Abstract
Both linked and un-linked co-transformation can be used to overcome limitations of methods, such as re-transformation or sexual crossing of transgenic plants, to enable transfer of multiple genes to a single plant. Un-linked co-transformation can also facilitate the production of selectable marker-free transgenic plants. In this study, transgenic white clover plants were generated by Agrobacterium-mediated linked co-transformation using a single T-DNA of 9803 bp expressing: an isopentenyl transferase (IPT) gene for delayed leaf senescence under the control of an organ specific MYB32 promoter from Arabidopsis, a white clover nodule enhanced malate dehydrogenase (neMDH) gene for aluminium tolerance controlled by the endogenous Phosphate Transporter 1 (PT1) promoter, and the coat protein gene from Alfalfa Mosaic Virus (CP-AMV) controlled by the 35S promoter from Cauliflower Mosaic Virus. The selectable marker gene encoding hygromycin phosphotransferase (hph) was borne on a separate T-DNA. Forty independent transgenic events carrying the triple stack were generated, with estimated co-transformation efficiencies of 0.22 to 0.23%. Forty three percent of the events generated had a single insertion, while two events were selectable marker-free. Transcript abundance studies of the three transgenes of interest demonstrated the transcriptional competence of the inserted T-DNA. This study illustrates the feasibility of transferring multiple genes in a large single T-DNA into white clover by Agrobacterium-mediated co-transformation. Furthermore, observations of consistently delayed leaf senescence, statistically significant increases in TrneMDH transcript, and presence of CP-AMV transcript, support further analysis of these events for delayed leaf senescence under drought conditions, aluminium tolerance, and resistance to AMV.
Key message
The generation of marker-free white clover plants with three transgenes in a single T-DNA by cotransformation in one step is reported here.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing incidence of adverse environmental phenomena such as extreme temperatures, drought, and floods has been attributed to climate change. These extreme weather events impact crop yields and limit productivity in marginal environments (Mickelbart et al. 2015). Furthermore, population growth and the consequent increased demand for food have made it incumbent on researchers, pre-breeders, and breeders to enhance crop yields (Foley et al. 2011; Varshney et al. 2011). Development of genetically modified crops with adaptive traits is an important strategy to address these demands (Ricroch and Hénard-Damave 2017). After more than twenty years of progress in this area, the second generation of transgenic crops is emerging, typically expressing multiple transgenes for multiple traits. Such events have the potential to enhance tolerance to multiple biotic and abiotic stresses (Que et al. 2010; Mickelbart et al. 2015), increase yields by modification of complex multigenic traits (Dockter and Hansson 2015), and confer higher nutritive value by modification of metabolic pathways (Farre et al. 2014).
Typical methods for generation of multi-gene transgenic plants include re-transformation, sexual crossing of transgenic parent lines, and co-transformation. Re-transformation involves the sequential transformation of transgenic plants with additional transgenes. Both sexual crossing of transgenic lines and re-transformation can be labour intensive as screening for the presence of inserted transgenes at each generation is necessary (Halpin 2005; Naqvi et al. 2009). Additionally, when using these techniques, transgenes typically occupy unlinked genomic sites, which makes the production of lines homozygous for the transgenes difficult (Halpin 2005; Dafny-Yelin and Tzfira 2007; Naqvi et al. 2009). Furthermore, given that different selectable marker genes are required at each transformation round, and the number of available selectable marker genes is limited, the incorporation of a high number of transgenes by re-transformation can be challenging (Halpin 2005; Dafny-Yelin and Tzfira 2007; Farre et al. 2014).
Some of these limitations can be overcome by co-transformation. Here, transgenes can either be linked as a stack in a single transformation plasmid, or un-linked where different plasmids are used in a single transformation step (Farre et al. 2014). By using co-transformation, all the transgenes of interest can be incorporated in the first generation (T0), which saves time relative to re-transformation and sexual crossing strategies (Halpin 2005; Farre et al. 2014). Linked co-transformation provides the additional advantage of introducing the transgenes of interest into a single site in the genome (Halpin 2005; Farre et al. 2014). As a consequence, the inserted genes segregate together in subsequent generations, which simplifies the production of plants homozygous for the transgenes (Halpin 2005; Farre et al. 2014). Although insertion of multiple genes linked at a single locus can be attained by direct DNA transfer using un-linked co-transformation (Chen et al. 1998), this is rarely observed when using Agrobacterium-mediated transformation (Naqvi et al. 2009). As a consequence, linked co-transformation is the preferred strategy for transferring multiple genes with Agrobacterium (Naqvi et al. 2009).
The use of linked co-transformation for incorporating a high number of transgenes can, however, be difficult. Availability of unique restriction sites for cloning multiple genes in a single T-DNA was historically a limitation (Halpin 2005). Also, transformation efficiency declines with increasing DNA molecule length as a consequence of a greater likelihood of T-DNA fragmentation and rearrangement (Hamilton et al. 1996). Naqvi et al (2009) propose a T-DNA upper size limit of 30 kb for Agrobacterium-mediated linked co-transformation using standard binary vectors. Agrobacterium-mediated insertion of three genes linked in a single T-DNA of 22 kb was reported in potato (Zhu et al. 2012), and five transgenes linked in a single T-DNA were introduced into rice (Cao et al. 2004, 2005). Other examples of transgenic crops, approved for commercial use, generated by Agrobacterium-mediated linked co-transformation of three or more genes are summarized by Dafny-Yelin and Tzfira (2007). A combination of linked and unlinked co-transformation can be a sensible strategy when three or more transgenes are introduced (Dafny-Yelin and Tzfira 2007). For example, the construction of a β-carotenoid pathway in rice was engineered by combining linked and unlinked co-transformation for the simultaneous Agrobacterium-mediated insertion of four genes (Ye et al. 2000).
In white clover (Trifolium repens L.), transformation efficiencies are typically low in comparison to other crop species. Previous work in the species reported Agrobacterium-mediated transformation efficiencies ranging between 0.3 and 10% (Voisey et al. 1994; Christiansen et al. 2000; Ding et al. 2003; Rahimi-Ashtiani 2015; Rahimi-Ashtiani et al. 2015). Additionally, co-transformation studies reported the introduction of two genes by un-linked co-transformation, and of three genes by combining linked and un-linked co-transformation, with efficiencies estimated at 1.1 and 0.54% respectively (Vala 2012). Therefore, the number of genes that can be incorporated into white clover through linked or unlinked co-transformation, may ultimately be limited by declining transformation efficiency.
In this study, white clover plants were transformed using Agrobacterium with three genes linked in a single T-DNA: the Isopentenyl transferase (IPT) gene from Agrobacterium tumefaciens, conferring delayed leaf senescence (Lin et al. 2010; Kant et al. 2015); a nodule enhanced malate dehydrogenase (neMDH) from white clover, conferring enhanced aluminium tolerance (Labandera et al. 2005; Labandera 2007); and the AMV (Alfalfa Mosaic Virus) coat protein gene (CP-AMV), encoding resistance to AMV (Panter et al. 2012). Concomitantly a separate vector carrying either the selectable marker gene hph encoding hygromycin phosphotransferase, and the reporter gene GFP encoding green fluorescent protein, or one with hph only, was co-transformed. Transfer of the selectable marker gene using un-linked co-transformation enables the insertion of the selectable marker at a different site in the genome. As a consequence, marker-free events can be generated by segregation in subsequent generations. The incorporation of all three transgenes of interest, and T-DNA copy number, was determined by molecular analysis. Up to five transgenes were incorporated into white clover by combining linked and un-linked co-transformation. Transcriptional competence of the inserted T-DNA was evaluated by measuring transcript levels of the three transgenes of interest. Furthermore, delayed leaf senescence was assessed to confirm functionality of the IPT transgene. This work validated the feasibility of combining linked and unlinked co-transformation for transferring multiple genes into white clover in a single transformation step. The generation of transgenic events carrying a functional T-DNA, conferring a triple stack of desirable traits, enables continued evaluation of their agronomic potential.
Materials and methods
Plant material and propagation
White clover (Trifolium repens cv ‘Storm’, Heritage Seeds, Dandenong South, Australia) was used for transformations. Plants were grown in a glasshouse at 22 °C day/16 °C night, and a 16 h light/8 h dark/night cycle, in standard potting mix and vegetatively propagated by stolon cuttings.
Transformation vectors
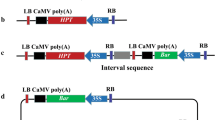
The sequence of the RNA4 coding region from AMV (CP-AMV transgene, Panter et al. (2012)) was synthesised within a CaMV35S-p:CP-AMV:nos-t cassette, with flanking att recombination sites for Gateway™ cloning (Gateway®, Thermo Fisher Scientific). The TrPT1 promoter sequence and the nodule-enhanced malate dehydrogenase coding sequence (neMDH transgene) from white clover from Labandera (2007) were synthesised within a TrPT1-p:TrneMDH:TrneMDH-t cassette with flanking att sites. The AtMYB32-p:ipt:ocs-t cassette was derived by synthesis of the Isopentenyl Transferase (IPT) gene from Agrobacterium encoded on the octopine Ti plasmid (Li et al. 1992), under the control of the MYB32 promoter from Arabidopsis (Preston et al. 2004). The pPZP200 binary vector (Hajdukiewicz et al. 1994) was digested with PmeI and Gateway™-enabled by adding the Gateway™ RfA cassette (Invitrogen) to this site, generating pDPI000001. The AtMYB32-p:ipt:ocs-t cassette was inserted into the multiple cloning site of the pDPI000001 binary vector backbone by digestion with enzymes SbfI and HindIII, creating pCLV000031. The CaMV35S-p:CP-AMV:nos-t cassette was transferred to pDONR221™ P5-P2, and the TrPT1-p:TrneMDH:TrneMDH-t cassette was transferred to pDONR221™ P1-P5 by a Gateway™ BP Clonase™ reaction according to the manufacturer's protocol (Gateway®, Thermo Fisher Scientific), generating pCLV000030 and pCLV000029 respectively. A multisite Gateway™ LR Clonase™ reaction was then performed to combine pCLV000031, pCLV000030 and pCLV000029 into the final transformation vector, pCLV000032 containing the AtMYB32-p:ipt:ocs-t, TrPT1-p:TrneMDH:TrneMDH-t, and CaMV35S-p:CP-AMV:nos-t cassettes (Fig. 1a).
Vectors used for white clover transformation. a Vector pCLV000032 containing the three transgenes of interest b vector pCLV000081 containing hph and the reporter gene turbo green fluorescent protein (tuGFP) c vector pCLV000080 containing the selectable marker gene hph for resistance to hygromycin. attB1 and attB2 Gateway att sites, RB right border of T-DNA, LB left border of T-DNA, KanR kanamycin resistance gene, SpecR/StrepR spectinomycin/streptomycin resistance gene, OriV origin of vegetative replication, OriT origin of transfer, IPT Isopentenyl transferase, TrneMDH T. repens nodule enhanced Malate dehydrogenase, CP-AMV Alfalfa mosaic virus coat protein gene, ocs terminator Octopine synthase terminator, nos terminator Nopaline synthase terminator, AtORF1 terminator Agrobacterium tumefaciens open reading frame 1 3′ untranslated region
Two different vectors carrying the selectable marker were used to test the effects of vector size on the transformation efficiencies. The pDPI000081 vector contains a CaMV19S-p:hph:AtORF1-t selection cassette and a CaMV35S:tuGFP:nos-t reporter gene cassette in a modified pBIN backbone (Bevan 1984) (Fig. 1b). The pDPI000080 vector contains a CaMV19S-p:hph:AtORF1-t selection cassette also in a modified pBIN backbone (Fig. 1c). Vector sequences were verified by restriction endonuclease analysis and independent Sanger sequencing, with sequences compared to those available in public databases using BLAST searches.
Agrobacterium and white clover transformation
Agrobacterium tumefaciens strain EHA105 was co-transformed according to Main et al. (1995) with pCLV000032 and either pDPI000080 or pDPI000081. Bacterial cultures were selected with 50 mg/l kanamycin, and 20 mg/l spectinomycin. Cotyledonary explants were co-transformed with this Agrobacterium isolate. Seeds were sterilized before transformation by rinsing with ethanol 80% for 5 min, 1.5% sodium hypochlorite (12.5 g/l active chlorine) for 16 min, followed by 8 distilled water washes. Sterilized seeds were kept in sterilized water overnight at 4 °C. Plant transformations and regeneration, including of non-transgenic isogenic controls were performed according to Ding et al. (2003), with some modifications. Explants were cultivated for 3 days in regeneration media (RM73) (Ding et al 2003), and transferred to selective RM73 containing 50 mg l−1 hygromycin and 250 mg l−1 cefotaxime at 25 °C under a 16/8 h photoperiod at a photon flux density of 80 µM m−2 s−1. Explants were transferred to fresh selection media every 2 weeks for subculture for a period of 8 weeks. Regenerated shoots were transferred into root-inducing medium (RIM73), and planted into standard potting mix after roots were developed.
Molecular analysis of transgenic white clover plants
Putative transgenic plants that survived selection under hygromycin were evaluated for presence of the transgenes of interest and the selectable marker hph by quantitative PCR (qPCR). A quantification cycle (Cq) threshold of 30 was used to distinguish transgene positive, and negative plants. Plants whose qPCR amplification was positive for sequences from any of the genes of interest, or the selectable marker gene, were considered transgenic, and the integration of an intact cassette was assumed when qPCR was positive for the three genes of interest.
High throughput DNA extraction was performed using a DNeasy 96 Plant Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Transgene presence was confirmed in putative events by qPCR. For each gene of interest, Taqman qPCR reactions were performed using Quantitect Probe PCR kit (Qiagen) with 20 µl reaction volumes, 600 nM of forward and reverse primers, 200 nM of probes (Table 1), and 1× master mix. The reaction conditions were: 10 min at 95 °C, and 40 cycles of 10 s at 95 °C, 30 s at 60 °C, and 10 s at 72 °C.
Copy number determination
The copy number of the insert was estimated by droplet digital PCR (ddPCR). For each transgene of interest, reaction mixtures were prepared in a 24 μl final volume, consisting of 600 nM of forward and reverse primers, 200 nM of probes, 12 μl of ddPCR 2× Master Mix (Bio-Rad, Hercules, CA, USA), and 20–50 ng of DNA. Primers and probes used for copy number estimations were the same as those for transgene detection (Table 1). Duplex PCRs were performed using Pyruvate dehydrogenase (PDH) as the internal reference (Narancio et al. in preparation). Emulsified 1 nl reaction droplets were generated using an AutoDG™ droplet generator (Bio-Rad, Hercules, CA, USA) and a DG8 cartridge (Bio-Rad) containing 20 μl of reaction mixture and 70 μl of ddPCR droplet generation oil (Bio-Rad) per well. The droplet emulsions were transferred to 96-well PCR plates and PCR performed using the same conditions as used in qPCR for transgene presence. The fluorescence of each thermal cycled droplet was measured using a QX100 droplet reader (Bio-Rad).
Transcript abundance analysis
Newly developed leaves were sampled from plants grown in the glasshouse at 22 °C day/16 °C night, and 16 h light/8 h dark. Samples were flash frozen and stored at − 80 °C until RNA extraction. RNA was extracted using the RNeasy kit (Qiagen). RNA was quantified using a NanoDrop N-D 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and RNA integrity monitored by 2% agarose gel electrophoresis and observing 25S/18S ribosomal RNA integrity. RNA was treated with DNase I (Sigma) to remove traces of DNA before cDNA synthesis. cDNA was synthesized using the iScript cDNA synthesis kit for RT-ddPCR (Bio-Rad). DNase I treated, no-RT controls were included in the assays to check for amplification of genomic DNA. For each gene of interest, a duplex PCR was performed using the endogenous reference gene EF1α (Narancio et al. 2018) (oligos used in these reactions are presented in Table 1). For each transgene of interest, reaction mixtures were prepared in a 24 μl final volume, consisting of 600 nM of forward and reverse primers, 200 nM of probes, 12 μl of ddPCR 2× Master Mix (Bio-Rad), and 3 µl of cDNA. Emulsified reaction droplets were generated, PCR performed, and thermal cycled droplets measured using the same conditions as used for copy number assays. Three to six biological replicates were used, and samples were obtained from randomized design for each gene of interest assay. the statistical significance of differences in TrneMDH transcript abundance between transgenic and non-transgenic lines was evaluated by one-tailed and two-tailed t tests, assuming equal variances, by GenStat for Windows edition 14 (VSN International, Hemel Hempstead, UK).
Detached leaf bioassay
Detached leaf bioassays were performed in 90 mm Petri dishes at 21 °C, with 16 h light/8 h dark at a photon flux density of 80 µM m−2 s−1. In order to evaluate leaves at a similar developmental stage, nine fully expanded leaves at the second node from the tip of the stolons were detached and placed on filter paper soaked in distilled water in the Petri dishes.
Digital images were taken when a clear contrast in colour was observed between transgenic and isogenic control leaves. Colour analysis was performed using Lemnagrid software (Lemnatec GmbH, Würselen, Germany) to estimate the proportion of green and senescing tissue. Briefly the steps involved were: identification of the object of analysis (foreground) and background pixels; noise reduction; assignment of colour bins for senescing (yellow, orange and brown) and non-senescing (green) tissue area; and, counting of pixels corresponding to the two colour bins to estimate the degree of leaf senescence.
Data from nine replicates (fully expanded leaves) for each genotype were used for the analysis, percentage data were arcsine square root-transformed, and significance was evaluated by single and two-tailed t test using GenStat for Windows edition 14. A paired single and two-tailed t test was performed, including all the studied events, to evaluate the significance of the effect of the transgene on transgenic plants.
Results
Plant transformation
The plants that survived selection were transferred to the glasshouse and tested, by qPCR, for integration of the three transgenes of interest and the selectable marker gene. Forty events were confirmed positive for the three transgenes of interest.
From the 40 events produced, 30 had available their respective isogenic non-transgenic controls, generated by dissecting cotyledonary explants from a single mature seed (Ding et al. 2003). Most of the transgenic plants exhibited normal morphology when compared to their respective non-transgenic isogenic siblings (Fig. 2). Two events confirmed positive for the three transgenes of interest, but lacked the selectable marker gene. Two combinations of vectors, pCLV000032/pCLV000080 and pCLV000032/pCLV000081, were used in transformations to determine whether T-DNA size has any effect on transformation efficiencies. Relative to pCLV000080, pCLV000081 contains an additional 1582 bp insert with the tuGFP gene driven by the CaMV35S promoter, and therefore has a T-DNA that is 58% larger. Co-transformation frequencies, estimated as the proportion of inoculated explants that yielded positive transformants for all four transgenes, were 0.22 and 0.23% for the pCLV000032/pCLV000080, and pCLV000032/pCLV000081 combinations, respectively (Table 2). Transformation frequencies for the selectable marker gene hph, were 1.76 and 1.29% for pCLV000080 and pCLV000081 vectors respectively. So, although vectors pCLV000080 and pCLV000081 have different T-DNA sizes, their transformation frequencies were similar.
Based on the observation that 75 mg l−1 hygromycin produces a 85% lethality in untransformed white clover cotyledonary explants (Rossello 2011), a lower hygromycin concentration of 50 mg l-1 was used for selection in order to allow survival of plants lacking the selectable marker. A high proportion of plants which did not carry the selectable marker gene hph survived antibiotic selection. From 231 putative events transplanted to soil, 143 carried the selectable marker, while 88 did not. This represents a 38% frequency of “escapes”.
Some putative events were qPCR positive for one or two of the transgenes of interest, consistent with insertion of an incomplete T-DNA (Fig. 3). Sixty seven percent of the transgenic events generated had a complete T-DNA that included the three transgenes of interest.
Transgene copy number
Copy number of the three transgenes of interest was estimated for the thirty events that had their respective non-transgenic isogenic controls (Table 3). Forty three percent of these events exhibited insertion of a single copy T-DNA. A dissimilar copy number of the three GOI was observed for some of the multicopy events, consistent with the occurrence of T-DNA rearrangements, and the insertion of partial transgene fragments into the genome.
Transcript abundance assays
Transcript abundance was determined in leaves under well watered conditions for the transgenes IPT and CP-AMV (Fig. 4), in 11 events, selected based on expresssion of delayed leaf senescence and low copy number. Given that the TrPT1 promoter used for this transgene is root specific (Labandera et al. 2005), TrneMDH transcript abundance was measured in root tips from transgenic events, and their respective non-transgenic isogenic controls (Fig. 4).
Transcript abundance of the inserted transgenes represented as ratios of concentration of target/concentration of reference. Error bars represent standard errors. Transcript abundance was estimated by relative quantification using droplet digital PCR (ddPCR), by calculating the ratio of concentration target (GOI transcript copies/µl)/concentration reference (EF1α transcript copies/µl). *Statistically significant at p values < 0.05 (n = 3 to 6). nd not detectable
High variability in transcript levels was observed, both between events, and between the three transgenes. IPT transcripts were detected in leaves of 9 of the 11 events evaluated. In those events where IPT transcripts were detected, the measured levels of IPT transcript were low. Transcript abundance was estimated to be between 180 and 4484 fold lower than that of the reference gene (EF1alpha). No IPT transcripts were detected in events 21 and 25.
CP-AMV transcript abundance exhibited high variation among events. Events 1 and 31 showed transcript abundance almost as high as those of the reference gene, and events 32 and 34 had CP-AMV transcript levels of a third of the reference gene. Conversely, events 9, 11 and 25 had very low CP-AMV transcript levels, and no transcripts were detected in events 4, 10 and 21.
No correlation was observed between copy number (Table 3) and transcript abundance (Fig. 4). The highest IPT transcript levels were measured in events 1 and 34 (ratio of conc. target/ conc. reference 0.00532 and 0.00262 respectively) whose T-DNA copy number are one, and low levels were observed in event 11 (conc. target/ conc. reference = 0.00085), whose copy number is approximately 7. No IPT transcripts were detected in events 21 and 25. Similarly, single copy events 1, 32, and 34 exhibited high CP-AMV transcription levels, while event 11 had very low CP-AMV transcript levels.
TrneMDH transcript abundance was assayed in root tips. Increased TrneMDH transcript abundance was observed in 12 events relative to their respective non-transgenic isogenic controls. Eight of these, events, 1, 8, 10, 11, 14, 20, 31 and 33, exhibited a significant increase (p value < 0.05), while event 26 showed a significant decrease.
Concomitant transcription of IPT and CP-AMV transgenes were detected in six events (1, 9, 11, 31, 32, and 34) from the 11 evaluated. From these, events 1, 11, and 31 exhibited concomitant increased TrneMDH transcript abundance relative to their respective non-transgenic isogenic controls. These results support the integrity of the entire T-DNA for these events, and provide further evidence of the transcriptional competence of the GOIs.
Detached leaf bioassay
Delayed leaf senescence was evaluated in transgenic events to test the effects of the IPT transgene. Levels of leaf senescence were quantified by image analysis using Lemnagrid software. Green pixels were assigned as non-senescent leaf areas, and yellow, orange and brown as senescent.
Delayed leaf senescence was observed in 11 of the 27 transgenic events evaluated relative to their respective non-transgenic isogenic controls (Figs. 5 and 6). The effect observed was consistent and event dependant. In events 10, 11, 14, and 28 delayed senescence was maintained for periods of 10 to 20 days, while in events 4, 5, 9, 21, 27, 31, and 34 it was observed for shorter periods of 3 to 10 days. No clear effect was observed in 13 events, while 3 events showed significant accelerated senescence (Fig. 6). Although some of these events exhibited significant delayed leaf senescence, this was not consistent in subsequent assays.
No clear association between IPT transcript abundance and delayed leaf senescence was observed. Seven out of nine events with detectable IPT transcript abundance exhibited significantly delayed leaf senescence. By contrast events 21 and 25, with levels of IPT transcript below the threshold of detection, also showed delayed leaf senescence. There was, however, an association with transgene copy number. Events with the highest T-DNA copy number: events 11 (7–8 copies), 14 (7–10), and 28 (5–7), along with event 10 (2–3 copies), exhibited the greatest degree of delayed leaf senescence.
A significant delay in leaf senescence was observed between transgenic and non-transgenic control plants when all 27 events were included in the analysis (p value < 0.05). These results provide collective evidence that supports the effectiveness of the introduced IPT transgene for delaying leaf senescence, independent of position effects, and the varied genetic backgrounds of the events.
Discussion
Successful insertion of a large T-DNA of 8.9 kb carrying three transgenes was routinely achieved. This study supports the efficacy of Agrobacterium-mediated linked co-transformation for transferring multiple genes into white clover. This strategy saves time and resources when compared to other methods, such as re-transformation or unlinked co-transformation (Halpin 2005; Farre et al. 2014). In addition, transgenes of interest are inserted at the same locus, which allows their linked segregation in subsequent generations.
Given that the selectable marker gene is located in a separate vector, simultaneous insertion of the two T-DNAs used is necessary for selection of the transformed events. However, the likelihood of this occuring is relatively low, which negatively impacts co-transformation frequencies. Furthermore, given that the likelihood of T-DNA breakages is correlated with insert size, the transfer of large T-DNA occurs at low frequency (Hamilton et al. 1996). It is believed that these, and other factors contributed to the low transformation frequency observed in this study.
Higher co-transformation frequencies were reported in previous studies in white clover where smaller T-DNAs carrying one or two genes were used (Rossello 2011; Vala 2012). Although factors such as Agrobacterium strain, plant genotype, and operator can be confounding, comparison between the current and previous studies provide an approximation of the effects of T-DNA size on transformation frequencies in white clover. A negative correlation between the total length of transferred T-DNAs and the co-transformation frequency is clearly observed (Fig. 7).
Levels of non-senescent tissue in detached leaf bioassays in transgenic events and their non-transgenic isogenic controls. Digital images were taken when highest contrast in colour was observed between transgenic and isogenic control leaves. (*) significant at p value < 0.001. (+) significant at p value < 0.01
Co-transformation efficiencies presented as a function of the size of the total T-DNA transferred, calculated as the sum of sizes of the two T-DNAs. Each symbol represent a different experiment: (times) AtMYB32:ipt + 35S:hph (cv. Sustain) (Vala 2012); (asterisk) TrPT1:TrneMDH + 35S:hph (cv. Sustain) (Rossello 2011); (open circle) TrPT1:TrneMDH::AtMYB32:ipt + 35S:hph (cv. Sustain) (Vala 2012); (diamond) AtMYB32:ipt::TrPT1:TrneMDH::35S:CP-AMV + 19S:hph::35S:GFP (cv. Storm) (current study); (plus) AtMYB32:ipt::TrPT1:TrneMDH::35S:CP-AMV + 19S:hph (cv. Storm) (current study)
Although the use of unlinked co-transformation for incorporating the selectable marker gene has a negative impact on transformation frequencies, it affords the possibility of producing selectable marker-free events. In events where insertion of the different T-DNAs has occured at separate loci, selectable marker-free events can be generated in subsequent generations by sexual crossing and segregation of transgenes.
Remarkably, two selectable marker-free primary events were produced. This may be explained by the relatively high proportion of plants (38%) lacking the selectable marker genes that survived (“escaped”) antibiotic selection. This was favoured by a relatively low hygromycin concentration used at the selection stages, 50 mg l−1, based on the observation that 75 mg l−1 hygromycin produces a 85% lethality in untransformed white clover cotyledonary explants within 2 weeks (Rossello 2011).
Twenty events, of the 30 generated with a matching isogenic control, exhibited one or two T-DNA copies. Low copy number is a desirable feature, as it minimizes the difficulties that segregation of the inserted genes at unlinked loci would bring in subsequent generations. Previous work reported a 50% frequency of single T-DNA insertions in white clover by Agrobacterium-mediated transformation (Rossello 2011). A frequency of 30–40% was observed for single T-DNA insertions in rice (Sallaud et al. 2003), approximately 40% was reported in maize (Ishida et al. 1996), and 31.5% was observed in soybean (Olhoft et al. 2004). Compared to these species, for this work in white clover, the frequency of single T-DNA insertions was broadly similar. However, in addition to the species transformed, many elements such as target tissue used, cultivar or genotype, Agrobacterium strain and transformation method can affect the frequency of single T-DNA insertion (Grevelding et al. 1993; Kohli et al. 2003).
Transcript abundance analysis was performed to confirm the integrity and functionality of the inserted T-DNA. Detection of transcripts from the three transgenes of interest demonstrated that they are transcriptionally competent. The inability to detect CP-AMV or IPT transcripts, nor an increase in TrneMDH transcript levels in events 21 and 25 suggests that the three transgenes of interest may be silenced. A lack of expression of integrated T-DNA has been previously reported (Gelvin 2003). T-DNA can be inserted in different regions of the genome, with contrasting chromatin structures, which could be either transcriptionally active or silent. Transcription can also be positively or negatively affected depending on whether the T-DNA is inserted proximally or distally relative to endogenous transcriptionally activating elements (Birch 1997; Gelvin 2003). Furthermore, transgene silencing can be triggered by DNA methylation (Matzke and Matzke, 1998), and by changes in environmental conditions (Zhong 2001).
CP-AMV transcript levels showed high variability across events. Considering that this transgene is controlled by the constitutive promoter CaMV35S, high transcription abundance was expected. However, no transcription activity was observed in some events, and others exhibited low transcription levels. Different types of transgene silencing could be taking place in these events. Previous studies report the occurrence of transgene silencing when certain transcript levels are exceeded, as a consequence of using strong constitutive promoters or due to a high number of inserted T-DNA copies (Lechtenberg et al. 2003). Alternatively, promoter homology-dependent silencing could provide a credible explanation of such observations. In these cases, sequence homology between transgenes can trigger promoter methylation, which results in transgene silencing (Matzke et al. 1996). In this work, possible silencing of the CaMV35S:CP-AMV transgene was observed in events co-transformed with the vector pDPI000081 carrying GFP controlled by the constitutive CaMV35S promoter (events 4, 9, 10, 11, 20, 21, 25). Interestingly, only event number 1, which lacked the selectable marker gene, and events 31, 32 and 34, co-transformed using vector pDPI000080 without the CaMV35S:tuGFP transgene, exhibited a high CP-AMV transgene expression. These results are consistent with possible silencing caused by the duplication of the CaMV35S promoter, used for controlling two different transgenes. If this was the case, it is predicted that CP-AMV transgene transcription levels may be restored by removal of the T-DNA carrying the CaMV35S:GFP transgene in subsequent generations by sexual crossing, providing the silencing has not become irreversible.
IPT transcripts were detected in all the events tested, except for events 21 and 25. However, where detectable, levels were very low. Semi-quantitative RT-PCR performed in Arabidopsis for AtMYB32 transcript quantification, revealed low transcript levels in leaves compared to flowers (Preston et al. 2004). These results are consistent with low activity of the AtMYB32 promoter in leaves in Arabidopsis. The low transcript abundance observed for this work in white clover events can further be explained by the localized activity of the AtMYB32 promoter, being confined mostly to vascular tissues (Lin et al. 2003). High cytokinin production can induce negative effects in plant development, such as impairment of root growth, and a loss of apical dominance (Smigocki and Owens, 1989). Therefore, in order to produce transgenic events with the desired phenotype, cytokinin levels should not be too high, to avoid negative effects, but sufficient to confer delayed leaf senescence. Arguably, the low IPT transcript levels observed here are consistent with these requirements, and should result in the desired phenotype.
The observation of delayed leaf senescence in 11 of the 27 events evaluated provides further evidence of the functionality of the AtMYB32:IPT cassette. These results demonstrate that despite the low IPT transcript abundance observed in the events generated, these levels are sufficient to elevate cytokinin levels and to significantly delay leaf senescence. IPT transcript abundance analysis confirmed transcription in events 11, 31 and 34, in which leaf senescence was greatly delayed. By contrast, transcript was below the limits of detection in events 21 and 25, which also exhibited delayed leaf senescence. Although IPT transcript levels were not measured in all of the generated events, the absence of delayed leaf senescence in a number of events is indicative of low or nonexistent IPT transcription. Furthermore, the inconsistent observation of delayed leaf senescence in some events, and the failure to detect transcript in some events that exhibited delayed leaf senescence, lead us to speculate that transcript levels may vary due to other factors, such as diurnal, spatial or developmental dependency.
Eight events showed a statistically significant increase in TrneMDH transcript levels when compared to their controls (p-value = 0.05), and event 26 exhibited a statistically significant decrease. The increase in TrneMDH transcript levels observed validates the use of the TrPT1:TrneMDH cassette to increase malate production in roots. It is anticipated that events exhibiting increased TrneMDH transcript levels will have elevated malate production, and consequently the potential for enhanced Al3+ tolerance.
Although the presence of transcript for the three transgenes, and the observed delayed leaf senescence provide evidence of functionality of the inserted T-DNA, evaluations at the biochemical and phenotypic level will need to be carried out in the future. Cytokinin quantification, exudation of organic acids, and translation of the protein CP-AMV, would provide further evidence of the competence of the inserted transgenes. Ultimately, evaluations of aluminium tolerance, and AMV resistance, and for the IPT transgene effects such as increased seed yield and enhanced stress tolerance (Lin et al. 2010), will enable identification of events with the desired agronomic performance.
In conclusion, insertion of a large T-DNA for stacking multiple transgenes in a single round of transformation has been evaluated and validated in white clover. Although the estimated co-transformation efficiencies were relatively low, we consider that these values demonstrate the feasibility of inserting an even higher number of transgenes in future studies. Nevertheless, an evaluation of labour and time costs should be carried out, in the knowledge that increasing T-DNA size would likely have an escalating negative impact on transformation frequencies.
Change history
21 October 2020
In the original article, the order of transgene names in the column headings of Table 3 was incorrect. The correct Table 3 is printed below. The error does not change the outcome of the study. We apologize for any inconvenience.
Abbreviations
- Atmyb32 :
-
Arabidopsis thaliana MYB 32
- CaMV35S :
-
Cauliflower mosaic virus 35S
- CP-AMV:
-
Coat protein Gene from Alfalfa Mosaic Virus
- ddPCR:
-
Droplet digital PCR
- EF1alpha :
-
Eukaryotic elongation factor alpha 1
- GFP:
-
Green fluorescent protein
- GOI:
-
Gene of interest
- hph :
-
Hygromycin phosphotransferase
- IPT :
-
Isopentenyl transferase
- RIM:
-
Root-inducing media
- RM:
-
Regeneration media
- PDH :
-
Pyruvate dehydrogenase
- qPCR:
-
Quantitative PCR
- T-DNA:
-
Transfer DNA
- TrneMDH :
-
Trifolium repens nodule-enhanced malate dehydrogenase
- TrPt1 :
-
Trifolium repens phosphate transporter gene
- tuGFP:
-
Turbo green fluorescent protein
References
Bevan M (1984) Binary Agrobacterium vectors for plant transformation nucleic. Nucleic Acids Res 12:8711–8721
Birch RG (1997) Plant transformation: problems and strategies for practical application. Breed Forage Plants Genome Era 48:297–326. https://doi.org/10.1146/annurev.arplant.48.1.297
Cao MX, Huang JQ, Wei ZM et al (2004) Engineering higher yield and herbicide resistance in rice by mediated multiple gene transformation. Crop Sci 44:2206. https://doi.org/10.2135/cropsci2004.2206
Cao MX, Huang JQ, Wei ZM et al (2005) Agrobacterium-mediated multiple gene transformation in rice using a single vector. J Integr Plant Biol 47:233–242. https://doi.org/10.1111/j.1744-7909.2005.00015.x
Chen L, Marmey P, Taylor NJ et al (1998) Expression and inheritance of multiple transgenes in rice plants. Nat Biotechnol 16:1060–1064. https://doi.org/10.1038/3511
Christiansen P, Gibson JM, Moore A et al (2000) Transgenic Trifolium repens with foliage accumulating the high sulphur protein, sunflower seed albumin. Transgenic Res 9:103–113. https://doi.org/10.1023/A:1008967409302
Dafny-Yelin M, Tzfira T (2007) Delivery of multiple transgenes to plant cells. Plant Physiol 145:1118–1128. https://doi.org/10.1104/pp.107.106104
Ding Y-L, Aldao-Humble G, Ludlow E et al (2003) Efficient plant regeneration and Agrobacterium-mediated transformation in Medicago and Trifolium species. Plant Sci 165:1419–1427. https://doi.org/10.1016/j.plantsci.2003.08.013
Dockter C, Hansson M (2015) Improving barley culm robustness for secured crop yield in a changing climate. J Exp Bot 66:3499–3509. https://doi.org/10.1093/jxb/eru521
Farre G, Blancquaert D, Capell T et al (2014) Engineering complex metabolic pathways in plants. Ann Rev Plant Biol. https://doi.org/10.1146/annurev-arplant-050213-035825
Foley JA, Ramankutty N, Brauman KA et al (2011) Solutions for a cultivated planet. Nature 478:337–342. https://doi.org/10.1038/nature10452
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “Gene-Jockeying” tool. Microbiol Mol Biol Rev 67:16–37. https://doi.org/10.1128/MMBR.67.1.16
Grevelding C, Fantes V, Kemper E et al (1993) Single-copy T-DNA insertions in Arabidopsis are the predominant form of integration in root-derived transgenics, whereas multiple insertions are found in leaf discs. Plant Mol Biol 23:847–860. https://doi.org/10.1007/BF00021539
Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25:989–994. https://doi.org/10.1007/BF00014672
Halpin C (2005) Gene stacking in transgenic plants: the challenge for 21st century plant biotechnology. Plant Biotechnol J 3:141–155. https://doi.org/10.1111/j.1467-7652.2004.00113.x
Hamilton CM, Frary A, Lewis C, Tanksley SD (1996) Stable transfer of intact high molecular weight DNA into plant chromosomes. Proc Natl Acad Sci U S A 93:9975–9979. https://doi.org/10.1073/pnas.93.18.9975
Ishida Y, Saito H, Ohta S et al (1996) High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol 14:745–750. https://doi.org/10.1038/nbt0696-745
Kant S, Burch D, Badenhorst P et al (2015) Regulated expression of a cytokinin biosynthesis gene IPT delays leaf senescence and improves yield under rainfed and irrigated conditions in canola (Brassica napus L.). PLoS ONE. https://doi.org/10.1371/journal.pone.0116349
Kohli A, Twyman RM, Abranches R et al (2003) Transgene integration, organization and interaction in plants. Plant Mol Biol 52:247–258. https://doi.org/10.1023/A:1023941407376
Labandera CM (2007) Development and evaluation of transgenic white clover (Trifolium repens) for enhanced aluminium tolerance and phosphorus acquisition efficiency. La Trobe University
Labandera CM, Lin Y-H, Ludlow EJ, et al (2005) Discovery, isolation and characterisation of promoters in white clover (Trifolium repens L.). In: MO Humphreys (ed) ‘Proceedings of the Fourth International Symposium on the Molecular Breeding of Forage and Turf.’ Wageningen Academic Publishers: Wageningen, Aberystwyth, Wales, p 168
Lechtenberg B, Schubert D, Forsbach A et al (2003) Neither inverted repeat T-DNA configurations nor arrangements of tandemly repeated transgenes are sufficient to trigger transgene silencing. Plant J 34:507–517. https://doi.org/10.1046/j.1365-313X.2003.01746.x
Li Y, Hagen G, Guilfoyle TJ (1992) Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Dev Biol 153:386–395. https://doi.org/10.1016/0012-1606(92)90123-X
Lin Y-H, Ludlow E, Kalla R et al (2003) Organ-specific, developmentally-regulated and abiotic stress-induced activities of four Arabidopsis thaliana promoters in transgenic white clover (Trifolium repens L.). Plant Sci 165:1437–1444. https://doi.org/10.1016/j.plantsci.2003.08.011
Lin Y-H, Ludlow E, Schrauf G, et al (2010) LXRTM transgenic white clover plants (Trifolium repens L.) with delayed leaf senescence, increased seed yield and improved stress tolerance. In: Ed. Raul Rios (ed) Proceedings of the Sixth International Symposium on the Molecular Breeding of Forage and Turf’. INTA, Buenos Aires, Argentina
Main GD, Reynolds S, Gartland JS (1995) Electroporation protocols for Agrobacterium. In: Davey MR (ed) KMA Gratland. Methods in molecular biology. Humana Press, Totowa, pp 405–412
Matzke AJM, Matzke MA (1998) Position effects and epigenetic silencing of plant transgenes. Curr Opin Plant Biol 1:142–148. https://doi.org/10.1016/S1369-5266(98)80016-2
Matzke MA, Matzke AJM, Eggleston WB (1996) Paramutation and transgene silencing: a common response to invasive DNA? Trends Plant Sci 1:382–388. https://doi.org/10.1016/1360-1385(96)10039-X
Mickelbart MV, Hasegawa PM, Bailey-serres J (2015) Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat Publ Gr 16:237–251. https://doi.org/10.1038/nrg3901
Naqvi S, Farre G, Sanahuja G et al (2009) When more is better: multigene engineering in plants. Trends Plant Sci 15:48–56. https://doi.org/10.1016/j.tplants.2009.09.010
Narancio R, John U, Mason J, Spangenberg G (2018) Selection of optimal reference genes for quantitative RT-PCR transcript abundance analysis in white clover (Trifolium repens L.). Funct Plant Biol 45:737–744. https://doi.org/10.1071/FP17304
Olhoft PM, Flagel LE, Somers DA (2004) T-DNA locus structure in a large population of soybean plants transformed using the Agrobacterium-mediated cotyledonary-node method. Plant Biotechnol J 2:289–300. https://doi.org/10.1111/j.1467-7652.2004.00070.x
Panter S, Chu PG, Ludlow E et al (2012) Molecular breeding of transgenic white clover (Trifolium repens L.) with field resistance to Alfalfa mosaic virus through the expression of its coat protein gene. Transgenic Res 21:619–632. https://doi.org/10.1007/s11248-011-9557-z
Preston J, Wheeler J, Heazlewood J et al (2004) AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J 40:979–995. https://doi.org/10.1111/j.1365-313X.2004.02280.x
Que Q, Chilton M-DM, de Fontes CM et al (2010) Trait stacking in transgenic crops: challenges and opportunities. GM Crops 1:220–229. https://doi.org/10.4161/gmcr.1.4.13439
Rahimi-Ashtiani S (2015) Biosynthesis of proanthocyanidins in white clover (Trifolium repens L.): single cell omics for designing pathway. La Trobe University Bundoora
Rahimi-Ashtiani S, Sahab S, Panter S, Mason J, Spangenberg G (2015) Clovers (Trifolium spp.). In: Wang K (ed) Agrobacterium protocols. Methods in molecular biology, vol 1223. Springer, New York
Ricroch AE, Hénard-Damave MC (2017) Next biotech plants: new traits, crops, developers and technologies for addressing global challenges. Crit Rev Biotechnol 36:675–690. https://doi.org/10.3109/07388551.2015.1004521
Rossello FJ (2011) Production and characterization of transgenic white clover for Alfalfa Mosaic Virus resistance and aluminium tolerance. La Trobe University
Sallaud C, Meynard D, van Boxtel J et al (2003) Highly efficient production and characterization of T-DNA plants for rice (Oryza sativa L.) functional genomics. Theor Appl Genet 106:1396–1408. https://doi.org/10.1007/s00122-002-1184-x
Smigocki AC, Owens LD (1989) Cytokinin-to-auxin ratios and morphology of shoots and tissues transformed by a chimeric isopentenyl transferase gene. 808–811
Vala B (2012) Transgenic white clover for enhanced yield and performance: Trait dissection, trait stacking, and phenomics. La Trobe University
Varshney RK, Bansal KC, Aggarwal PK et al (2011) Agricultural biotechnology for crop improvement in a variable climate: hope or hype? Trends Plant Sci 16:363–371. https://doi.org/10.1016/j.tplants.2011.03.004
Voisey CR, White DWR, Dudas B et al (1994) Agrobacterium-mediated transformation of white clover using direct shoot organogenesis. Plant Cell Rep 13:309–314
Ye X, Al-Babili S, Klo A, et al (2000) Engineering the provitamin A (B-Carotene) biosynthetic pathway into (Carotenoid-Free) rice endosperm. 287:303–306
Zhong GY (2001) Genetic issues and pitfalls in transgenic plant breeding. Euphytica 118:137–144. https://doi.org/10.1023/A:1004048019670
Zhu S, Li Y, Vossen JH et al (2012) Functional stacking of three resistance genes against Phytophthora infestans in potato. Transgenic Res 21:89–99. https://doi.org/10.1007/s11248-011-9510-1
Acknowledgements
We thank Dr. Daniel Isenegger for critical reading of the manuscript.
Funding
This project was funded by Agriculture Victoria, and Dairy Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by Sergio J. Ochatt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Narancio, R., Ding, YL., Lin, YH. et al. Application of linked and unlinked co-transformation to generate triple stack, marker-free, transgenic white clover (Trifolium repens L.). Plant Cell Tiss Organ Cult 142, 635–646 (2020). https://doi.org/10.1007/s11240-020-01891-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01891-6