Abstract
Background
Generation of marker-free transgenic plants is very important to the regulatory permission and commercial release of transgenic crops. Co-transformation methods that enable the removal of selectable marker genes have been extensively used because they are simple and clean. Few comparisons are currently available between different strain/plasmid co-transformation systems, and also data are related to variation in co-transformation frequencies caused by other details of the vector design.
Results
In this study, we constructed three vector systems for the co-transformation of allotetraploid Brassica napus (B. napus) mediated by Agrobacterium tumefaciens and compared these co-transformation methods. We tested a mixed-strain system, in which a single T-DNA is harbored in two plasmids, as well as two “double T-DNA” vector systems, in which two independent T-DNAs are harbored in one plasmid in a tandem orientation or in an inverted orientation. As confirmed by the use of PCR analysis, test strips, and Southern blot, the average co-transformation frequencies from these systems ranged from 24 to 81% in T0 plants, with the highest frequency of 81% for 1:1 treatment of the mixed-strain system. These vector systems are valuable for generating marker-free transgenic B. napus plants, and marker-free plants were successfully obtained in the T1 generation from 50 to 77% of T0 transgenic lines using these systems, with the highest frequency of 77% for “double T-DNA” vector systems of pBID RT Enhanced. We further found that marker-free B. napus plants were more frequently encountered in the progeny of transgenic lines which has only one or two marker gene copies in the T0 generation. Two types of herbicide resistant transgenic B. napus plants, Bar+ with phosphinothricin resistance and Bar+EPSPS+GOX+ with phosphinothricin and glyphosate resistance, were obtained.
Conclusion
We were successful in removing selectable marker genes in transgenic B. napus plants using all three co-transformation systems developed in this study. It was proved that if a appropriate mole ratio was designed for the specific length ratio of the twin T-DNAs for the mixed-strain method, high unlinked co-insertion frequency and overall success frequency could be achieved. Our study provides useful information for the construction of efficient co-transformation system for marker-free transgenic crop production and developed transgenic B. napus with various types of herbicide resistance.
Similar content being viewed by others
Background
Allotetraploid oilseed rape Brassica napus L. (B. napus) is one of the most important sources of plant oil and it is a major crop used for generating protein-rich products worldwide. To meet the increasing demand, work has been undertaken to improve existing cultivars and to generate new elite cultivars of B. napus using genetic engineering approaches, which provide an alternative approach to creating novel varieties with improved traits and desirable characteristics that can’t be obtained by traditional breeding methods [1]. Genetic transformation techniques have been used successfully for B. napus improvement by employing an Agrobacterium tumefaciens (A. tumefaciens)-mediated system [2, 3] since the first reports of successful B. napus transformation [4, 5]. Many important traits, including herbicide [6], insect [7] and fungal [8] resistance, as well as improved composition of oil [9, 10] and proteins [11] have been introduced into B. napus by transgenic methods. The safety of transgenic B. napus is facing the same questions and debates as for other transgenic crops, and one concern is over the continued presence of a selectable marker gene in the final plant product [12, 13].
Gene transfer requires the simultaneous transformation of a antibiotic or herbicide resistance selectable marker gene to select positive transgenic plants carrying the gene of interest. Once the transgenic plants have been regenerated and characterized, the selectable marker gene is no longer necessary. Moreover, transgenic plants with marker genes not only impair public acceptance due to the concerns of public health but also increase the environmental risk of the introgression of marker genes into weedy relatives and non-transgenic crops [14]. These factors complicate the regulatory process for the commercialization of genetically modified plants [15, 16]. In addition, marker genes limit the process of gene stacking through re-transformation [16]. Therefore, producing marker-free transgenic B. napus varieties, where the marker gene has been eliminated, will be beneficial to their development and eventual commercial release. Genetically modified B. napus varieties without marker genes have been commercially released in a small number of countries, including the United States, Canada, Australia and Chile thus far [17].
To date, a number of methods that enable the removal of selectable marker genes have been developed, including co-transformation [18, 19], site-specific recombination [20,21,22,23,24,25,26,27], homologous recombination [28], transposon-mediated repositioning [29] and gene editing [30]. Among these methods, co-transformation has been extensively used because it is a simple and clean technique and it doesn’t leave behind residual DNA sequences such as invert repeats and recombination sites in transgenic plants from which the selectable marker gene has been eliminated with a high frequency [16, 31, 32]. Co-transformation involves the simultaneous integration of a selectable marker gene and a gene of interest from different T-DNAs, as well as their subsequent recombination and segregation in the progeny, if the two genes are integrated into unlinked loci [31, 33]. Co-transformation has been applied in many species, including soya bean [34], tobacco [35], maize [18], sorghum [36], rice [37], durum wheat [19] and B. napus [38]. Elimination of selectable marker genes after co-transformation can be realized by using a single Agrobacterium strain system with a plasmid that contains two independent T-DNA regions (the one strain/one plasmid method or the “double T-DNA” vector system) [35], or by using a two Agrobacterium strain system harboring two plasmids that each contain an independent T-DNA (the two strains/two plasmids method or the mixed-strain system) [38]. Zhou et al. [39] and Komari et al. [40] reported that the “double T-DNA” vector system might be more effective than the mixed-strain method in producing co-transformants.
Although co-transformation strategies have been developed over the past 30 years, it is more popular in model plants, such as tobacco and rice, than in other plants. Co-transformation strategies have not yet been applied widely in B. napus, and there are few comparisons between different strain/plasmid systems, and also few studies examine variation in co-transformation frequencies caused by other details of the vector design studies. In order to obtain guidelines as to how to construct an efficient co-transformation system to produce marker-free B. napus, we established three systems and provide a comprehensive and scientific comparison of these co-transformation systems in the same Agrobacterium-mediated transformation platform with respect to B. napus and evaluated these systems with the same criteria. In the first system, the two strains/two plasmids method was applied, and the different strains were mixed in a series of ratios. In the second and third systems, the one strain/one plasmid method was employed. Double T-DNAs, arrayed in a tandem orientation or in an inverted orientation relative to each other, were introduced into one binary vector and were carried by one Agrobacterium strain (Table 1). Gene of interest and selectable marker gene from different T-DNAs were able to be simultaneously integrated in T0 and marker-free plants can be yielded after their subsequent recombination and segregation in T1. We examined the combination of co-transformation and subsequent marker-free efficacy by studying the molecular and genomic organization of the transgenic plants. Our results indicate that the systems developed in this study were effective in removing selectable marker genes, in this particular case herbicide resistant B. napus plants. Our study has obtained information about these co-transformation systems which will be valuable in future B. napus breeding projects using genetic engineering approaches.
Methods
Plant material
The allotetraploid B. napus genotype used for genetic transformation in this study is Zhongshuang 6 (ZS6), a semi-winter variety provided by the Oil Crops Research Institute of Chinese Academy of Agricultural Sciences in Wuhan, China. ZS6 is an elite Chinese cultivar due to its low erucic acid and glucosinolate levels and high oil content (39.08%). Transgenic plants and the wild type were grown in pots containing a mixture of moss peat (PINDSTRUP, Denmark) and field soil (3:1). Plants were maintained in a greenhouse under growth conditions of 20 °C ± 2 °C under a 16/8 h photo-period at a light intensity of 44 umol m−2 s−1 and 60–90% relative humidity.
Expression vectors with two independent T-DNA regions
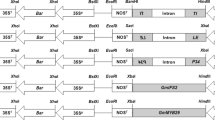
For the mixed-strain system, two commercial expression vectors, pCAMBIA1300 and pCAMBIA3300 (https://cambia.org/, YOUBIO, Hunan, China), were used for delivering two T-DNAs from mixtures of A. tumefaciens strains, each containing only one T-DNA. They are commonly used binary expression vectors in plant genetic engineering and are harmless in E. coli, Agrobacterium and plants [41]. pCAMBIA1300 contained one T-DNA with the Hygromycin phosphotransferase (HPT) gene, which was under the control of the CaMV35S promoter and followed by the CaMV poly(A) signal sequence. pCAMBIA3300 harbored one T-DNA region with the phosphinothricin N-acetyltransferase gene (Bar), which is under the control of the same CaMV35S promoter and followed by the CaMV poly(A) signal (Fig. 1a, b, Additional file 2: Fig. S1). In this study, the Bar gene of pCAMBIA3300 was regarded as a gene of interest and the HPT gene of pCAMBIA1300 as a selectable marker gene (Table 1).
Vector maps of three co-transformation systems. The mixed-strain system includes vectors of pCAMBIA3300 (a) and pCAMBIA1300 (b) showing the independent T-DNA region with Bar and HPT expression cassettes. The “double T-DNA” vector system of pDB1300-3300 (c) shows two T-DNA regions with the Bar and HPT expression cassettes. The “double T-DNA” vector system of pBID RT Enhanced (d) shows two T-DNA regions with Bar, EPSPS and GOX V247 expression cassettes
The “Double T-DNA” binary vector pDB1300-3300, which carried two independent T-DNAs in a tandem orientation, with one T-DNA containing a selectable marker HPT gene and the other a Bar gene (Table 1, Fig. 1c), was constructed as follows: the plasmid pCAMBIA1300 was double digested with EcoRI (Thermo Fisher Scientific, America) and SacI (Thermo Fisher Scientific, America). A 387 bp fragment of the GUS (β-glucuronidase) gene, which was used as an interval sequence between the left border and right border, was amplified from pBI121 with primers RB + GUS + LB-L: TTTGAATTCGCGGGTAAACCTAAGAGAAAAGAGCGTTTATTAGTGGGCAGATGAACATGGCAT (containing the right border sequence) and RB + GUS + LB-R: ACCGAGCTCAATTTGTTTACACCACAATATATCCTGCCACCAGATAACGGTTCAGGCACAGCA (containing the left border sequence) and then ligated to the digested pCAMBIA1300 to form an intermediate vector pCAMBIA1300-1. pCAMBIA1300-1 was then digested with BamHI and HindIII. The CaMV poly(A) signal sequence, Bar gene and CaMV35S promoter were amplified from pCAMBIA3300 with primers polyA + Bar + 35S-L: TTTGGATCCTGACGCTTAGACAACTTAATAACACA and polyA + Bar + 35S-R: TTTAAGCTTTCGTGCCAGCTGCATTAATGAA, and inserted into the digested pCAMBIA1300-1. The expression vector pDB1300-3300 harboring two T-DNA regions in a tandem orientation was thus constructed (Additional file 2: Fig. S2). All the primers were synthesized by Sangon Biotech (Shanghai, China).
The “Double T-DNA” expression vector pBID RT Enhanced contains two independent T-DNA regions in an inverted orientation, with the Bar gene in the first T-DNA region and the CP4 EPSPS and GOX V247 genes in the second. CP4 EPSPS was taken as the selectable marker gene during the period of genetic transformation in this system (Fig. 1d, Table 1). CP4 EPSPS originates from Salmonella typhimurium and encodes a 5-enolpyruvate phenyloxalate-3-phosphate lipase, rendering plant resistance to herbicide glyphosate. However, glyphosate residues have some negative impacts on, e.g., sensitive pollination and reproductive development of plants [42]. This is the rationale for generating glyphosate resistant B. napus with the glyphosate-degrading gene GOX V247 transferred together with the CP4 EPSPS gene to confer resistance to glyphosate while reducing glyphosate residues [43]. In addition to being marker-free, the plants were also expected to create single and multiple herbicide resistant B. napus by these systems. The vector pBID RT Enhanced was constructed on the basis of an initial skeleton of pCAMBIA3300 and an additional artificially synthesized fragment. First, plasmid pCAMBIA3300 containing the Bar gene was phosphorylated to ensure its multiple cloning site (MCS) was invalidated, and a new MCS was inserted between the pBR322 bom and pVS1 oriV sites. The synthetic sequence containing the left border, Enhanced CaMV 35S promoter, CP4 EPSPS gene, NOS terminator, Enhanced CaMV 35S promoter, GOX V247 gene, NOS terminator and right border (Additional file 1: Table S1), with a total length of 5776 bp, was inserted into the new MCS in pCAMBIA3300. Finally, the desired expression vector, pBID RT Enhanced, was constructed (Additional file 2: Fig. S3).
Genetic transformation
The four expression vectors were introduced into A. tumefaciens GV3101 (Weidi Biotechnology, Shanghai, China) by electroporation system of MicroPulser™ (Bio-Rad, America) respectively. Then etiolated hypocotyl segments of B. napus cv. ZS6 were transformed with A. tumefaciens strain GV3101 harboring the vectors. The detailed steps of this genetic transformation were described by Liu et al. [44]. Embryonic calli and regenerated shoots were screened on selection media containing 25 mg/L hygromycin (Sigma, America) for the mixed-strain method and the “double-T-DNA” vector of pDB1300-3300. For transformation of the “double-T-DNA” vector of pBID RT Enhanced, because hypocotyl segments are sensitive to glyphosate, embryonic calli grew on selection media not containing glyphosate and regenerated shoots were screened on regeneration media containing 80 mg/L glyphosate (Bayer, Germany) in order to make transformed cells more competitive than untransformed cells. Rooted shoots were transferred to soil for further analysis.
For the transformation of mixed strains containing the independent plasmids of pCAMBIA1300 or pCAMBIA3300, seven treatments were set up with varying concentration proportion of the strain harboring pCAMBIA1300 to the strain harboring pCAMBIA3300. The concentration proportions were 1:8, 1:4, 1:2, 1:1, 2:1, 4:1 and 8:1. First, each vector was introduced into A. tumefaciens GV3101 by electroporation system of MicroPulser™ (Bio-Rad, America), and positive clones were selected on LB agar plates at 37 °C, supplemented with appropriate concentrations of antibiotics (gentamicin: 50 mg/L, rifampicin: 50 mg/L and kanamycin: 50 mg/L, Sigma, America), and verified by PCR. A single positive A. tumefaciens colony containing pCAMBIA1300 or pCAMBIA3300 was obtained, and each colony was cultured in 50 mL LA medium with 50 mg/L kanamycin until the Optical Density (OD) reached 0.2. The strains were then mixed according to the concentration proportion described above with a final volume of 50 mL for infection.
PCR-based genotyping, test strip detection of transgenic B. napus plants
Genomic DNA was extracted from leaves of T0 and T1 transgenic plants using a Plant Genomic DNA Kit (DP305, TIANGEN, China) following the manufacturer’s instructions. The presence of the Bar gene in transgenic plants was demonstrated by PCR amplification of a 440 bp fragment using the primer pair 5′-GAAGTCCAGCTGCCAGAAAC-3′ and 5′-GCACCATCGTCAACCACTAC-3′. The presence of the HPT gene in transgenic plants was verified by PCR amplification of a 486 bp fragment with the primer pair 5′-ACTTCTACACAGCCATCGGT-3′ and 5′-GCAAACTGTGATGGACGACA-3′. The EPSPS and GOX V247 genes were evidenced by PCR amplification of a 459 bp fragment using the primer pair 5′-CGTTGAGACTGATGCTGACG-3′ and 5′-TTGAGCTTGAGACCGTTTGC-3′ as well as a 503 bp fragment using the primers 5′-TTGAGAGCACGACCTTCAGT-3′ and 5′-CCAAAGTGGCTTCTTGACCC-3′. All the primers were synthesized by Sangon Biotech (Shanghai, China) and the PCR machines were C1000™ (Bio-Rad, America).
Test strips can be used to detect protein effectively [45, 46] Putative transgenic B. napus plants and their self-pollinated or ZS6-crossed offspring were identified based on the detection of the Bar or CP4 EPSPS protein with test strip kits developed by the Oil Crops Research Institute, Chinese Academy of Agricultural Sciences (Wuhan, China); the procedure was carried out according to the manufacturer’s instructions.
For the assessment of T0 seedlings, the statistics summarized in Tables 2 and 3 were derived from the intersection of the PCR and test strip detection results. For all T1 seedlings from the verified T0 plants, the statistics in Tables 4 and 5 were based on only PCR results.
Southern blot analysis of transgenic B. napus plants
For Southern blot analysis of transgenic B. napus T0 and T1 plants, more than 100 μg of total genomic DNA was obtained using a standard CTAB method [47]; a total of 30 μg of DNA from each sample was digested with suitable restriction endonuclease. The digested DNA samples and DNA molecular weight marker II, with Digoxin-labelled (DIG-labelled) (Roche, Germany). were electrophoresed on 0.8% agarose gel (Biowest, Spain), transferred onto a nylon Hybond-N+ membrane (Roche, Germany) and hybridized with DIG-labelled probes. The hybridization and detection steps were carried out according to the instructions for the DIG High Prime DNA Labeling and Detection Starter Kit II (Roche, Germany).
DNA samples from the mixed-strain system and the “double T-DNA” system of pDB1300-3300 were all digested with HindIII (Takara, Japan), and hybridized with probes of Bar (262 bp) and HPT (556 bp). For DNA samples from the “double T-DNA” system of pBID RT Enhanced, samples digested with HindIII were hybridized with a probe of Bar (262 bp) and samples digested with EcoRI (Takara, Japan) were hybridized with a probe of EPSPS (579 bp).
Results
Co-transformation using the three systems resulted in different co-transformation frequencies in the T0 generation
Co-transformation frequencies of the three systems was examined in putative T0 transformants based on genomic PCR test strip detection. For the mixed-strain system, we tested the presence of HPT or/and Bar genes by genomic PCR (Fig. 2a, b), and performed test strip detection of the Bar protein (Fig. 2c). The detection result of individual plants from pCAMBIA1300/3300 (X:X) was shown in Table 2. Among 379 T0 putative root regeneration plants obtained from all transformed strains, 370 plants were confirmed to be HPT-positive. Among the 370 positive-tested plants, 173 plants were confirmed to have the Bar gene, so the average Bar and HPT gene co-transformation rate was 47% (Table 2). Our results also showed that co-transformation frequencies varied with the ratio of pCAMBIA1300/3300. With the increase of pCAMBIA1300/3300 ratio, the co-transformation frequency increased first and then decreased. It was notable that the Bar and HPT gene co-transformation frequency was 81% when the ratio of pCAMBIA1300/3300 was 1:1; this was much higher than that obtained with the other six ratios (Table 2, Fig. 3). Co-integrated T0 transgenic plants were identified by Southern blot to detect the integration status and copy numbers of Bar and HPT genes (Fig. 4). Blot results revealed that the Bar gene was a single copy in seven out of 15 (47%) T0B. napus plants (Fig. 4a lanes 2, 3, 4, 7, 8, 13 and 14). The HPT gene was integrated as a single copy in 3 out of 15 transgenic plants (Fig. 4b lanes 1, 3 and 15), and as three copies in most plants (Fig. 4b lanes 2, 4, 6, 7, 8 and 13). Therefore, it indicated that the Bar gene and the HPT gene were inserted into the genomes of the same transgenic plants in different copy numbers.
Detection of T0 transformants from the mixed-strain system and “double T-DNA” system of pDB1300-3300. PCR detection was done for Bar (a) and HPT (b) genes, M: DL1000 DNA marker; 1–22: individual T0 plants from pCAMBIA1300/3300 of a variety of ratios (X:X) and plants from pDB1300-3300); P: expression vector pCAMBIA3300 for (a) and pCAMBIA1300 for (b); WT: wild type ZS6. Test strips detection was done for the Bar protein (c), 1–17: individual T0 plants from pCAMBIA1300/3300 of a variety of ratios and plants from pDB1300-3300; P: positive transgenic B. napus plant of Ms8 with the Bar protein; WT: wild type ZS6. The figure represented part of the results
Southern blot detection in plants. Bar (a) and HPT (b) gene copy numbers were detected in T0 transgenic B. napus plants (lanes 1-15) from pCAMBIA1300/3300 of a variety of ratios (X:X) with the Bar+HPT+ genotype from the mixed-strain system. Bar (c) and HPT (d) gene were detected for identification of marker-free plants in the T1 generation from the mixed-strain system, 1, 9 and 11 are T1 progeny with the Bar+HPT− genotype from T0 plants as indicated in lanes 1, 9 and 11 of a, b. Bar (e) and HPT (f) gene copy numbers were detected in T0 plants with the Bar+HPT+ genotype (lanes 1-2). Bar (e) and HPT (f) gene were detected in T1 individual plants including Bar+HPT+ plants and marker-free Bar+HPT− plants from T0 plant as indicated in lanes 1 of e, f (lanes 3-7) from the “double T-DNA” system of pCAMBIA1300-3300. M: DNA molecular weight marker II, DIG-labelled; WT: wild type ZS6; P: expression vector pCAMBIA3300 for (a, c, e) and pCAMBIA1300 for (b, d, f)
For the “double T-DNA” vector system of pDB1300-3300, the putative T0 transformants were analyzed by PCR for the insert (Fig. 2a, b lanes 20 to 22) and test strip detection for Bar protein expression (Fig. 2c lanes 11 to 17). All the 26 putative T0 root regeneration plants were confirmed to be HPT-positive by PCR, and among the 26 plants, 17 were confirmed to have the Bar gene. Therefore, the co-transformation frequency was 65% for the pDB1300-3300 vector (Table 2). To confirm these results, two T0Bar+HPT+ lines were then identified by Southern blot for detecting the integration status and copy numbers of Bar and HPT genes. Blot results revealed that the Bar gene had three and six copies in the two T0B. napus plants (Fig. 4e lanes 1 and 2). However, the HPT gene was integrated with a single copy and three copies, respectively (Fig. 4f lanes 1 and 2). The Bar gene and the HPT gene were inserted into the genomes of the same transgenic plants in different copy numbers.
For the “Double T-DNA” vector system of pBID RT Enhanced, a total of 76 putative root regeneration plants were obtained and the gene integration condition was detected by PCR for the presence of Bar, EPSPS and GOX genes and by test strips for the expression of Bar and EPSPS proteins (Fig. 5, Table 3). The results revealed that among 76 T0 putative root regeneration plants, 57 plants were confirmed to be EPSPS-positive. Among the 57 positive-tested plants, 18 plants were confirmed to have the Bar gene, so the co-transformation frequency was 32% for the pBID RT Enhanced vector (Table 3). Next, co-integrated T0 transgenic plants were identified by Southern blot to detect the integration status and copy numbers of Bar and EPSPS genes (Fig. 6a, b). Blot results revealed that the Bar gene was integrated as a single copy in four out of 17 (24%) transgenic plants (Fig. 6a lanes 5, 13, 15 and 16), and as three copies in slightly more than half plants (Fig. 6a). However, the EPSPS gene was a single copy in nine out of 17 T0B. napus plants (Fig. 6b). This indicated that the Bar gene and EPSPS genes were inserted in different copy number into the genomes of individual transgenic plants.
Detection of T0 transgenic plants from the “double T-DNA” system of pBID RT Enhanced. Putative T0 transformants were detected by PCR for Bar (a), EPSPS (b), and GOX (c) genes, M: DL 2000 DNA marker; 1-17: individual T0 plants; P: expression vector pBID RT Enhanced; WT: wild type ZS6. Putative T0 transgenic B. napus plants were detected with test strips for Bar (d) and EPSPS (e) protein, P: positive transgenic B. napus plant of MS8 with the Bar protein for d and GT73 with the EPSPS protein for e; WT: wild type ZS6
Southern blot detection in plants from the “double T-DNA” system of pBID RT Enhanced. Bar (a) and EPSPS (b) gene copy numbers were detected in T0 transgenic B. napus plants, 1-17: T0 individual plants with the Bar+EPSPS+ genotype. Bar (c, e) and EPSPS (d, f) gene detection for identification of marker-free and herbicide resistant plants in the T1 generation, 1-12: T1 individual plants with the Bar+EPSPS− genotype, 13-24: T1 individual plants with the Bar+EPSPS+ genotype. M: DNA molecular weight marker II, DIG-labelled; WT: wild type ZS6; P: expression vector pBID RT Enhanced
Screening for marker-free transgenic B. napus plants in the T1 generation produced by the three systems
Marker-free efficacy was then assessed in T1 by frequency of marker-free lines, which was calculated as percentage of lines with marker-free plants production out of total number of identified lines, and frequency of marker-free plants in a line, which was calculated as percentage of marker-free plants out of all identified plants in a T1 line. For the mixed-strain system, ten T0 plants were randomly chosen from the 173 plants with positive Bar and HPT genes and then self-pollinated. The genotyping of every T1 plant at the seedling stage was performed for the presence of the HPT and Bar genes by PCR in order to identify marker-free transgenic B. napus plants. There were four types of segregated transgenic plants in the T1 generation: Bar+HPT+, Bar−HPT+, Bar+HPT− and Bar−HPT− (Additional file 2: Fig. S4, Table 4). Most of the plants were Bar+HPT+, and 19 marker-free plants Bar+HPT− with resistance to phosphinothricin were identified in the six out of 10 T1 transgenic lines, so the frequency of marker-free lines was 60%. The frequency of marker-free plants in the 5 lines varied from 3 to 36%, with an average frequency of 11% (Table 4). To confirm these results, seven marker-free B. napus in T1 progeny with the Bar+HPT− genotype derived from three transgenic T0Bar+HPT+ were selected and their genotype was verified by Southern blot with non-transformed wild-type ZS6 as a control (Fig. 4c, d). All of the seven T1Bar+HPT− progeny exhibited one to three Bar bands without HPT bands and showed three different band patterns, representing these T1 progeny derived from three independent T0 transgenic lines. Two of the three T0 lines exhibited more than three HPT bands (Fig. 4b lanes 9 and 11) and these multiple bands were associated with multiple copies of the HPT gene in T0. In theory genes with multiple copies would be segregated in the next generation with a lower frequency than one-copied gene would, while these two T0 lines produced marker-free B. napus (Fig. 4c, d lanes 9 and 11) in the T1 generation as the T0 line with one HPT copy did (Fig. 4b lane 1 and Fig. 4c, d lane 1). In the case of hybridization with the Bar and HPT probes, one T1 progeny did not exhibit a signal, likely due to a false-positive PCR result.
For the “double T-DNA” vector system of pDB1300-3300, two randomly selected T0 plants with positive Bar and HPT genes were self-pollinated, and the T-DNA segregation in T1 seedlings was analyzed by testing the presence of the HPT and Bar genes by PCR. There were four types of segregated transgenic plants in the T1 generation: Bar+HPT+, Bar−HPT+, Bar+HPT− and Bar−HPT− (Additional file 2: Fig. S5, Table 4). One out of the two T0 transgenic lines produced five marker-free progeny Bar+HPT− with resistance to phosphinothricin, so the frequency of marker-free lines was 50%. The frequency of marker-free plants in the line was 20% (Table 4). To confirm these results, five T1B. napus plants of one transgenic T0Bar+HPT+ were then identified by Southern blot (Fig. 4e, f) for confirming marker-free plants. Blot results revealed that all of the five T1Bar+HPT− progeny exhibited three Bar bands with zero or one HPT bands. We observed that one of two T0 lines which exhibited three copies of the HPT gene (Fig. 4e, f lane 2) did not produce marker-free B. napus in the T1 generation. However, the other T0 line exhibited one copy of the HPT gene (Fig. 4e, f lane 1) produced marker-free B. napus in the T1 generation (Fig. 4e, f lanes 3 and 7).
For the “double T-DNA” vector system of pBID RT Enhanced, eight randomly chosen T0Bar+EPSPS+GOX+ plants were selfed or crossed with wild type ZS6. Seedlings of 13 T1 or F1 generations were then subjected to segregation analysis in order to identify marker-free transgenic B. napus plants (Additional file 2: Fig. S6, Table 5). Every T1 plant was tested for the presence of Bar, EPSPS and GOX genes by PCR. Four types of transgenic plants were detected in the T1 generation: Bar+EPSPS+GOX+, Bar−EPSPS+GOX+, Bar+EPSPS−GOX− and Bar−EPSPS−GOX− (Additional file 2: Fig. S6, Table 5). Most of the T1 plants were Bar+EPSPS+GOX+, and a total of 37 marker-free plants Bar+EPSPS−GOX− were identified in the 10 out of 13 transgenic T1 or F1 transgenic lines, so the frequency of marker-free lines was 77%. The frequency of marker-free plants in the 5 selfed lines varied from 6 to 41%, with an average frequency of 19%. The frequency of marker-free plants in the 5 backcrossed lines varied from 3 to 53%, with an average frequency of 23%. The average frequency of marker-free plants in all the marker-free lines was 21% (Table 5). To confirm these results, 12 marker-free B. napus in the T1 or F1 generation with the Bar+EPSPS−GOX− genotype were selected for Southern blot analysis (Fig. 6c, d). Due to linked sites of EPSPS and GOX in the same T-DNA, EPSPS was chosen to represent the presence of these two genes. All of the 12 T1Bar+EPSPS−GOX− progeny exhibited one to two Bar bands without EPSPS band and showed four different band patterns, representing these T1 or F1 progeny derived from four independent T0 lines, which exhibited a single EPSPS copy (Fig. 6b lanes 2, 6, 8, and 15). In addition to Bar+EPSPS−GOX− marker-free plants with resistance to phosphinothricin, Bar+EPSPS+GOX+ transgenic B. napus with resistance to phosphinothricin and glyphosate were obtained. Therefore, 12 of T1 or F1 progeny with the Bar+EPSPS+GOX+ genotype were chosen and verified by Southern blot with the non-transformed wild-type ZS6 used as a control (Fig. 6e, f). The 12 T1 or F1Bar+EPSPS+GOX+ progeny exhibited one to four Bar bands and one to two EPSPS bands, while showing six different band patterns, representing these T1 progeny derived from six independent T0 lines (Fig. 6e, f).
Discussion
Co-transformation using the three systems
In the transformation process, T-DNAs are randomly integrated into plant genomes [48]. If a transgenic plant harbors two T-DNAs in unlinked loci, genetic separation of an gene of interest from a selectable marker gene may be feasible by segregation in the successive generation [34], thereby providing a simple approach to eliminate selectable marker genes from transgenic plants. There have been successful examples of producing marker-free transgenic plants such as rice, tobacco (Nicotiana tabacum) and B. napus by co-transformation of two separate T-DNAs, one T-DNA including a gene of interest and the other a selectable marker gene [31, 49]. High efficiency of co-transformation and unlinked integration of T-DNAs with a gene of interest and a selectable marker gene in the T1 generation are prerequisites for the effective segregation of marker-free transgenic plants. Compared to the particle-bombardment method, which presents a very low level of effective selectable marker gene elimination due to complex and linked integrations [50, 51], Agrobacterium-mediated co-transformation shows simple integration patterns and is more suitable for selectable marker gene elimination. In our study, the highest frequency (81%) co-transformation was attained by using the mixed-strain method as compared with the “double T-DNA” vector system of pDB1300-3300 (65%) or pBID RT Enhanced (32%) in T0 plants. The co-transformation frequency of 81% is higher than most results obtained for other plant species, such as a co-transformation frequency range of 38.5 to 60.0% in wheat [19], 43.2 to 66.7% in sorghum [36] and 67% in rice [37], although there was a report that a high co-transformation efficiency of 86% in rice was achieved by placing the gene of interest into a 12-copy vector and the selectable marker gene in a single-copy co-integrated vector [52]. Another “double T-DNA” binary system developed for rice yielded 90% co-transformation, in which the first T-DNA was delimited by the A. tumefaciens borders and the second T-DNA was delimited by the A. rhizogenes borders [53].
The molar ratio of the T-DNA of the gene of interest to the T-DNA of the selectable marker gene influences the co-transformation frequency [54]. The co-transformant frequencies varied from 24 to 81% with different mixed ratios of A. tumefaciens strains via the mixed-strain method. Co-transformants containing both Bar and HPT genes occurred at an increasing frequency when the ratio of pCAMBIA1300 and pCAMBIA3300 was increased from 1:8 to 1:1. When the ratio of pCAMBIA1300 and pCAMBIA3300 continued to be increased from 1:1 to 8:1, the co-transformation frequency decreased. The average co-transformation frequency for the mixed-strain method was 47%, which was lower than the frequency obtained with the “double T-DNA” vector system of pDB1300-3300 (65%) but higher than that with the “double T-DNA” vector system of pBID RT Enhanced (32%). It was reported that the “double T-DNA” vector system might be more effective than the mixed-strain method in producing co-transformants [39, 40]. This view was not supported by our results, probably because co-transformation of T-DNAs from two different Agrobacterium strains is the equivalent of producing two independent transformation events. However, in either the “double T-DNA” vector system or the mixed-strain system, integration of each T-DNA is an independent process. Another possible reason is that the research which reported that the mixed-strain method might be less effective [39, 40] may have been unable to produce the highest co-transformation frequency due to limited mixed proportional gradients in the mixed-strain method. In their study, two mixing ratios were used, which were 1:1 and 3:1 of the T-DNA of the gene of interest to the T-DNA of the selectable marker gene, and higher co-transformation frequency was attained when the ratio was 3:1 [39, 40]. In our study, seven mixing ratios were tried—a more comprehensive approach. When compared to “double T-DNA” system, Agrobacterium-mediated co-transformation involving two T-DNAs on different plasmids (mixed-strain method) offers an advantage of altering to achieve the optimal ratio of T-DNA, including the selectable marker gene, to the T-DNA, including the gene of interest, to attained the highest co-transformation frequency.
In addition, the length of the T-DNA is an important element that should be considered. It has been reported that two T-DNA cassettes with different sizes in an expression vector led to different integration copy numbers in transgenic tobacco [35]. Compared with shorter T-DNA, longer T-DNA tends to have lower integration efficiency, thus affecting the final co-transformation frequency. For example, in the “double T-DNA” expression vector system of pDB1300-3300, one T-DNA region harboring shorter Bar genes was 1781 bp in length, and the other T-DNA containing the selectable marker gene HPT was 2437 bp (Fig. 1c, Additional file 2: Fig. S2). The co-transformation frequency of this vector system was 65% (Table 2). However, in the “double T-DNA” expression vector system of pBID RT Enhanced, one T-DNA region containing Bar gene was 1870 bp in length, but the other T-DNA region containing the selectable marker gene CP4 EPSPS and the GOX V247 gene was 5775 bp, which is about three times greater (Fig. 1d, Additional file 2: Fig. S3). The co-transformation frequency of this vector system was 32% (Table 3). The incomparable size of the two T-DNAs may have resulted in fewer copies of the CP4 EPSPS gene than the Bar gene being integrated into the B. napus genome (Fig. 5a, b) [35]. In a “double T-DNA” vector that McCormac et al. [35] constructed, the gene of interest was placed in a shorter T-DNA and the selectable marker gene was placed in a T-DNA that was twice as long, thereby increasing integration efficiency of the unselected gene of interest. In the mixed-strain systems that Zhou et al. [39] and Komari et al. [40] reported, the T-DNA of the interested gene is 1.3 times and 1.6 times longer than that of the selected marker gene respectively, and when the molar ratio of the T-DNA of the gene of interest to the T-DNA of the selectable marker gene was 3:1, higher co-transformation frequency was attained than when the molar ratio was 1:1. Hence, it may be that if the length of two independent T-DNA regions is adjusted to an optimal ratio, stable 1:1 gene co-integration could be achieved. It is likely that it was the appropriate molar ratio based on the length ratio of the two T-DNAs that yielded a high co-transformation frequency of 81% in the mixed-strain method reported here. Further investigations could be carried out to determine how the T-DNA length affects the co-transformation frequency by setting a series of length gradients of T-DNAs.
Marker-free situations achieved in the three systems
It has been reported that marker-free transgenic plants are more easily acquired for the progeny of T0 plants with a single or a few copies of selectable marker genes [19]. In our study marker-free plants tended to be obtained in some T1 lines where there was only one to two copies of the marker gene, i.e., the HPT gene in the T0-1 line in the mixed-strain system (Fig. 4c, d), the HPT gene in the T0-1 line in the “double T-DNA” system of pDB1300-3300 (Fig. 4e, f), and the EPSPS gene in the “double T-DNA” system of pBID RT Enhanced (Fig. 6c, d). Taking the pBID RT Enhanced vector as an example, the longer T-DNA cassette with EPSPS and GOX genes tended to integrate into the genome with fewer copies, and the shorter T-DNA cassette with the Bar gene tended to integrate into the genome with more copies, which were inherited with different copy numbers via independent assortment in the T1 generation. The efficiency of obtaining marker-free plants may indeed be closely related to the copy number of selectable marker genes.
However, marker-free plants were also obtained from T0 plants which had multiple copies of the marker gene. For example, marker-free transgenic plants were obtained from the two T0 lines despite their being five or more copies of the HPT marker gene in the mixed-strain system (Fig. 4 lanes 9 and 11). Theoretically we might have expected the two T0 lines to produce marker-free plants in the segregation generations at a very low frequency. However, it may be that the multiple copies were integrated into one or two sites in a closely linked manner, allowing marker-free plants to be more easily obtained.
Unlinked co-insertion frequency and overall success frequency in the three systems
There were two important parameters for unlinked co-insertion frequency: the co-transformation frequency in T0 and the frequency of marker-free lines in T1. When using the mixed-strain system, co-transformation frequencies of 24 to 81% were obtained from a series of treatments (Table 2), and the frequency of marker-free lines was 60% (Table 4). Taking these collective results together, it gives a frequency of 14 to 49% (24% or 81% multiplied by 60%) of useful unlinked co-insertion events (Additional file 1: Table S2). When employing the “double T-DNA” system of pDB1300-3300, an average co-transformation frequency of 65% was obtained (Table 2), and the frequency of marker-free lines was 50% (Table 4), giving a frequency of 33% of useful unlinked co-insertion events among pDB1300-3300 transformed lines (Additional file 1: Table S2). When utilizing the “double T-DNA” system of pBID RT Enhanced, the co-transformation frequency of 32% was obtained (Table 2) and the frequency of marker-free lines was 77% (Table 4), giving a frequency of useful unlinked co-insertion events was 25% (Additional file 1: Table S2). All these results are comparable to 24% for barley [49] and 30% for rice [40]. It is difficult to compare different frequencies of independent co-insertion to other studies because of the size of the twin T-DNA binary vectors, the frequency of linked co-delivery of the double T-DNAs, and the complexity in the construction of the vectors. The A. tumefaciens strain types and species of the recipient plants are the major factors that affect the efficiency of these methods.
The higher positive transformant frequencies of 98% and 100% were obtained when using the mixed-strain method and the “double T-DNA” vector system of pDB1300-3300 (Table 2), and this is consistent with previous study which indicated that transformation with selectable marker gene HPT led to a higher positive transformant frequency (90.33%) compared with NPTII and Bar (80.23% and 65.53% respectively) [3]. The highest frequency of co-transformation of 81% was obtained when using the mixed-strain method (Table 2), probably because this method offers the advantage of altering the ratio of T-DNAs when compared to “double T-DNA” system. The highest frequency of marker-free lines of 77% were attained from the “double T-DNA” vector system of pBID RT Enhanced (Table 5) because marker-free plants are more easily produced from T0 plants with only one or two marker gene copies and in this system the longer T-DNA cassette including EPSPS and GOX genes tends to have lower integration efficiency. Some “double T-DNA” vectors have been constructed by inserting only “right border and left border” sequences into the polylinker of a binary vector [49]. In our study, the spacer between the two T-DNAs of the pDB1300-3300 vector was a GUS gene segment of 387 bp (Additional file 1: Fig. S2) and the distance between the two T-DNAs of the pBID RT Enhanced vector was more than 2 kb (Additional file 2: Fig. S3), and the T-DNAs were arranged in an inverted orientation. These designs may reduce the frequency of T-DNA integration into linked loci. However, two independent T-DNAs in a mixed-strain system tend to integrate into different loci in any event. The highest frequency of single copy gene of interest lines of 47% was obtained in T0 generation when using the mixed-strain method (Fig. 2a). The overall success in producing marker-free plants from initial transformation was summarized and taking these collective results together, it gives a overall success frequency of 7 to 22% in the mixed-strain system and 4% in the “double T-DNA” vector system of pBID RT Enhanced (Additional file 1: Table S2). Consequently it was expected that if a specific mole ratio (1:1 ratio in our study) was designed for the length ratio of the twin T-DNAs for the mixed-strain method, high unlinked co-insertion frequency and overall success frequency could be achieved.
Three types of vector systems created two types of herbicide resistant B. napus materials
Herbicide resistance is one of the most important traits utilised in plant biotechnology; on the one hand used as a selectable marker during genetic engineering and on the other hand widely used as a character trait to improve agricultural efficiency by controlling weeds. Herbicide resistant crops continue to be developed by introducing genes that can confer resistance specifically to different kinds of herbicides [55]. In addition to the vector systems developed to achieve marker-free B. napus plants, in our study two types of herbicide resistant B. napus materials were simultaneously created from the segregated progeny of “double T-DNA” binary vector pBID RT Enhanced transformed T0 plants, including Bar+EPSPS−GOX− plants with resistance to phosphinothricin and Bar+EPSPS+GOX+B. napus with resistance to both phosphinothricin and glyphosate (Fig. 6c–f). Bar+HPT− plants with resistance to phosphinothricin were also produced from the segregated progeny of the mixed-strain system and from the “double T-DNA” binary vector of pCAMBIA1300-3300 (Fig. 4c–f). The systems in our study provided an effective and convenient approach to develop transgenic B. napus with various types of herbicide resistance.
Conclusion
Removing selection markers and unnecessary vector fragments in early generations of the transgenic crop development would eliminate the biosafety concerns surrounding these sequences and facilitate the release of commercial genetically modified B. napus varieties [19]. In our study, three systems for co-transformation were developed, including the mixed-strain method and “double T-DNA” vector systems of pDB1300-3300 and pBID RT Enhanced, which were arranged in opposite orientations and had a different length. We were successful in producing marker-free transgenic B. napus plants using all three co-transformation systems and compared and evaluated these co-transformation methods. If a appropriate mole ratio was designed for the specific length ratio of the twin T-DNAs for the mixed-strain method, high unlinked co-insertion frequency and overall success frequency could be achieved. These systems provide an effective and convenient approach to developing herbicide resistant transgenic B. napus. The three vector systems can be used for experimental purposes, for crop improvement and for commercial needs. These systems have the potential to transform many plant species, including dicotyledonous and monocotyledonous plants in practical application.
Availability of data and materials
The datasets supporting the conclusions and a description of the complete protocols are included within the article.
References
Giddings G, Allison G, Brooks D, Carter A. Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol. 2000;18:1151–5.
Boszoradova E, Libantova J, Matusikova I, Poloniova Z, Jopcik M, Berenyi M, Moravcikova J. Agrobacterium-mediated genetic transformation of economically important oilseed rape cultivars. Plant Cell Tiss Org. 2011;107(2):317–23.
Liu F, Xiong X, Wang P, Lei L, Zeng X, Zhu L, Li Y, Luo J, Fu D, Fu P, Wu G. Effects of non-procedural factors in Brassica napus genetic transformation. Oil Crop Sci. 2017;2:106–21.
Fry J, Barnason A, Horsch RB. Transformation of Brassica napus with Agrobacterium tumefaciens based vectors. Plant Cell Rep. 1987;6(5):321–5.
Block MD, Brouwer DD, Tenning P. Transformation of Brassica napus and Brassica oleracea Using Agrobacterium tumefaciens and the expression of the bar and neo genes in the transgenic plants. Plant Physiol. 1989;91(2):694–701.
Stewart JCN, Adang MJ, All JN, Raymer PL, Ramachandran S, Parrott WA. Insect control and dosage effects in transgenic canola containing a synthetic bacillus thuringiensis cryIAc gene. Plant Physiol. 1996;112(1):115–20.
Wang J, Chen Z, Du J, Sun Y, Liang A. Novel insect resistance in Brassica napus developed by transformation of chitinase and scorpion toxin genes. Plant Cell Rep. 2005;24(9):549–55.
Wang YG, Culley D, Hadwiger LA, Fristensky B. Constitutive expression of pea defense gene DRR206 confers resistance to blackleg (Leptosphaeria maculans) disease in transgenic canola (Brassica napus). Mol Plant Microbe Interact. 1999;12(5):410–8.
Zou J, Katavic V, Giblin EM, Barton DL, Mackenzie SL, Keller WA, Hu X, Taylor DC. Modification of seed oil content and acyl composition in the brassicaceae by expression of a yeast sn-2 acyltransferase gene. Plant Cell. 1997;9(6):909–23.
Scarth R, Tang J. Modification of oil using conventional and transgenic approaches. Crop Sci. 2006;46(3):1225–36.
Chavadej S, Brisson N, Mcneil JN, Luca VD. Redirection of tryptophan leads to production of low indole glucosinolate canola. Proc Natl Acad Sci USA. 1994;91(6):2166–70.
Kopertekh L, Broer I, Schiemann J. Developmentally regulated site-specific marker gene excision in transgenic B. napus plants. Plant Cell Rep. 2009;28(7):1075–83.
Schiemann J, Dietz-Pfeilstetter A, Hartung F, Kohl C, Romeis J, Sprink T. Risk assessment and regulation of plants modified by modern biotechniques: current status and future challenges. Annu Rev Plant Biol. 2019;70(25):1–28.
Zechendorf B. What the public thinks about biotechnology. Biotechnology. 1994;12:870–3.
Hare PD, Chua NH. Excision of selectable marker genes from transgenic plants. Nat Biotechnol. 2002;20(6):575–80.
Miki B, Mchugh S. Selectable marker genes in transgenic plants: applications, alternatives and biosafety. J Biotechnol. 2004;107(3):193–232.
ISAAA. Global status of commercialized biotech, GM crops in 2017: biotech crop adoption surges as economic benefits accumulate in 22 years. ISAAA Briefs. 2017;2017(53):213–31.
Miller M, Tagliani L, Wang N, Berka B, Bidney D, Zhao ZY. High efficiency transgene segregation in co-transformed maize plants using an Agrobacterium tumefaciens 2 T-DNA Binary System. Transgenic Res. 2002;11(4):381–96.
Wang K, Liu H, Du L, Ye X. Generation of marker-free transgenic hexaploid wheat via an Agrobacterium-mediated co-transformation strategy in commercial Chinese wheat varieties. Plant Biotechnol. 2016;15(5):614–23.
Nanto K, Ebinuma H. Marker-free site-specific integration plants. Transgenic Res. 2007;17(3):337–44.
Nandy S, Srivastava V. Site-specific gene integration in rice genome mediated by the FLP-FRT recombination system. Plant Biotechnol J. 2011;9(6):713–21.
Mészáros K, Eva C, Kiss T, Bányai J, Kiss E, Téglás F, Láng L, Karsai I, Tamás L. Generating marker-free transgenic wheat using minimal gene cassette and cold-inducible Cre/Lox system. Plant Mol Biol Rep. 2014;31(6):1474–84.
Hoff T, Schnorr KM, Mundy J. A recombinase-mediated transcriptional induction system in transgenic plants. Plant Mol Biol. 2001;45:41–6.
Zuo J, Niu QW, Moller S, Chua N. Chemical-regulated, site-specific DNA excision in transgenic plants. Nat Biotech. 2001;19(2):157–61.
Kopertekh L, Schulze K, Frolov A, Strack D, Broer I, Schiemann J. Cre-mediated seed-specific transgene excision in tobacco. Plant Mol Biol. 2010;72:597–605.
Zuo J, Niu QW, Chua NH. Technical advance: an estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 2000;24:265–73.
Sreekala C, Wu L, Gu K, Wang D, Tian D, Yin Z. Excision of a selectable marker in transgenic rice (Oryza sativa L.) using a chemically regulated Cre/loxP system. Plant Cell Rep. 2005;24(2):86–94.
Zubko E, Scutt C, Meyer P. Intrachromosomal recombination between attP regions as a tool to remove selectable marker genes from tobacco transgenes. Nat Biotechnol. 2000;18(4):442–5.
Goldsbrough AP, Lastrella CN, Yoder JI. Transposition mediated re-positioning and subsequent elimination of marker genes from transgenic tomato. Nat Biotechnol. 1993;11(11):1286–92.
Murovec J, Guček K, Bohanec B, Avbelj M, Jerala R. DNA-free genome editing of Brassica oleracea and B. rapa protoplasts using CRISPR-Cas9 ribonucleoprotein complexes. Front Plant Sci. 2018;9:1–9. https://doi.org/10.3389/fpls.2018.01594.
Daley M, Knauf VC, Summerfelt KR, Turner JC. Co-transformation with one Agrobacterium tumefaciens strain containing two binary plasmids as a method for producing marker-free transgenic plants. Plant Cell Rep. 1998;17(6–7):489–96.
Tuteja N, Sahoo RK, Raveendar S, Reddy IBL. Recent advances in development of marker-free transgenic plants: regulation and biosafety concern. J Biosci. 2012;37(1):167–97.
Hervé C, Rouan D, Guerche P, Montané MH, Yot P. Molecular analysis of transgenic rapeseed plants obtained by direct transfer of two separate plasmids containing, respectively, the cauliflower mosaic virus coat protein gene and a selectable marker gene. Plant Sci. 1993;91(2):181–93.
Xing A, Zhang Z, Sato S, Staswick P, Clemente T. The use of the two T-DNA binary system to derive marker-free transgenic soybeans. Vitro Cell Dev-Pl. 2000;36(6):456–63.
Mccormac AC, Fowler MR, Chen DF, Elliott MC. Efficient co-transformation of Nicotiana tabacum by two independent T-DNAs, the effect of T-DNA size and implications for genetic separation. Transgenic Res. 2001;10(2):143–55.
Lu L, Wu X, Yin X, Morrand J, Chen X, Folk WR, Zhang ZJ. Development of marker-free transgenic sorghum [Sorghum bicolor (L.) Moench] using standard binary vectors with bar as a selectable marker. Plant Cell Tiss Org. 2009;99(1):97–108.
Rao MVR, Parameswari C, Sripriya R, Veluthambi K. Transgene stacking and marker elimination in transgenic rice by sequential Agrobacterium-mediated co-transformation with the same selectable marker gene. Plant Cell Rep. 2011;30(7):1241–52.
Block MD, Debrouwer D. Two T-DNA’s co-transformed into Brassica napus by a double Agrobacterium tumefaciens infection are mainly integrated at the same locus. Theor Appl Genet. 1991;82(3):257–63.
Zhou HY, Chen XG, Xiao GF, Wei XL, Zhu Z. Generating marker-free transgenic tobacco plants by Agrobacterium-mediated transformation with double T-DNA binary vector. Chinese Bull Bot. 2003;45(9):1103–8.
Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T. Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J. 1996;10(1):165–74.
Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liuy B, Wang XC, Chen QJ. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014;14(1):327–38.
Meilan R, Han KH, Ma C, DiFazio SP, Eaton JA, Hoien EA, Stanton BJ, Crockett RP, Taylor ML, James RR, Skinner JS, Jouanian L, Pilate G, Strauss SH. The CP4 transgene provides high level of tolerance to Roundup herbicide in field-grown hybrid poplars. Canad J Res. 2002;32:967–76.
Peñaloza-Vazquez A, Mena GL, Herrera-Estrella L, Bailey AM. Cloning and sequencing of the genes involved in glyphosate utilization by Pseudomonas pseudomallei. Appl Environ Microb. 1995;61(2):538–43.
Liu F, Xiong X, Wu L, Fu D, Hayward A, Zeng X, Cao Y, Wu Y, Li Y, Wu G. BraLTP1, a lipid transfer protein gene involved in epicuticular wax deposition, cell proliferation and flower development in Brassica napus. PLoS ONE. 2014;9:e110272.
Corey PF, Michaels AA, Sommer RG. Test-strip containing merocyanine and nitro or nitroso substituted polyhalogenated phenol-sulfonephthaleins as protein indicators. European Patent EP0517050; 1996.
Free HM, Collins GF, Free AH. Triple-test strip for urinary glucose, protein, and PH. Clin Chem. 1960;6(4):352–61.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989.
Chyi YS, Jorgensen RA, Goldstein D, Tanksley SD, Loaiza-Figueroa F. Locations and stability of Agrobacterium-mediated T-DNA insertions in the Lycopersicon genome. Mol Gen Genet. 1986;204(1):64–9.
Matthews PR, Wang MB, Waterhouse PM, Thornton S, Fieg SJ, Gubler F, Jacobsen JV. Marker gene elimination from transgenic barley, using co-transformation with adjacent ‘twin T-DNAs’ on a standard Agrobacterium transformation vector. Mol Breeding. 2001;7(3):195–202.
Zhao Y, Qian Q, Wang HZ, Huang DN. Co-transformation of gene expression cassettes via particle bombardment to generate safe transgenic plant without any unwanted DNA. Vitro Cell Dev-Pl. 2007;43(4):328–34.
Wang D, Zhao Q, Zhu D, Ao G, Yu J. Particle-bombardment-mediated cotransformation of maize with a lysine rich protein gene (sb401) from potato. Euphytica. 2006;150:75–85.
Sripriya R, Sangeetha M, Parameswari C, Veluthambi B, Veluthambi K. Improved Agrobacterum-mediated co-transformation and selectable marker elimination in transgenic rice by using a copy number pBIN19-derived binary vector. Plant Sci. 2011;180(6):766–74.
Breitler JC, Meynard D, Van BJ, Royer M, Bonnot F, Cambillau L, Guiderdoni E. A novel two T-DNA binary vector allows efficient generation of marker-free transgenic plants in three elite cultivars of rice (Oryza sativa L.). Transgenic Res. 2004;13(3):271–87.
Bettany AJE. Effect of selectable gene to reporter gene ratio on the frequency of co-transformation and co-expression of uidA and hpt transgenes in protoplast-derived plants of tall fescue. Plant Cell Tiss Org. 2002;68(2):177–86.
Han YJ, Kim J. Application of CRISPR/Cas9–mediated gene editing for the development of herbicide–resistant plants. Plant Biotech Rep. 2019;13:447–57.
Acknowledgements
We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the National Program of Transgenic Variety Development of China (2018ZX08010-05B and 2019ZD080018) and the Central Public-interest Scientific Institution Basal Research Fund, a Major Research Project of CAAS Science and the Technology Innovation Program.
Author information
Authors and Affiliations
Contributions
GW and FL designed the experiments. GW, FL and PW selected the material. PW, XX and PF performed the experiments. FL and PW analyzed the data. FL wrote the paper. GW, HG and XD commented on the manuscript. FL revised the text and structure. GW and FL provided project management and acquired the project funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have consented to publication of this article.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table
S1. The sequences of six units included in the artificially synthetic fragment. Table S2. The unlinked co-insertion frequency and overall success frequency in the three systems.
Additional file 2: Fig. S1.
Structures of pCAMBIA1300 and pCAMBIA3300 that contain single independent T-DNA regions. Fig. S2. Construction of pDB1300-3300, which contains two independent T-DNA regions in a tandem orientation. Fig. S3. Construction of pBID RT Enhanced containing two independent T-DNA regions in an inverted orientation. Fig. S4 PCR detection of Bar (a) and HPT (b) genes for screening of marker-free transgenic plants in the T1 generation using the mixed strains method. M: DL1000 DNA marker; 1-19: segregated T1 plants produced by self pollination from T0 co-transformants from pCAMBIA1300/3300 of a variety of ratios; P: expression vector pCAMBIA3300 for (a) and pCAMBIA1300 for (b); WT: wild type ZS6. The arrows indicated marker-free individuals Fig. S5 PCR detection of Bar (a) and HPT (b) genes for screening of marker-free transgenic plants in the T1 generation from the “double T-DNA” vector pDB1300-3300. M: DL1000 DNA marker; 1-18: segregated T1 plants produced by self pollination from T0 co-transformants; P: expression vector pDB1300-3300; WT: wild type ZS6. The arrows indicated marker-free individuals Fig. S6 PCR detection of Bar (A), EPSPS (B) and GOX (C) genes for screening of marker-free transgenic plants in the T1 generation. M: DL 1000 DNA marker; 1-17: individual T1 plants; P: expression vector pBID RT Enhanced; WT: wild type ZS6. The arrows indicated marker-free individuals.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, F., Wang, P., Xiong, X. et al. Comparison of three Agrobacterium-mediated co-transformation methods for generating marker-free transgenic Brassica napus plants. Plant Methods 16, 81 (2020). https://doi.org/10.1186/s13007-020-00628-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13007-020-00628-y