Abstract
Context
The previous studies suggested a possible increased risk of hypercalcaemia and reduced bone mineral density (BMD) in Williams’ syndrome (WS). However, an extensive study regarding bone metabolism has never been performed.
Objective
To investigate bone health in young adults with WS.
Design
Cross-sectional study.
Settings
Endocrinology and Metabolic Diseases and Medical Genetic Units.
Patients
29 WS young adults and 29 age- and sex-matched controls.
Main outcome measures
In all subjects, calcium, phosphorus, bone alkaline phosphatase (bALP), parathyroid hormone (PTH), 25-hydroxyvitamin D (25OHVitD), osteocalcin (OC), carboxyterminal cross-linking telopeptide of type I collagen (CTX), 24-h urinary calcium and phosphorus, femoral-neck (FN) and lumbar-spine (LS) BMD and vertebral fractures (VFx) were assessed. In 19 patients, serum fibroblast growth factor-23 (FGF23) levels were measured.
Results
WS patients showed lower phosphorus (3.1 ± 0.7 vs 3.8 ± 0.5 mg/dL, p = 0.0001) and TmP/GFR (0.81 ± 0.32 vs 1.06 ± 0.25 mmol/L, p = 0.001), and an increased prevalence (p = 0.005) of hypophosphoremia (34.5 vs 3.4%) and reduced TmP/GFR (37.9 vs 3.4%). Moreover, bALP (26.3 ± 8.5 vs 35.0 ± 8.0 U/L), PTH (24.5 ± 12.6 vs 33.7 ± 10.8 pg/mL), OC (19.4 ± 5.3 vs 24.5 ± 8.7 ng/mL), and FN-BMD (− 0.51 ± 0.32 vs 0.36 ± 0.32) were significantly lower (p < 0.05), while CTX significantly higher (401.2 ± 169.3 vs 322.3 ± 122.4 pg/mL, p < 0.05). Serum and urinary calcium and 25OHVitD levels, LS-BMD and VFx prevalence were comparable. No cases of hypercalcemia and suppressed FGF23 were documented. Patients with low vs normal phosphorus and low vs normal TmP/GFR showed comparable FGF23 levels. FGF23 did not correlate with phosphorus and TmP/GFR values.
Conclusions
Adult WS patients have reduced TmP/GFR, inappropriately normal FGF23 levels and an uncoupled bone turnover with low femoral BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Williams’ syndrome (WS; OMIM #194050) is a rare genetic disorder caused by a hemizygous microdeletion on chromosome 7q11.23 with a prevalence ranging from 1/7500 to 1/20,000 live births [1,2,3]. The clinical phenotype is widely heterogeneous and is mainly characterized by unique facial dysmorphisms and personality, mild intellectual disability, growth retardation, impaired glucose metabolism, thyroid dysfunction, and cardiovascular abnormalities [4, 5]. In the past, some studies found an increased risk of hypercalcaemia in WS patients. In particular, mild hypercalcaemia [serum calcium < 11.5 mg/dL (2.88 mmol/L)] has been reported in 5–50% of WS patients [6,7,8,9], and some cases of severe hypercalcemia have been described in childhood [10]. The causal factor has not been clarified and various mechanisms, including a pathological metabolism of vitamin D or calcitonin, have been hypothesized [11, 12]. In addition, a previous not-controlled study suggested that in WS, a reduced bone mineral density (BMD) could be present [13], but data regarding calcium–phosphorous metabolism, bone turnover, and the presence of asymptomatic vertebral fractures (VFx) in WS patients are completely lacking.
Therefore, the aim of our study was to assess bone involvement in a population of young adults with WS compared with a control group by evaluating calcium–phosphorus metabolism, bone turnover indexes, the BMD, and the prevalence of morphometric VFx.
Subjects and methods
Subjects
From February to June 2016, 33 WS patients referred to the Medical Genetic Unit of our hospital were evaluated for the enrolment in the study. The diagnosis of WS was made clinically and confirmed in all cases by the typical ELN gene hemizygosity shown by FISH. None of the patients’ parents had the clinical features of WS, thus suggesting that all cases were de novo presentations. The exclusion criteria were the following: past or current history of diseases known to affect bone tissue (i.e., thyrotoxicosis, bowel diseases, chronic hepatic or renal diseases, alcoholism, rheumatologic or hematologic diseases, hypogonadism in males, and postmenopausal status in females) and intake of diuretics or drugs influencing bone metabolism. Eventually, 29 WS young patients (13 females and 16 males, mean age 28.8 ± 5.5 years) were included in the study. During the same period of time, 29 subjects matched for age and sex were consecutively enrolled as controls on the basis of the above-mentioned exclusion criteria from our outpatient clinics for endocrine diseases, where they were referred for unrelated diseases (multinodular goiter with normal thyroid function without others’ pathological conditions).
In all patients and controls, besides medical reports, clinical history and general chemistry profile, serum calcium, phosphorus, albumin, bone alkaline phosphatase (bALP), parathyroid hormone (PTH), 25-hydroxyvitamin D (25OHVitD), osteocalcin (OC), and serum carboxyterminal cross-linking telopeptide of type I collagen levels (CTX), urinary calcium and phosphorus in 3- and 24-h urine collection were measured. In 19 patients, we also evaluated serum fibroblast growth factor 23 (FGF23) levels on samples stored in a − 20 °C freezer until the time of testing. In all subjects, the BMD at femoral neck (FN) and lumbar spine (LS) and VFx were assessed.
Methods
Weight and height were measured by standard procedures; body mass index (BMI) was calculated according to the formula weight (kg)/[height (m)]2 [14].
Serum and urinary samples were collected between 8 a.m. and 9 a.m. after 8-h fasting. All samples were analysed in the same laboratory. Serum and urinary calcium and phosphorus, albumin, and bALP were measured by the standard techniques [reference intervals: serum calcium 8.4–10.2 mg/dL (2.1–2.6 mmol/L), serum phosphorus 2.7–4.5 mg/dL (0.87–1.45 mmol/L), bALP 15–60 U/L]. Total calcium was corrected for serum albumin (Caalb adj) according to the formula Caalb adj (milligrams per deciliter) = total calcium + [(4.4 − albumin in grams per deciliter) × 0.8] [15]. Measurement of ionized calcium was made using calcium ion-selective electrode direct potentiometry (reference interval 1.13–1.32 mmol/L). Hypercalciuria was defined as urinary calcium excretion of ≥ 4-mg/kg body weight/day in both sexes [16]. Serum intact PTH levels were measured by a two-step automated sandwich chemiluminescent immunoassay (CLIA; DiaSorin), with intra- and interassay coefficients of variation (CVs) < 10% [reference interval 6.5–36.8 pg/mL (6.5–36.8 ng/L)]. Serum 25(OH)VitD concentration was measured by radioimmunoassay (RIA; DiaSorin), with intra- and interassay CVs of 7.2 and < 12%, respectively [reference interval 30–120 ng/mL (75–300 nmol/L)]. Serum OC [normal values, 5–65 ng/mL (µg/L)] was measured by the Invitrogen human Osteocalcin Enzyme Amplified Sensitivity Immunoassay (Life Technologies) and serum CTX [normal values, 140–1350 pg/mL (ng/L)] was determined by the Serum CrossLaps ELISA (Immunodiagnostic System Ltd) according to the manufacturer’s assay procedure. Serum intact FGF23 was analysed by a two-site enzyme-linked immunosorbent assay (ELISA; Kainos Laboratories inc, Tokyo, Japan) with a reference interval of 10–50 pg/mL [1.33–6.65 pmol/L] [17]. The ratio of tubular maximum reabsorption of phosphate to glomerular filtration rate (TmP/GFR), normal values 2.5–4.2 mg/dL [0.80–1.35 mmol/L], was determined using the Walton and Bijvoet nomogram [18].
Bone mineral density (BMD) was measured by dual-energy X-ray Absorptiometry (DXA; Hologic Discovery QDR series, Waltham, MA, USA) at LS (in vivo precision 1.0%) and FN (in vivo precision 1.8%). Individual BMD values were expressed as SD units (Z-scores). Fractured vertebrae were excluded from BMD measurement.
Conventional spinal radiographs in the lateral (T4–L4) and anteroposterior projections were obtained in all patients by a standardized technique. Two physicians, blinded to the BMD and biochemical data, independently reviewed the radiographs. The questionable cases were discussed to reach an agreed diagnosis. VFx were diagnosed on visual inspection using the semi-quantitative visual assessment described by Genant et al., defined as a reduction of > 20% in anterior, middle, or posterior vertebral height [19].
Statistical analysis
Statistical analysis was performed by SPSS version 21.0 (SPSS Inc, Chicago, IL). The results are expressed as mean ± standard deviation (SD) or medians ± standard error (SE) in the case of non-Gaussian distribution. The normality of distribution was tested by the Kolmogorov–Smirnov test. The comparison of continuous variables was performed using the Student t test or Mann–Whitney U test as appropriate. Categorical variables were compared by the χ2 test or Fisher exact test as appropriate. The comparison of the continuous variables among patients and controls, among patients with low and normal phosphorus and TmP/GFR values and among patients with high and normal FGF23 values, was performed by one-way ANOVA. Logistic regression analysis was used to assess in patients the possible independent associations between the presence of low phosphorus and low TmP/GFR values (dependent variables) with age, BMI, sex, serum calcium, 25OHVitD, bALP, PTH, CTX, OC, FGF23 levels, urinary calcium levels, BMD and VFx (independent variables).
P values ≤ 0.05 were considered significant.
Results
The comparison of the basal clinical and biochemical characteristics between WS patients and controls is reported in Table 1. Age, sex, and BMI were comparable between patients and controls. As expected for the disease, the height of the patients was significantly lower compared to controls. Considering endocrine comorbidities associated with WS, 4 out of 29 (14%) patients had diabetes mellitus type 2 (one patient on metformin therapy, 500 mg tid, and three patients on diet therapy) and 5 out of 29 (17%) patients had primary hypothyroidism on replacement therapy with levothyroxine. Both diabetic and hypothyroid patients had a good control of the diseases (glycated hemoglobin < 53 mmol/mol, TSH values between 0.9 and 4.0 µIU/mL). Moreover, 12 out of 29 (41%) WS patients had one or more cardiovascular abnormalities (five patients with supravalvular aortic stenosis, one patients with supravalvular pulmonary stenosis, one patient with peripheral pulmonary stenosis, one patient with aortic narrowing, six patients with mitral valve prolapse, one patient with aortic regurgitation, and two patients with ventricular or atrial septal defects) and 19/29 (65%) were hypertensive. The fracture history was collected in all patients: no peripheral low trauma fractures were reported, and on the other side, no patients told of any accident or traumatic fall.
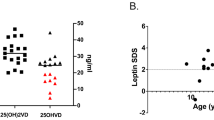
All patients and controls had normal renal function. No differences were found in serum calcium levels between patients and controls, even if the formers showed slightly higher serum calcium levels than the letters. In particular, no patient was hypercalcemic at basal evaluation. The ionized calcium levels were significantly higher in patients than in controls albeit always in the normal range (p = 0.017) (Fig. 1). In particular, female WS patients tended to have higher blood calcium values than males (p = 0.06).
No difference was found in 24-h urinary calcium levels between patients and controls but interestingly, female WS patients had an increased 24-h urinary calcium excretion than males (p = 0.005). This difference was absent in controls. 5 (17%) WS patients had hypercalciuria, and of note, four out of five patients with hypercalciuria were female.
Compared with controls, WS patients showed significantly lower serum phosphorus levels, an increased prevalence of hypophosphatemia [serum phosphorus < 2.7 mg/dL (< 0.87 mmol/L)] (p = 0.005), a significantly lower TmP/GFR and increased prevalence of reduced TmP/GFR [< 2.5 mg/dL (< 0.80 mmol/L)] (p = 0.005). None of the ten patients with low phosphorus levels was symptomatic for hypophosphatemia and no differences were seen in phosphorus serum levels between male and female WS patients. The bALP, PTH, and OC levels were significantly lower, while the CTX levels were significantly higher in patients than in controls. No differences were found in 25OHVitD levels and in the prevalence of low 25OHVitD levels [i.e., serum vitamin D < 30 ng/mL (75 nmol/L)] between the two groups. Compared with controls, WS patients showed a reduced FN-BMD, while no differences were found in LS-BMD and in the prevalence of VFx between the two groups.
FGF23 was elevated [> 50 pg/mL (6.65 pmol/L)] in six out of 19 (31.5%) tested WS patients. All biochemical and bone metabolism parameters and, in particular, phosphorus levels were comparable between WS patients with normal or elevated FGF23 levels. No patients had suppressed FGF23 values.
The comparison of the basal clinical and biochemical characteristics between WS patients with low and normal TmP/GFR is reported in Table 2. Age, sex, BMI, serum and 24-h urinary calcium levels, PTH, 25OHVitD, bone turnover markers, FGF23 levels, BMD, and prevalence of VFx were comparable between patients with low and normal TmP/GFR.
No correlation was found between FGF23 and phosphorus levels (r = − 0.07, p = 0.79) and between FGF23 and TmP/GFR values (r = − 0.04, p = 0.88). A negative correlation was found between FGF23 and CTX levels (r = − 0.56 p = 0.01).
Discussion
In this study, we evaluated the bone involvement in a group of young adults with WS compared to an age- and sex-matched control group by assessing the calcium–phosphorus metabolism, the bone turnover indexes, the BMD, and the prevalence of morphometric VFx.
Our data show that, at least in adulthood, hypercalcemia is a rare event in WS and that an hypophosphatemic disorder with a reduced TmP/GFR together with an inappropriately normal production of FGF23, an uncoupled bone turnover and a low bone mass at FN is a feature of this syndrome.
The available studies reported hypercalcemia in 5–50% of WS patients, with a greater frequency of episodes in childhood and progressive reduction in adulthood [6,7,8,9], even if when paediatric reference intervals were applied, the occurrence of hypercalcemia in childhood dropped significantly [9]. The prevalence of hypercalcemia exclusively in adult WS patients was evaluated only in one study [13]. In this work, 1 out of 20 (5%) patients showed moderately high levels of calcium [10.6 mg/dL (2.65 mmol/L)] together with an inappropriately normal PTH levels [13], thus possibly related to the presence of a primary hyperparathyroidism associated to WS. In our study, no patient was hypercalcaemic, although ionized calcium values were significantly higher in patients than controls. Similarly, in a recent study, Stagi et al. reported significantly higher ionized and total calcium levels in WS patients compared with age- and sex-matched controls [20].
The noteworthy and novel result of our study is the finding in WS patients of significantly lower serum phosphorus levels with a higher prevalence of asymptomatic hypophosphatemia with a reduced TmP/GFR representative of a renal phosphate loss disorder. In addition, despite the low phosphorus values, none of our patients had suppressed FGF23 levels, suggesting an inappropriate secretion of this phosphatonin. In particular, FGF23 values were higher in 31.5% of patients and within the normal range in the remaining 68% of patients. Up to now, only one study showed in WS patients, serum phosphate levels lower in respect to controls, but no information was available regarding the prevalence of hypophosphatemia, the TmP/GFR and FGF23 levels [20].
The possibility that this inappropriate production of FGF23 could be caused by an augmented osteocytes/osteoblasts sensitivity to serum phosphorus levels or to an impaired action or catabolism of FGF23 remain to be evaluated. In fact, several factors are involved in the complex paracrine and endocrine activity of FGF23 in the kidney, including the protein Klotho [21,22,23] and also the status of FGF23 O-glycosylation of threonine178 and FGF23 phosphorylation of serine180 that significantly influence its expression and degradation [24]. Our data, however, did not show any correlation between FGF23, phosphorus, and TmP/GFR values. This finding could be related to the low number of patients analysed, but it is conceivable that other phosphatonins, that were not assessed in our study (i.e., FGF7, MEPE, and sFRP-4), could be involved. Unfortunately, we do not have the possibility to dose FGF23 levels in our control group for a comparison. Beside this limit of the study, an analytical bias related to the assay used for FGF23 dosage cannot be excluded. In fact, it is well known that current ELISA kit for intact FGF23 showed little analytical agreement [25, 26] and that intact FGF23 appears to have greater intra-individual variation compared with his C-terminal fragment [27]. Therefore, the lack of associations between FGF23 and phosphorus and/or TmP/GFR values could be linked to the type of assay used [25, 26].
Overall, it must be considered that several aspects of FGF23 regulation remain incompletely understood. Indeed, besides the stimulatory effect of PTH (directly or via 1,25-hydroxy-cholecalciferol) on FGF23 secretion, some data suggest that hypercalcemia also stimulates FGF23 production, whereas hypocalcemia prevents normally expected FGF23 responses to phosphate overload [28, 29]. Therefore, it is not possible to exclude that in our sample of WS patients, the tendency toward hypercalcemia may have contributed in maintaining the inappropriately normal FGF23.
An additional important finding of the present study is that WS patients showed an uncoupled bone turnover (low bALP and OC and high CTX levels) associated with a reduction of cortical but not trabecular bone density. However, this is not accompanied by a statistically significant increase in the VFx prevalence, even though it must be observed that the 10% of our WS patients had a VFx, while no fractured subject was found in the control group. The previous data showed an impaired bone apposition in both adolescent and adults WS patients [13, 20], and an increased bone resorption in adults WS patients [20], together with a significantly impaired bone mineral status at quantitative bone ultrasonometry evaluation [20] and an increased prevalence of osteopenia and osteoporosis [13]. In the present study, we show for the first time that WS patients have a compromised cortical bone density at femur, while the trabecular spinal bone density seems to be preserved. Notwithstanding the normal BMD at spine, the WS patients may have an increased prevalence of VFx (10%), even though the present study is probably not enough powered to reach the statistical significance. The possible discrepancy between the possibly increased VFx prevalence and the normal spinal BMD could be explained by the presence of a reduced trabecular microarchitecture, which is not entirely captured by the DXA evaluation [30].
The bone metabolism disorder in WS patients may be linked in part to the genetic predisposition itself. In fact, the haploinsufficiency of FDZ9, a gene involved in the early stages of osteoblastogenesis and bone formation [31], could explain the low levels of OC and bALP and the reduced BMD found in WS patients. Moreover, the alterations in phosphate homeostasis may affect the skeletal metabolism and in particular the osteoblast (OB) function, as demonstrated in OB cultures derived from Hyp mice (counterparts of X-linked hypophosphatemic rickets) characterized by low bone mineralization [32, 33]. It is also possible to hypothesize that the condition of hypophosphatemia and hyperphosphaturia activates bone resorption [34, 35]. This can explain the finding of elevated CTX and serum calcium levels and lower PTH levels in WS patients. The fact that CTX levels, though higher in WS patients than in controls, were still within the normal range in the former group, may be due to the fact that in WS bone, resorption is only slightly increased. It should also observed that our patients were all eugonadal. Therefore, the normal range of CTX given by the manufacturer may be not entirely representative of the population enrolled in the present study.
Another possible causative factor of bone metabolism disorder in WS patients may be related to the reduced physical activity compared to controls as demonstrated in a previous study [20]. Neither joint mobility problems, nor physical limitations were reported by our patients, but, unfortunately, a quantitative assessment of physical activity was not carried out.
Finally, interestingly, we found a negative association between FGF23 and CTX levels. This may be explained in part by the known inhibitory effect of FGF23 on PTH levels [36], but other factors (i.e., an excess in sclerostin production) not evaluated in our study may be involved.
In conclusion, the present study shows that in WS, there is a hypophosphatemic disorder with reduced TmP/GFR together with an inappropriate secretion of FGF23. Moreover, we found an uncoupled bone turnover associated with a significantly reduced cortical bone density at femur. Longitudinal studies on larger samples and with an adequate follow-up should be designed to identify the possible causative factors and the long-term complications of these findings.
References
Morris CA (2010) Introduction: Williams syndrome. Am J Med Genet C Semin Med Genet 154C(2):203–208
Strømme P, Bjornstad P, Ramstad K (2002) Prevalence estimation of Williams syndrome. J Child Neurol 17:269–271
Schubert C (2009) The genomic basis of the Williams–Beuren syndrome. Cell Mol Life Sci 66(7):1178–1197
Pober BR (2010) Williams–Beuren syndrome. N Engl J Med 362(3):239–252 (Review)
Masserini B, Bedeschi MF, Bianchi V, Scuvera G, Beck-Peccoz P, Lalatta F, Selicorni A, Orsi E (2013) Prevalence of diabetes and pre-diabetes in a cohort of Italian young adults with Williams syndrome. Am J Med Genet A 161A(4):817–821
Sforzini C, Milani D, Fossali E, Barbato A, Grumieri G, Bianchetti MG, Selicorni A (2002) Renal tract ultrasonography and calcium homeostasis in Williams–Beuren syndrome. Pediatr Nephrol 17(11):899–902
Amenta S, Sofocleous C, Kolialexi A, Thomaidis L, Giouroukos S, Karavitakis E, Mavrou A, Kitsiou S, Kanavakis E, Fryssira H (2005) Clinical manifestations and molecular investigation of 50 patients with Williams syndrome in the Greek population. Pediatr Res 57(6):789–795
Jurado LP, Peoples R, Kaplan P, Hamel BC, Francke U (1996) Molecular definition of the chromosome 7 deletion in Williams syndrome and parent-of-origin effects on growth. Am J Hum Genet 59(4):781–792
Sindhar S, Lugo M, Levin MD, Danback JR, Brink BD, Yu E, Dietzen DJ, Clark AL, Purgert CA, Waxler JL, Elder RW, Pober BR, Kozel BA (2016) Hypercalcemia in patients with Williams–Beuren syndrome. J Pediatr 178:254–260
Cagle AP, Waguespack SG, Buckingham BA, Shankar RR, Dimeglio LA (2004) Severe infantile hypercalcemia associated with Williams syndrome successfully treated with intravenously administered pamidronate. Pediatrics 114(4):1091–1095
Garabédian M, Jacqz E, Guillozo H, Grimberg R, Guillot M, Gagnadoux MF, Broyer M, Lenoir G, Balsan S (1985) Elevated plasma 1,25-dihydroxyvitamin D concentrations in infants with hypercalcemia and an elfin facies. N Engl J Med 312(15):948–952
Culler FL, Jones KL, Deftos LJ (1985) Imparied calcitonin secretion in patients with Williams syndrome. J Pediatr 107(5):720–723
Cherniske EM, Carpenter TO, Klaiman C, Young E, Bregman J, Insogna K, Schultz RT, Pober BR (2004) Multisystem study of 20 older adults with Williams syndrome. Am J Med Genet A. 131:255–264
Garrow JS, Webster J (1985) Quetelet’s index (W/H2) as a measure of fatness. Int J Obes 9(2):147–153
Figge J, Jabor A, Kazda A, Fencl V (1998) Anion gap and hypoalbuminemia. Crit Care Med 26(11):1807–1810
Hodgkinson A, Pyrah LN (1958) The urinary excretion of calcium and inorganic phosphate in 344 patients with calcium stone of renal origin. Br J Surg 46(195):10–18
Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S (2002) Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87(11):4957–4960
Walton RJ, Bijvoet OL (1975) Nomogram for derivation of renal threshold phosphate concentration. Lancet 2(7929):309–310
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148
Stagi S, Manoni C, Scalini P, Chiarelli F, Verrotti A, Cecchi C, Lapi E, Giglio S, Romano S, de Martino M (2016) Bone mineral status and metabolism in patients with Williams–Beuren syndrome. Hormones (Athens) 15(3):404–412
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444(7120):770–774
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281(10):6120–6123
Razzaque MS, Lanske B (2007) The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol 194(1):1–10 (Review)
Frishberg Y, Ito N, Rinat C, Yamazaki Y, Feinstein S, Urakawa I, Navon-Elkan P, Becker-Cohen R, Yamashita T, Araya K, Igarashi T, Fujita T, Fukumoto S (2007) Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res 22(2):235–242
El-Maouche D, Dumitrescu CE, Andreopoulou P, Gafni RI, Brillante BA, Bhattacharyya N, Fedarko NS, Collins MT (2016) Stability and degradation of fibroblast growth factor 23 (FGF23): the effect of time and temperature and assay type. Osteoporos Int 27(7):2345–2353
Smith ER, McMahon LP, Holt SG (2013) Method-specific differences in plasma fibroblast growth factor 23 measurement using four commercial ELISAs. Clin Chem Lab Med 51(10):1971–1981
Smith ER, Cai MM, McMahon LP, Holt SG (2012) Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab 97(9):3357–3365
Pool LR, Wolf M (2017) FGF23 and nutritional metabolism. Ann Rev Nutr 21(37):247–268
Rodriguez-Ortiz ME, Lopez I, Muñoz-Castañeda JR, Martinez-Moreno JM, Ramírez AP, Pineda C, Canalejo A, Jaeger P, Aguilera-Tejero E, Rodriguez M, Felsenfeld A, Almaden Y (2012) Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol 23(7):1190–1197
Eller-Vainicher C, Cairoli E, Zhukouskaya VV, Morelli V, Palmieri S, Scillitani A, Beck-Peccoz P, Chiodini I (2013) Prevalence of subclinical contributors to low bone mineral density and/or fragility fracture. Eur J Endocrinol 169(2):225–237
Albers J, Schulze J, Beil FT, Gebauer M, Baranowsky A, Keller J, Marshall RP, Wintges K, Friedrich FW, Priemel M, Schilling AF, Rueger JM, Cornils K, Fehse B, Streichert T, Sauter G, Jakob F, Insogna KL, Pober B, Knobeloch KP, Francke U, Amling M, Schinke T (2011) Control of bone formation by the serpentine receptor Frizzled-9. J Cell Biol 192(6):1057–1072
Tenenhouse HS (1999) X-linked hypophosphataemia: a homologous disorder in humans and mice. Nephrol Dial Transplant 14(2):333–341 (Review)
Xiao ZS, Crenshaw M, Guo R, Nesbitt T, Drezner MK, Quarles LD (1998) Intrinsic mineralization defect in Hyp mouse osteoblasts. Am J Physiol 275(4 Pt 1):E700–E708
Bruin WJ, Baylink DJ, Wergedal JE (1975) Acute inhibition of mineralization and stimulation of bone resorption mediated by hypophosphatemia. Endocrinology 96(2):394–399
Baylink D, Wergedal J, Stauffer M (1971) Formation, mineralization, and resorption of bone in hypophosphatemic rats. J Clin Invest 50(12):2519–2530
Krajisnik T, Björklund P, Marsell R, Ljunggren O, Akerström G, Jonsson KB, Westin G, Larsson TE (2007) Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol 195(1):125–131
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Palmieri, S., Bedeschi, M.F., Cairoli, E. et al. Bone involvement and mineral metabolism in Williams’ syndrome. J Endocrinol Invest 42, 337–344 (2019). https://doi.org/10.1007/s40618-018-0924-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0924-y