and ESI (ranged between 12.90 and 18.86 min) of GPPC altered in following order: UF/D-GPPC > IE-GPPC > SE-GPPC which suggested that UF/D-GPPC were more effective at emulsion forming. In general, EAI of isolates directly correlate with surface charge and solubility while negative correlation has been reported between EAI and surface hydrophobicity (Karaca et al. 2011). Similar to our results, Karaca et al. (2011) found that pea protein isolated by IE had superior EAI and ESI compared to SE. Meanwhile, Boye et al. (2010a) mentioned that EAI of yellow pea isolates extracted by both IE and UF methods did not have noticeable differences. However, those authors showed that the ESI of Kabuli chickpea, green lentils and Desi chickpea protein isolates extracted by UF was better than IE method.
Least gelation concentration (LGC)
Protein gel is considered as a three-dimensional network consists a network that solvent are embedded within protein molecules (Corredig 2006). Globular proteins gel are formed following from reactions such as association or aggregation of protein molecules as a result of partial denaturation, leading to network formation that traps solvent ingredients (Corredig 2006; Wierenga and Gruppen 2010). The strength of proteins gel largely are impressed through electrostatic and hydrophobic interactions as well as hydrogen and covalent bonds (Adebiyi and Aluko 2011).Proteins with ability to form firm gel at low concentration are considered desirable.
In term of LGC, it was revealed that firm gel at lowest concentration (10% (W/V)) referred to UF/D-GPPC. Firm gel of IE-GPPC formed at 12% (W/V) concentration. Furthermore, SE-GPPC with 14% (W/V) concentration was needed to form firm gel (Table 5). Therefore, it can be deduced that the intensity of inter-molecular interactions in UF/D-GPPC gel was stronger relative to IE-GPPC and SE-GPPC to overcome repulsive forces. In this regard, Adebiyi and Aluko (2011) in comparison gel forming ability of soluble fractions of commercial pea protein in water, salt, alkaline and ethanol demonstrated that unlike water soluble and salt soluble fractions, alkaline soluble fraction formed a firm gel at the LGC of 10%. Therefore, it can be understood that protein quantity, quality and also type of protein fractions solubilized in various extraction methods had directly impact on LGC.
Conclusion
All proteins had similar amino acids profile, indicate that precipitation methods did not have considerable effect on amino acids profiles. GPPC prepared by UF/D method had higher total, charged hydrophilic, uncharged hydrophilic and hydrophilic amino acids relative to others. Likewise, GPPC processed by SE method had lowest values. The results demonstrated that the relatively high hydrophobic-hydrophilic balance in IE-GPPC resulted in low WBC. Meanwhile, high amino acid content and charged hydrophilic amino acid level of UF/D-GPPC participate at high WBC. On the other hand, the presence of high hydrophobic amino acids content at UF/D-GPPC raised OBC. Protein solubility decreased till pH reached to 4–5 and then increased by increasing pH. The highest solubility was observed for UF/D-GPPC which referred to its relatively high surface charge and low surface hydrophobicity. In term of interfacial tension, it was revealed that all isolates were capable to reduce interfacial tension relative to water. The results showed that the highest surface charge, surface hydrophobicity, FC and FS belonged to IE-GPPC. However, emulsifying results showed that the highest EC, EAI and ESI belonged to UF/D-GPPC. UF/D-GPPC was capable to form firm gel at lowest concentration (10% (W/V)). However, 14% (W/V) concentration of SE-GPPC was needed to form firm gel which demonstrated that UF/D-GPPC gel had strong inter-molecular interactions intensity.
References
Adebiyi AP, Aluko RE (2011) Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem 128:902–908. https://doi.org/10.1016/j.foodchem.2011.03.116
AOAC (2000) Official methods of analysis, 17th edn. Association of Official Analytical Chemists International, Gaithersburg
Aydemir LY, Yemenicioğlu A (2013) Potential of Turkish Kabuli type chickpea and green and red lentil cultivars as source of soy and animal origin functional protein alternatives. LWT Food Sci Technol 50:686–694. https://doi.org/10.1016/j.lwt.2012.07.023
Boye J, Aksay S, Roufik S, Ribéreau S, Mondor M, Farnworth E, Rajamohamed S (2010a) Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Int 43:537–546. https://doi.org/10.1016/j.foodres.2009.07.021
Boye J, Zare F, Pletch A (2010b) Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res Int 43:414–431. https://doi.org/10.1016/j.foodres.2009.09.003
Cano-Medina A, Jiménez-Islas H, Dendooven L, Herrera RP, González-Alatorre G, Escamilla-Silva EM (2011) Emulsifying and foaming capacity and emulsion and foam stability of sesame protein concentrates. Food Res Int 44:684–692. https://doi.org/10.1016/j.foodres.2010.12.015
Corredig M (2006) Protein–protein interactions in food science and technology, vol 154. Marcel Dekker, New York, p 283
Damodaran S (2017) Food proteins and their applications, vol 1. Routledge, Abingdon, pp 25–142
Du M et al (2018) Extraction, physicochemical characteristics and functional properties of Mung bean protein. Food Hydrocolloids 76:131–140. https://doi.org/10.1016/j.foodhyd.2017.01.003
Feyzi S, Milani E, Golimovahhed QA (2018) Grass pea (Lathyrus sativus L.) protein isolate: the effect of extraction optimization and drying methods on the structure and functional properties. Food Hydrocolloids 74:187–196. https://doi.org/10.1016/j.foodhyd.2017.07.031
Horax R, Hettiarachchy N, Kannan A, Chen P (2011) Protein extraction optimisation, characterisation, and functionalities of protein isolate from bitter melon (Momordica charantia) seed. Food Chem 124:545–550. https://doi.org/10.1016/j.foodchem.2010.06.068
Karaca AC, Low N, Nickerson M (2011) Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res Int 44:2742–2750. https://doi.org/10.1016/j.foodres.2011.06.012
Kaur M, Singh N (2007) Characterization of protein isolates from different Indian chickpea (Cicer arietinum L.) cultivars. Food Chem 102:366–374. https://doi.org/10.1016/j.foodchem.2006.05.029
Kinsella JE (1981) Functional properties of proteins: possible relationships between structure and function in foams. Food Chem 7:273–288. https://doi.org/10.1016/0308-8146(81)90033-9
Krause JP, Schultz M, Dudek S (2002) Effect of extraction conditions on composition, surface activity and rheological properties of protein isolates from flaxseed (Linum usitativissimum L). J Sci Food Agric 82:970–976. https://doi.org/10.1002/jsfa.1140
Lam A, Can Karaca A, Tyler R, Nickerson M (2018) Pea protein isolates: structure, extraction, and functionality. Food Rev Int 34:126–147. https://doi.org/10.1080/87559129.2016.1242135
Linares E, Larre C, Lemeste M, Popineau Y (2000) Emulsifying and foaming properties of gluten hydrolysates with an increasing degree of hydrolysis: role of soluble and insoluble fractions. Cereal Chem 77:414–420. https://doi.org/10.1094/CCHEM.2000.77.4.414
Liu C, Wang X, Ma H, Zhang Z, Gao W, Xiao L (2008a) Functional properties of protein isolates from soybeans stored under various conditions. Food Chem 111:29–37. https://doi.org/10.1016/j.foodchem.2008.03.040
Liu L, Hung T, Bennett L (2008b) Extraction and characterization of chickpea (Cicer arietinum) albumin and globulin. J Food Sci 73(5):299–305. https://doi.org/10.1111/j.1750-3841.2008.00773.x
Makri E, Papalamprou E, Doxastakis G (2005) Study of functional properties of seed storage proteins from indigenous European legume crops (lupin, pea, broad bean) in admixture with polysaccharides. Food Hydrocolloids 19:583–594. https://doi.org/10.1016/j.foodhyd.2004.10.028
McClements DJ (2004) Protein-stabilized emulsions. Curr Opin Colloid Interface Sci 9(5):305–313. https://doi.org/10.1016/j.cocis.2004.09.003
Mondor M, Tuyishime O, Drolet H (2012) Production of pea protein concentrates by ultrafiltration: influence of hollow-fibre module. Innov Food Sci Emerg Technol 14:135–138. https://doi.org/10.1016/j.ifset.2012.02.003
Papalamprou E, Doxastakis G, Biliaderis C, Kiosseoglou V (2009) Influence of preparation methods on physicochemical and gelation properties of chickpea protein isolates. Food Hydrocolloids 23:337–343. https://doi.org/10.1016/j.foodhyd.2008.03.006
Pearce KN, Kinsella JE (1978) Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem 26:716–723. https://doi.org/10.1021/jf60217a041
Shevkani K, Singh N, Kaur A, Rana JC (2015) Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocolloids 43:679–689. https://doi.org/10.1016/j.foodhyd.2014.07.024
Sosulski F, McCurdy A (1987) Functionality of flours, protein fractions and isolates from field peas and faba bean. J Food Sci 52:1010–1014. https://doi.org/10.1111/j.1365-2621.1987.tb14263.x
Stone AK, Avarmenko NA, Warkentin TD, Nickerson MT (2015a) Functional properties of protein isolates from different pea cultivars. Food Sci Biotechnol 24:827–833. https://doi.org/10.1007/s10068-015-0107-y
Stone AK, Karalash A, Tyler RT, Warkentin TD, Nickerson MT (2015b) Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res Int 76:31–38. https://doi.org/10.1016/j.foodres.2014.11.017
Tian S, Kyle WS, Small DM (1999) Pilot scale isolation of proteins from field peas (Pisum sativum L.) for use as food ingredients. Int J Food Sci Technol 34:33–39. https://doi.org/10.1046/j.1365-2621.1999.00236.x
Ulloa JA, Rosas-Ulloa P, Ulloa-Rangel BE (2011) Physicochemical and functional properties of a protein isolate produced from safflower (Carthamus tinctorius L.) meal by ultrafiltration. J Sci Food Agric 91:572–577. https://doi.org/10.1046/j.1365-2621.1999.00236.x
Wierenga P, Gruppen H (2010) New views on foams from protein solutions. Curr Opin Colloid Interface Sci 15:365–373. https://doi.org/10.1016/j.cocis.2010.05.017
Withana-Gamage TS, Wanasundara JP, Pietrasik Z, Shand PJ (2011) Physicochemical, thermal and functional characterisation of protein isolates from Kabuli and Desi chickpea (Cicer arietinum L.): a comparative study with soy (Glycine max) and pea (Pisum sativum L.). J Sci Food Agric 91:1022–1031. https://doi.org/10.1002/jsfa.4277
Wu H, Wang Q, Ma T, Ren J (2009) Comparative studies on the functional properties of various protein concentrate preparations of peanut protein. Food Res Int 42:343–348. https://doi.org/10.1016/j.foodres.2008.12.006
Yoshie-Stark Y, Wada Y, Wäsche A (2008) Chemical composition, functional properties, and bioactivities of rapeseed protein isolates. Food Chem 107:32–39. https://doi.org/10.1016/j.foodchem.2007.07.061
Yu L, Yang W, Sun J, Zhang C, Bi J, Yang Q (2015) Preparation, characterisation and physicochemical properties of the phosphate modified peanut protein obtained from Arachin conarachin L. Food Chem 170:169–179. https://doi.org/10.1016/j.foodchem.2014.08.047
Yuan D-B, Yang X-Q, Tang C-H, Zheng Z-X, Ahmad I, Yin S-W (2009) Physicochemical and functional properties of acidic and basic polypeptides of soy glycinin. Food Res Int 42:700–706. https://doi.org/10.1016/j.foodres.2009.02.005
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hayati Zeidanloo, M., Ahmadzadeh Ghavidel, R., Ghiafeh Davoodi, M. et al. Functional properties of Grass pea protein concentrates prepared using various precipitation methods. J Food Sci Technol 56, 4799–4808 (2019). https://doi.org/10.1007/s13197-019-03930-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03930-3