Abstract
Background
In adults with Klinefelter syndrome (KS), impaired bone health with reduced bone mineral density (BMD) has been described even in the presence of testosterone replacement therapy. The aim of the present study was to characterize bone health in young patients with KS.
Patients and methods
20 participants aged 16.10 ± 4.28 years with KS (7 with testosterone replacement therapy) were included in the KliBONE study (DRKS 00024870). Medical history, clinical, radiographic and biochemical parameters of bone health and metabolism were obtained. Radiographic bone health index (BHI) was assessed via automated digital X-ray radiogrammetry of the left hand or via dual energy X-ray absorptiometry (DXA) of the lumbar spine and left femur in participants ≥ 16 years. Peripheral blood mononuclear cells were differentiated into osteoclasts and quantified in 7 participants and 7 healthy controls.
Results
Mean BHI SDS was − 1.42 ± 1.22 and mean BMD z-score at the lumbar vertebrae (L1-4) was − 0.92 ± 1.00. 25-OH-vitamin D levels < 20 ng/ml were detected in 8/20. Other parameters of bone metabolism (bone-specific alkaline phosphatase, PTH, ß-crosslaps and osteocalcin) were within age-appropriate reference ranges. Serum leptin SDS was elevated (mean 2.15 ± 1.19). The number of osteoclasts in participants with KS did not differ from that of controls.
Conclusion
BHI SDS and BMD z-scores were lower than expected in young individuals with KS despite age-appropriate bone turnover markers and no apparent pathology in osteoclast differentiation. The cause of the early-onset bone phenotype requires further investigation.
Similar content being viewed by others
Introduction
Klinefelter syndrome (KS) is caused by an extra X chromosome in male individuals, resulting in the karyotype 47,XXY (and variants) and affects approximately one in 500 men [1, 2]. In all non-mosaic forms gonadal failure develops, usually detectable around mid to late puberty and results over time in hypergonadotropic hypogonadism with small, firm testes, azoospermia and infertility [1]. Additionally, men with KS have orginally been described as individuals with a tall and feminine body habitus and marked gynecomastia [1]. However, the clinical spectrum is much wider and the presentation is highly heterogeneous which leads to a diagnostic delay of KS especially in childhood. It is estimated that only up to 40% of the affected men will receive the diagnosis of KS in their lifetime and only about 20% are being diagnosed during childhood and adolescence [2, 3]. A wide variety of organ systems has been reported to be affected in KS, resulting in increased morbidity and a reduced quality of life due to varying levels of impairment in cardiovascular, endocrine, metabolic, cognitive, and neuropsychological health [1, 4, 5].
Ferlin et al. reviewed that adult men with KS have an increased prevalence of metabolic bone disease and reduced bone mineral density (BMD). The combined rate of osteopenia and osteoporosis in men with KS is increased up to 40% compared to men without extra X chromosomes of the same age [6]. The underlying mechanism of the reduced bone mineral density is yet unclear and cannot be attributed to hypogonadism only, as osteoporosis and osteopenia also occur in men with KS with normal or normal-low testosterone levels [7, 8]. Data regarding the influence of testosterone replacement therapy (TRT) on bone mass, are discussed controversially. Late initiation of TRT does not appear to normalize BMD in adults with KS, but early initiation (before the age of 20 years) seems to normalize BMD [9, 10].
Pediatric studies on bone health in KS are rare but suggest that cortical bone mass or BMD are already impaired at a prepubertal age, and thus before the onset of hypogonadism [11]. An unfavorable body composition with increased adipose tissue, an increased prevalence of type 2 diabetes and metabolic syndrome as well as reduced motivation for exercise, have been described in children and young adults and pathogenetically discussed [12,13,14]. Aksglaede et al. described significantly increased body fat mass despite normal lean body mass and BMI for age, suggesting that an unfavorable muscle-fat ratio may already be present in childhood [15]. Physical activity has a significant influence on bone health. In a recent study by our group, we identified a marked impairment of physical fitness in a small group of adolescents with KS [4], echoing the findings of Skakkebæk et al. who showed a tendency towards physical inactivity in KS adults, [14] whereas others found no difference in the quantitative amount of exercise between patients with KS and controls [16].
Reduced levels of vitamin D in KS seem to be frequent in children as well as in adults [16, 17]. Recent data also identified a reduced BMD in boys with KS compared to boys with 46,XY [18]. However, changes in bone health have not been investigated in bone biopsies or on a cellular level, so far. The aim of this study was to characterize bone health in children and adolescents with KS in depth in a pilot cohort.
Materials and methods
Cohort
Individuals with KS were enrolled from April 2021 to March 2023 in the KliBONE study (DRKS No.: DRKS00024870). Participants were recruited during annual screening visits and in cooperation with national patient groups for 47,XXY [19, 20]. Inclusion criteria were a genetically confirmed diagnosis of KS, age between 9 and 25 years and signed informed consent from participants and/or their legal guardians if applicable. The study protocol was approved by the Ethics committee of the Medical Faculty, Ruhr-University Bochum (#21-7164) and the study was conducted in accordance with the principles of the Declaration of Helsinki.
Clinical parameters
Clinical and anamnestic parameters were assessed as previously described [21, 22]. In brief, the following parameters were obtained: age at study participation, age at diagnosis, birth weight and length, parental heights, current medication, medical history including detailed information on the start, frequency, and dose of testosterone replacement therapy if applicable. Standing height (in cm) was measured to two decimal places using a wall-mounted stadiometer (Ulmer Stadiometer, Busse Design, Elchingen, Germany), weight (in kg) was measured to two decimal places as well (Seca, Hamburg, Germany). BMI (kg)/(height in m)^2 was calculated and z-scores for BMI, height and weight based on the KiGGS data (9–18 years) and the WHO data (> 18 years) [23, 24] were calculated. The pubertal status (Tanner stages) and the determination of testicular volume using a Prader orchidometer were assessed by experienced pediatric endocrinologists. Body impedance was measured using the TANITA Body composition analyzer (Model DC-360, TANITA Europe B. V., Amsterdam, the Netherlands). No participants with mosaic chromosomal status were included.
Biochemical tests
As previously described [4], serum, plasma and spot-urine aliquots were assessed in the central laboratory of the St. Josef-Hospital Bochum and at the MVZ Dr Eberhard & Partner Dortmund, Germany. The respective samples were obtained in the mornings (before 10 am, non-fasting) and then stored at -80 °C until the analysis. The following parameters were assessed: hemoglobin (g/dl); thyroid-stimulating hormone; TSH (uIE/ml); free triiodothyronine, fT3 (pg/ml); thyroxine, fT4 (ng/dl); calcium (mmol/l); phosphate (mg/dl); 25-OH vitamin D ( 25OHD, ng/ml); 1,25-(OH)2 vitamin D (pg/ml); total serum alkaline phosphatase, TSAP (U/l); bone specific alkaline phosphatase, BAP (ug/l); parathyroid hormone, PTH (pg/ml); osteocalcin, OC (ng/ml); insulin-like growth factor-1, IGF-1 (ng/ml); beta-crosslaps, CTX (pg/ml); leptin (ng/ml); free and total testosterone (ng/ml); follicle stimulating hormone, FSH (IU/ml) and luteinizing hormone, LH (IU/ml). In addition, deoxypyridinoline, DPD (mg/g creatinine), calcium and creatinine were measured in spot urine and the calcium: creatinine ratio (in mg/mg) was calculated subsequently. Leptin SDS was calculated using the reference values of Blum et al. [25]. We provide information of inter and intra assay precision in suppl. Table 3.
Dual X-Ray absorptiometry (DXA) and Bone Health Index (BHI)
Bone mineral density was assessed using DXA in six patients aged 16–25 years (Lunar Prodigy, GE-Healthcare, Madison, WI, USA). The BMD was assessed at the lumbar spine (L1-L4; anteroposterior view) and at the total left femur. Z-scores were calculated for the lumbar spine measurements based on normative values measured by the manufacturer for the corresponding age without correction for height.
The Bone Health Index (BHI) was obtained from a conventional radiograph of the left hand from an anterior-posterior view in 14 individuals aged 9–17 years. Digital images were saved in DICOM (Digital Imaging and Communications in Medicine) format and analyzed for skeletal age, BHI and BHI SDS using BoneXpert software (BoneXpert version 2, Visiana, Holte, Denmark) as previously described by Thodberg et al. [26]. As a radiogrammetric method, the BHI describes bone mass as a function of the cortical thickness of three metacarpals and the width and length of the metacarpals. BHI standard deviations are calculated based on a large Caucasian reference cohort. BHI SDS has been shown to correspond to lumbar BMD SDS using DXA in pediatric cohorts [26,27,28].
Questionnaires
To obtain information about fractures and skeletal-associated pain, participants completed a specific questionnaire which was adapted from the questionnaire of the Child and Adolescent Health Survey (KiGGS) from the Robert Koch Institute (RKI) [29]. The questions of the RKI are based on the pain-related questionnaire according to Perquin et al. [30, 31]. Based on 35 questions, the 3-month pain prevalence, localization, frequency of occurrence, intensity and the first occurrence of pain were recorded. In addition, questions were asked about individual factors related to the development, consequences, and negative impact of pain to determine whether increased pain limits movement and exercise in this cohort. In a second step, information on the number and type of fractures in the previous history was asked [31].
Osteoclast differentiation & TRAP-staining
Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA whole blood samples by density centrifugation from 7 study subjects and 7 matched controls. PBMCs were seeded in triplicates at a density of 1 × 106/cm2 in α-MEM (Pan Biotech, Aidenbach, Germany), 10% FBS (Gibco™, ThermoFisher, Darmstadt, Germany), 1% Pen-Strep (Pan Biotech, Aidenbach, Germany), and 25 ng/ml macrophage colony-stimulating factor (M-CSF; PeproTech, Hamburg, Germany). After 3 days, 50 ng/ml receptor activator of NF-κB ligand (RANKL; PeproTech, Hamburg, Germany) was added additionally. Cells were incubated at 37 °C and 5% CO2 with changes of medium every 2–3 days. To assess osteoclast differentiation, cells were stained for tartrate-resistant acid phosphatase (TRAP, Acid Phosphatase Kit 387-A; Sigma-Aldrich, St. Louis, MO) after 14 days of differentiation. Four sections of each well were photographed using a Axiocam 305 color, Zeiss camera. Osteoclasts of all sections were quantified using ImageJ software (Fiji; National Institutes of Health, Bethesda, USA). Multinucleated (≥ 3 nuclei), TRAP-positive cells were counted as osteoclasts.
Statistical analysis
Statistical analysis was performed using Jamovi 2.3 version 1.6 for Mac (The Jamovi project [2021]) [32]. Data are presented as mean ± standard deviation (SD) of mean, data for osteoclast counts are presented as median and 25th and 75th percentile. Data were tested for normal distribution using the Shapiro-Wilk test. Figures were created using GraphPad Prism version 9.5.1 for macOS (GraphPad Software, San Diego, California USA) [33].
Results
Participants
Twenty individuals with KS (mean age of 16.10 ± 4.28, range 9.26–25.40 years) were included in this pilot cohort study. The descriptive statistics are shown in Table 1, the individual results are shown in suppl. Tables 1 and 2.
The pubertal status was determined as following: Tanner 1 = 2; Tanner 2 = 2; Tanner 3 = 2; Tanner 4 = 4; Tanner 5 = 10. 7 participants received a testosterone replacement therapy (testosterone undecanoate 250 mg/3–4 weekly (n = 3); testosterone undecanoate 1000 mg/3 monthly (n = 1), testosterone gel 25–50 mg/transdermal daily (n = 3)). Additional medication included: Lisdexamfetamin (n = 2), methylphenidate (n = 2) and salmeterol/fluticasone (n = 1).
The average body fat percentage was 22.93 ± 9.09%, with 5 participants in the overfat range according to age-appropriate norm values (defined as 95th to 98th percentile for < 18 years according to McCarthy et al. or body fat % 20–25 for ≥ 18 years according to Gallagher et al.) and 5 participants in the obese range (defined as > 98th percentile for < 18 years or body fat % > 25 for ≥ 18 years) [34, 35].
One or more fractures had occurred in 4 participants, all of those were fractures of the wrists. Regarding skeletal pain, 9 participants reported general pain and 8 back pain within the last three months.
Biochemical results
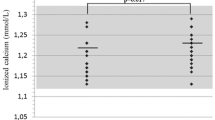
A vitamin D deficiency (serum 25OHD < 20 ng/ml) was present in 8 participants. Three participants supplemented vitamin D3. Serum calcium and phosphate values were unremarkable and serum PTH was within the normal range in all but one participant. Calcium excretion in the urine was in the normal range (Fig. 1A, supp. Table 2).
(A) Hormonal parameters of calcium-phosphate metabolism; circles: PTH = parathyroid hormone (pg/ml; left y-axis), squares: 1,25(OH)2VD = 1,25 dihydroxy vitamin D (pg/ml; left y-axis), triangles: 25OHD = 25 hydroxy vitamin D (ng/ml; right y-axis); red filling indicate readings outside of the age-appropriate reference range. (B) Leptin was elevated (expressed as SDS, n = 19); the dotted line represents the upper range of normal
The osteoanabolic markers osteocalcin (OC) and bone specific alkaline phosphatase (BAP) as well as the bone resorption markers beta-crosslinks and deoxypyridinoline in urine were within age-appropriate norms with one exception (supp. Figure 1, supp. Table 2). Leptin SDS was elevated with a mean of 2.15 ± 1.19 (range − 0.78–4.00; Fig. 1B). Parameters of thyroid function, Cortisol and IGF-1 were within normal ranges. For comprehensive biochemical results refer to supp. Table 2.
Measurement of BHI/BMD
The mean BMD z-scores were within the normal range in 6 participants (BMD L1-L4 z-score: -0.92 ± 1.00, range − 1.70–0.90, left femur BMD z-score − 0.63 ± 0.98, range − 1.50–1.20). In participants aged 9 to 17 years (n = 14), the BHI SDS was determined and was lower than expected (-1.42 ± 1.22, range − 3.04–0.94, p-value < 0.001) (Table 1; Fig. 2).
Results for radiographic measurements: Radiogrammatic assessment of the bone health index (BHI) SDS from X-rays of the left hand (n = 14; blue squares; blue square at BHI SDS = -1.12 corresponds to the BHI for two participants aged 14 years) and z-scores of bone mineral density (BMD) scans at the lumbar spine (L1-L4) using DXA methodology in 6 participants (orange squares)
Osteoclast differentiation in vitro
The number of osteoclasts did not differ in participants with KS (36 [11, 108]; median [P25, P75]) compared to healthy controls (36 [11, 152]; Fig. 3A). Osteoclasts from participants with KS showed a greater variance in their measured size (10.0 [7.12, 19.9] x103 µm2) compared to controls (10.3 [8.92, 11.3] x103 µm2). However, these differences were not statistically significant (Fig. 3B).
Comprehensive assessment
Data on this cohort expand to previously published data on cardiorespiratory fitness during ergometer testing. No statistically significant association between BHI-SDS/BMD Z-score and Fat mass, testosterone levels or maximum workload (Watt) SDS during the ergometer testing was detectable. The relevant items are combined in Table 2.
Discussion
Data on bone health and osteopathologies in men with KS have been largely limited to adults. In this cross-sectional study, we examined bone health comprehensively in a small cohort of boys and adolescents. The main finding of our study is a lower-than-expected bone density measured as BMD or BHI, despite age-appropriate bone turnover markers and unremarkable osteoclast differentiation.
While it is well known that skeletal growth and bone mineralization are influenced substantially by genetic background, hormonal factors, an unfavorable body composition and reduced physical activity may also influence bone quality [36, 37]. Hypogonadism in form of low testosterone in men is associated with decreased bone mass [36, 38]. In KS, the onset of gonadal failure is highly variable but usually does not occur before the onset of puberty. Accordingly, previous studies have shown a reduced BMD post puberty and in adulthood [1, 6, 8, 39]. Stagi et al. examined bone health in 40 Italian children and adolescents with KS and found reduced levels of 25-OH vitamin D and bone formation markers such as bone-specific alkaline phosphatase (BAP) and osteocalcin, as well as higher parathyroid hormone (PTH) levels [16]. The influence of testosterone therapy on the skeletal phenotype in boys and adolescents with KS has been discussed controversially, but most recent study results show an improvement in BMD with androgen therapy and provide evidence that testosterone levels may play a role already in prepubertal age [10, 18]. However, the present study suggests that BMD is in the low-normal range already in prepubertal boys and BMD was also reduced in the majority of participants with TRT. These results are in line with a recent report by Krabbe et al. suggesting additional contributors than low testosterone to reduced BMD in KS [18]. In addition, Vogiatzi et al. also showed low cortical bone mass in prepubertal or pubertal boys with KS and further showed that treatment with oxandrolone improved cortical BMD at the hand expressed as BHI SDS after 2 years compared to the placebo group [10]. In summary, these results suggest that the cause of reduced BMD in KS is multifactorial and that testosterone deficiency is relevant, but not the only, factor.
In addition to hypogonadism, vitamin D deficiency and a resulting calcium deficiency is often discussed in poor bone health. Nearly 40% of the individuals studied in this cohort showed a vitamin D deficiency (defined as 25-OH vitamin D levels < 20 nmol/l). However, these numbers are similar to other reports both in childhood and adulthood and may not explain an effect of vitamin D deficiency to the skeletal system [17, 40]. In fact, a secondary elevation of PTH indicating a relevant calcium deficiency, was only observed in 1 participant thus making it unlikely that the low vitamin D levels contribute to the low BMD in a relevant way. Other laboratory markers of bone formation and resorption were mostly within normal ranges in this cohort. Of note, these results contradict those of Stagi et al. who reported reduced BMD as well as reduced levels of bone formation markers (BAP and OC) in prepubertal individuals with KS [16].
While the rate and type of the reported fractures were unremarkable in this cohort, about 40% of the participants reported back pain within the last 3 months. Back pain in childhood is considered a potential symptom of vertebral fractures, but participants were asymptomatic during clinical investigation and the lumbar DXA scans were unremarkable. Individuals with KS report musculoskeletal pain more frequently than the general population [5] but it is feasible that the reported back pain is an early manifestation of a bone disease which is more prevalent in KS [6].
Physical activity is closely linked to bone health [41,42,43]. The transmission of mechanical stimuli to the skeletal system mediated by osteocytes induces the maintenance and increase of bone strength [44, 45]. Koedijk et al. reviewed a negative correlation between sedentary behaviour and BMD at the femoral neck in young adults [46]. A Danish study on quality-of-life reported reduced physical activity in adults with KS [14] and is supported by recent findings of our group showing impaired cardiorespiratory fitness as well as increased sedentary behaviour in a small group of adolescents with KS compared to age matched male controls. In that study participants with KS achieved only one tenth of the WHO’s recommended weekly physical activity, a finding that may also have an impact on bone health [4, 47].
Interestingly, leptin levels in this study were elevated on average two standard deviations regardless of age. While the BMI was in the normal range, the percentage body fat was increased on average which may explain the higher leptin levels. As reviewed elsewhere before [48], leptin itself has a controversial effect on bone via two different signaling pathways: Leptin appears to have an osteo-catabolic effect via a central pathway by binding to hypothalamic receptors and activating two sympathomimetic-mediated processes, first by indirectly inhibiting osteoblast proliferation and second by upregulating RANKL expression [49]. In contrast, peripherally leptin interacts with bone marrow stem cells (BMCs), osteoblasts, and osteoclasts, via the leptin receptor [50, 51]. Thereby, it promotes the proliferation of BMCs from the bone marrow and the differentiation into the osteoblastic lineage, and directly inhibits RANKL secretion in osteoblasts [52, 53]. Interestingly, Thomas et al. could show that leptin is positively associated with BMD in women, but not in men [54]. It is feasible that in KS the differentiation of BMCs into the adipose lineage is favoured thereby creating a subnormal osteoblast-lineage.
Considering osteoclasts as a possible factor for increased bone resorption leading to low BMD in KS individuals, the ex vivo assay for differentiation showed no difference between osteoclasts of patients and control subjects regarding number and size of cells. Thus, the question whether an alteration in bone formation could be present and contribute to the lower bone density of people with KS prevails. Subsequent studies should therefore focus on the role of osteoblasts as a potential cause of reduced bone mass in KS.
Limitations
The study is limited by the small number of participants. Therefore, the generation of larger cohorts is necessary to strengthen the validity. For the clinical parameters published reference values and normative cut offs were used, but no control group established. Longitudinal data are necessary especially in comparison of individuals with/without testosterone substitution. For the development of longitudinal data, we encouraged all of the participants to join the International Registries For Rare Conditions Affecting Sex Development & Maturation (I-DSD, https://sdmregistries.org/i-dsd/). [55].
Conclusions
Participants with KS had lower than expected bone mineral density readings already at a prepubertal age despite slightly increased height and normal BMI, and normal parameters of bone metabolism. The elevated leptin levels indicate at a potential inverse relationship of bone and adipose tissue in this cohort. However, given the complex and diverse phenotype in individuals with KS, these results underline the theory that the reduced BMD is caused by a multifactorial process which requires further investigations.
Data availability
No datasets were generated or analysed during the current study.
References
Kanakis GA, Nieschlag E (2018) Klinefelter syndrome: more than hypogonadism. Clinical and experimental, vol 86. W.B. Saunders, Metabolism
Bojesen A, Juul S, Gravholt CH (2003) Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. ;88(2)
Abramsky L, Chapple J (1997) 47,XXY (Klinefelter Syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. ;17(4)
Spiekermann J, Sinningen K, Hanusch B, Kleber M, Schündeln MM, Kiewert C et al (2023) Cardiorespiratory fitness in adolescents and young adults with Klinefelter syndrome – a pilot study. Front Endocrinol (Lausanne). ;14
Franik S, Fleischer K, Kortmann B, Stikkelbroeck NM, D’Hauwers K, Bouvattier C et al (2023) Quality of life in men with Klinefelter syndrome: a multicentre study. Endocr Connect. ;12(10)
Ferlin A, Schipilliti M, Di Mambro A, Vinanzi C, Foresta C (2010) Osteoporosis in Klinefelter’s syndrome. Mol Hum Reprod 16(6):402–410
Zitzmann M, Aksglaede L, Corona G, Isidori AM, Juul A, T’Sjoen G et al (2021) European academy of andrology guidelines on Klinefelter Syndrome Endorsing Organization: European Society of Endocrinology. Vol. 9, Andrology
Bojesen A, Birkebæk N, Kristensen K, Heickendorff L, Mosekilde L, Christiansen JS et al (2011) Bone mineral density in Klinefelter syndrome is reduced and primarily determined by muscle strength and resorptive markers, but not directly by testosterone. Osteoporos Int. ;22(5)
Kübler A, Schulz G, Cordes U, Beyer J, Krause U (1992) The influence of Testosterone Substitution on Bone Mineral density in patients with Klinefelter’s syndrome. Experimental Clin Endocrinol Diabetes. ;100
Vogiatzi MG, Davis SM, Ross JL (2021) Cortical bone Mass is low in boys with Klinefelter Syndrome and improves with Oxandrolone. J Endocr Soc. ;5(4)
De Schepper J, Ernst C, Gies I (2017) Bone health index in boys and adolescents with klinefelter syndrome. Osteoporos Int. ;28
Gravholt CH, Jensen AS, Høst C, Bojesen A (2011) Body composition, metabolic syndrome and type 2 diabetes in Klinefelter syndrome. Vol. 100, Acta Paediatrica, International Journal of Paediatrics
Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P et al (2006) The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. ;29(7)
Skakkebæk A, Moore PJ, Chang S, Fedder J, Gravholt CH (2018) Quality of life in men with Klinefelter syndrome: the impact of genotype, health, socioeconomics, and sexual function. Genet Sci 20(2):214–222
Aksglaede L, Molgaard C, Skakkebæk NE, Juul A (2008) Normal bone mineral content but unfavourable muscle/fat ratio in Klinefelter syndrome. Arch Dis Child 93(1):30–34
Stagi S, Di Tommaso M, Manoni C, Scalini P, Chiarelli F, Verrotti A et al (2016) Bone mineral status in children and adolescents with klinefelter syndrome. Int J Endocrinol. ;2016
Ferlin A, Selice R, Di Mambro A, Ghezzi M, Di Nisio A, Caretta N et al (2015) Role of vitamin D levels and vitamin D supplementation on bone mineral density in Klinefelter syndrome. Osteoporos Int. ;26(8)
Krabbe L, Valdemar H, Holm PJ, Laub AL, Holm JT, Peter C et al (2023) Reproductive hormones, Bone Mineral content, body composition and testosterone therapy in boys and adolescents with Klinefelter syndrome
Glöckner L Deutsche Klinefelter-Syndrom Vereinigung e.V. [Internet]. [cited 2022 Sep 30]. https://www.klinefelter.de/cms/index.php
Köpl B 47xxy Klinefelter Syndrom e.V. – Klinefelter Syndrom e.V. [Internet]. [cited 2022 Sep 30]. https://www.47xxy-klinefelter.de/
Schündeln MM, Goretzki SC, Hauffa PK, Wieland R, Bauer J, Baeder L et al (2014) Impairment of bone health in pediatric patients with hemolytic anemia. PLoS ONE. ;9(10)
Schündeln MM, Hauffa PK, Munteanu M, Kiewert C, Unger N, Bauer JJ et al (2020) Prevalence of osteopathologies in Children and adolescents after diagnosis of Acute Lymphoblastic Leukemia. Front Pediatr. ;8
Rosario AS, Kurth BM, Stolzenberg H, Ellert U, Neuhauser H (2010) Body mass index percentiles for children and adolescents in Germany based on a nationally representative sample (KiGGS 2003–2006). Eur J Clin Nutr. ;64(4)
World Health Organization (2000) Obesity: preventing and managing the global epidemic. World Health Organization: Tech Rep Ser WHO Technical Report Series, 894:894
Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Müller J et al (1997) Plasma leptin levels in healthy children and adolescents: dependence on body Mass Index, Body Fat Mass, gender, Pubertal Stage, and Testosterone*. J Clin Endocrinol Metab. ;82(9)
Thodberg HH, Van Rijn RR, Tanaka T, Martin DD, Kreiborg S (2010) A paediatric bone index derived by automated radiogrammetry. Osteoporos Int. ;21(8)
Martin DD, Heckmann C, Neuhof J, Jenni OG, Ranke MB, Binder G (2012) Comparison of radiogrammetrical metacarpal indices in children and reference data from the First Zurich Longitudinal Study. Pediatr Radiol. ;42(8)
Schündeln MM, Marschke L, Bauer JJ, Hauffa PK, Schweiger B, Führer-Sakel D et al (2016) A piece of the puzzle: the bone health index of the BoneXpert software reflects cortical bone mineral density in pediatric and adolescent patients. PLoS ONE. ;11(3)
Hölling H, Schlack R, Kamtsiuris P, Butschalowsky H, Schlaud M, Kurth BM (2012) Die KiGGS-Studie: Bundesweit repräsentative Längs- und Querschnittstudie Zur Gesundheit Von Kindern Und Jugendlichen Im Rahmen Des Gesundheitsmonitorings am Robert Koch-Institut. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 55:6–7
Perquin CW, Hazebroek-Kampschreur AAJM, Hunfeld JAM, Van Suijlekom-Smit LWA, Passchier J, Van Der Wouden JC (2000) Chronic pain among children and adolescents: physician consultation and medication use. Clin J Pain. ;16(3)
Ellert U, Neuhauser H, Roth-Isigkeit A (2007) Schmerzen Bei Kindern Und Jugendlichen in Deutschland: Prävalenz Und Inanspruchnahme Medizinischer Leistungen: Ergebnisse Des Kinder- Und Jugendgesundheitssurveys (KiGGS). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 50(5–6):711–717
The jamovi project (2021) jamovi (Version 1.6) [Computer Software]. [Internet]. [cited 2022 Sep 30]. https://www.jamovi.org/
GraphPad Prism version 9.5.1. for macOS, GraphPad Software, San Diego, California USA [Internet]. [cited 2023 Mar 17]. https://www.graphpad.com/
McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM (2006) Body fat reference curves for children. Int J Obes 30(4):598–602
Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y (2000) Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. ;72(3)
Finkelstein JS, Klibanski A, Neer RM, Greenspan SL, Rosenthal DI, Crowley WF (1987) Osteoporosis in men with idiopathic hypogonadotropic hypogonadism. Ann Intern Med. ;106(3)
Kindler JM, Lewis RD, Hamrick MW (2015) Skeletal muscle and pediatric bone development, vol 22. Current Opinion in Endocrinology, Diabetes and Obesity
Seeman E (1995) The dilemma of osteoporosis in men. Am J Med. ;98(2 SUPPL. 1)
Ferlin A, Schipilliti M, Vinanzi C, Garolla A, Di Mambro A, Selice R et al (2011) Bone mass in subjects with klinefelter syndrome: role of testosterone levels and androgen receptor gene cag polymorphism. J Clin Endocrinol Metab. ;96(4)
Akcan N, Poyrazoğlu Ş, Baş F, Bundak R, Darendeliler F (2018) Klinefelter syndrome in childhood: variability in clinical and molecular findings. JCRPE J Clin Res Pediatr Endocrinol 10(2):100–107
Brooke-Wavell K, Skelton DA, Barker KL, Clark EM, De Biase S, Arnold S et al (2022) Strong, steady and straight: UK consensus statement on physical activity and exercise for osteoporosis. Br J Sports Med. ;56(15)
Pelegrini A, Klen JA, Costa AM, Bim MA, Claumann GS, De Angelo HCC et al (2020) Association between sedentary behavior and bone mass in adolescents. Osteoporos Int. ;31(9)
Zhang S, Huang X, Zhao X, Li B, Cai Y, Liang X et al (2022) Effect of exercise on bone mineral density among patients with osteoporosis and osteopenia: a systematic review and network meta-analysis. 31, J Clin Nurs
Manske SL, Lorincz CR, Zernicke RF (2009) Bone health: part 2, physical activity. 1, Sports Health
Choi JUA, Kijas AW, Lauko J, Rowan AE (2022) The Mechanosensory Role of Osteocytes and implications for Bone Health and Disease States. Frontiers in Cell and Developmental Biology, vol 9. Frontiers Media S.A.
Koedijk JB, van Rijswijk J, Oranje WA, van den Bergh JP, Bours SP, Savelberg HH et al (2017) Sedentary behaviour and bone health in children, adolescents and young adults: a systematic review. Vol. 28, Osteoporosis International
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G et al (2020) World Health Organization 2020 guidelines on physical activity and sedentary behaviour. British Journal of Sports Medicine, vol 54. BMJ Publishing Group, pp 1451–1462
Chen XX, Yang T (2015) Roles of leptin in bone metabolism and bone diseases. Journal of Bone and Mineral Metabolism, vol 33. Springer Tokyo, pp 474–485
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL et al (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell. ;111(3)
Flier JS (1997) Leptin expression and action: New experimental paradigms. Vol. 94, Proceedings of the National Academy of Sciences of the United States of America
Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY et al (2011) Leptin in human physiology and pathophysiology, vol 301. American Journal of Physiology - Endocrinology and Metabolism
Reseland JE, Syversen U, Bakke I, Qvigstad G, Eide LG, Hjertner et al (2001) Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Miner Res. ;16(8)
Astudillo P, Ríos S, Pastenes L, Pino AM, Rodríguez JP (2008) Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) is characterized by impaired leptin action. J Cell Biochem. ;103(4)
Thomas T, Burguera B, Melton LJ, Atkinson EJ, O’Fallon WM, Riggs BL et al (2001) Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone. ;29(2)
International Registries For Rare Conditions Affecting Sex Development & Maturation (I-DSD) [Internet]. [cited 2024 Aug 18]. https://sdmregistries.org/
Acknowledgements
We thank the participants and families of this study and the patient organizations (Deutsche Klinefelter Syndrom Vereinigung e.V. and 47,XXY Klinefelter Syndrom e.V.) for their support of this study.
Funding
The KliBone study was supported by funding from the ‘Deutsche Gesellschaft für Sozialpädiatrie und Jugendmedizin’ and the ‘Elsbeth Bonhoff Foundation’.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
The authors have accepted responsibility for the entire content of this manuscript and approved its submission. Study design: CG, JH, JS Study conduct and recruitment: CG, JS, JH, BH, KS, CK, HS, EI. Data analysis and interpretation: JS, JH, CG. Drafting manuscript: JS, JH, CG Revising manuscript content: All authors. Approving final version of manuscript: All authors.
Corresponding author
Ethics declarations
Statement of ethics
This study protocol was reviewed and approved by Ruhr-University Bochum Ethics Committee (#21-7164). Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spiekermann, J., Höppner, J., Ibnukhsein, E. et al. Description of bone health in adolescents and young persons with Klinefelter syndrome – results from a pilot study. Mol Cell Pediatr 11, 9 (2024). https://doi.org/10.1186/s40348-024-00182-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40348-024-00182-w