Abstract
Background
Escherichia coli is a bacterial species widely distributed among mammals and avian species, and also a member of the normal intestinal microbiota. However, some E. coli strains of different pathotypes can cause disease in both humans and animals. Atypical enteropathogenic E. coli (aEPEC) can infect both animals and humans or influence the severity of other ongoing infections.
Results
In the present study, a total of 332 samples were collected from ducks, geese, turkeys, chickens, and pigeons from the Hungarian Veterinary Diagnostic Directorate, two slaughterhouses, two pigeon keepers and one backyard chicken farm. E. coli was isolated and verified from 319 samples. The isolates were screened by PCR for diarrheagenic E. coli pathotypes. Altogether seven atypical enteropathogenic E. coli (aEPEC) strains were identified: two from four-week-old dead turkeys, two from force-fed geese, and three from pigeons. No further pathotypes were identified in the collection. The atypical EPEC strains were classified phylogenetically to B1, B2, and F, and four out of the seven aEPEC isolates proved to be multidrug resistant. Serotypes of aEPEC strains were uniform collected from same farms and showed diversity between their origins with O76, O145, O109 serogroups.
Conclusions
This is the first report in the literature about aEPEC in goose (Anser anser domestica). Furthermore, this is the first isolation of aEPEC from turkeys and pigeons in Hungary. The uneven distribution of aEPEC in different age groups of poultry suggests that aEPEC disappears with growing up, but stress (e.g.: force-feeding) and concurrent diseases might promote its reappearance in the intestine.
Similar content being viewed by others
Background
E. coli is a bacterial species widely distributed among mammals and birds. The majority of E. coli strains take part in maintaining the normal function of the healthy intestinal tract and protect it from invasion by pathogenic bacteria. However, certain E. coli strains can cause mild or more severe diseases as facultative pathogenic bacteria in animals and humans as well. E. coli strains are categorized into extraintestinal (ExPEC) and intestinal (DEC) pathogenic groups depending on the site of the infection caused by them. ExPEC strains are classified into three categories, namely uropathogenic E. coli (UPEC), meningitis-associated E. coli (MNEC), and avian pathogenic E. coli (APEC). All DEC infect mainly the intestinal tract, but the infection mechanism and process vary by pathotype. Therefore, DEC was divided into six pathogenic groups, namely enteropathogenic E. coli (EPEC), verotoxigenic/shigatoxigenic E. coli (VTEC/STEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), and diffusely adherent E. coli (DAEC). These pathotypes were identified on the basis of their key virulence factors (eae-EPEC, eae and stx-VTEC, stA and lt1-ETEC, ipaH-EIEC, aggR-EAEC) and their histological effects (DAEC) [1, 2].
E. coli is a common cause of human infection and diarrhea in the world [3, 4]. In such cases, poultry are an important source of human exposure because chickens, turkeys and waterfowl are kept in high numbers and their products are consumed in the largest volume in the world as a meat source. Wild birds and free-range poultry also have a high chance of spreading possibly pathogenic E. coli strains. Poultry carry pathogenic E. coli in their intestines [5, 6] or the bacteria may be present on poultry-derived products [7,8,9] in the priority order of EPEC, VTEC, ETEC, EIEC, and DAEC. EPEC is an important pathotype based upon the frequency of infections caused by it in humans both in the developing and the developed countries [3, 4], and sometimes it causes mass outbreaks [10].
EPEC was divided into typical EPEC (tEPEC) and atypical EPEC (aEPEC) according to the pilus (bundle-forming pilus, BFP) forming ability (encoded by bfpA gene and its EAF plasmid carrier) of the bacterium, which is missing from aEPEC strains [4, 11]. The frequency of typical EPEC in epidemics and diarrhea cases decreased in the last few decades, and this pathotype is harbored permanently only by humans. The role of tEPEC in human infections has been taken over by aEPEC. Atypical EPEC has increasing frequency in diarrhea cases. This position of aEPEC is promoted by its wide presence in several animal species including poultry, which can raise the possibility of zoonotic risk [3].
To date, there is little information about the effect of aEPEC on animal species. However, many studies have demonstrated that aEPEC can also cause diarrhea in different animal species and influence the outcome of these infections in dogs, cats, turkeys, and lambs [12,13,14,15,16]. Some authors have also suggested that these animal species could act as the source of human aEPEC infections [17, 18].
Broilers frequently harbor aEPEC strains and their meat can also carry this pathogenic E. coli after slaughter [7, 9, 19, 20]. However, so far we have only very limited information about the existence of aEPEC in waterfowl species and pigeons.
Therefore, our aim was to investigate the presence of DEC pathotypes in five common poultry species, mainly in waterfowl, and to determine the possible effect of age on aEPEC frequency.
Results
Bacterial strains
Overall, 332 swab samples were collected from poultry. Each sample came from one bird as an individual specimen. However, lactose-positive colonies were isolated only from 319 samples (n = 35 pigeons, n = 42 chickens, n = 87 ducks, n = 101 geese, n = 54 turkeys), and they were verified biochemically as E. coli. Escherichia coli strains originating from the Diagnostic Directorate (DD) came from a pigeon (n = 1), chickens (n = 29), ducks (n = 36), geese (n = 53), and turkeys (n = 4). Escherichia coli bacteria isolated at the Backyard (BY) from pigeons (n = 34) and chickens (n = 13). Escherichia coli were identified from ducks (n = 51), geese (n = 48) and turkeys (n = 50) from Slaughterhouse (SH) (Table 1).
Pathogenic groups
None of the E. coli isolates belonged to the VTEC, ETEC, EAEC and EIEC pathotypes because of the absence of stx1 and stx 2 (VTEC), sta and lt1 (ETEC), aggR (EAEC), ipaH (EIEC) virulence genes screened by PCR [1]. In seven samples, the eae (encoding intimin adhesin) gene was detected, and thus these samples were identified as the EPEC pathotype [11]. We further classified EPEC strains as aEPEC on the basis of the missing EPEC Adherence Factor (EAF) plasmid and its carried bfpA gene by PCR [11]. All aEPEC isolates carried tir (translocated intimin receptor) which is a key virulence factor of EPEC and EHEC. Our aEPEC strains isolated from turkeys (n = 2 from the DD, both 4 weeks old), pigeons (n = 1 from the DD, 6 months old, n = 2 from BY, both nestlings) and geese (n = 2 from SH, both 16 weeks old).
Phylogenetic, serogroups and antimicrobial resistance of the aEPEC isolates
Both of turkey aEPEC strains were MDR, but they represented different phylogenetic groups, namely B1 (O/not typable:H/not moving) and F (O76:H/not moving). One turkey aEPEC had an exceptional feature, showing resistance to 14 out of the 15 tested antimicrobials and being sensitive only to gentamicin. Both of the goose aEPEC strains were MDR and showed resistance to 9 and 11 antimicrobials, respectively. However, they belonged to the same phylogenetic and serogroup, B2 and O145:H(spontaneous agglutination) respectively. Pigeon aEPEC strains belonged to the B1 phylogenetic group. However, pigeon aEPEC strains were resistant against maximum four antimicrobials and one strain showed resistance to only two. Nestling pigeons originated from one farm and has same serotype (O109:H21). Atypical EPEC from 6 month old pigeon serotype was O(not typable):H35 (Table 2).
The prevalence of antimicrobial resistant aEPEC strains isolated from turkeys and geese was significantly (p = 0.0037) higher than that found in pigeons.
Discussion
Because of the scarcity of relevant information in the literature, our aim was to study the distribution of aEPEC in five important poultry species and the possible effects of age on its prevalence.
Several research groups have reported the high prevalence of aEPEC around slaughtering age in broilers (at 5–6 weeks of age) and on their carcass [5,6,7, 9, 19]. Furthermore, some authors have suggested that aEPEC strains present a potential risk of zoonosis [21,22,23]. However, we were curious about the presence of aEPEC in different age groups of chickens. We did not find atypical EPEC in young chicks (n = 14 from 3 farms) and adult chickens (n = 28 from 6 farms), although we could have presumed this from our previous studies and from the findings of other authors [7, 9, 19].
There was no high caseload of dead turkeys (n = 4 from one farm) at the DD in 2020, but two aEPEC strains were isolated from two four-week-old turkeys. This finding was not unique, as aEPEC had been reported previously in turkeys [24] and found to be associated with other co-infections [14, 25]. Atypical EPEC was not detected by us from the slaughterhouse samples (n = 50), where the age of turkeys was around 20 weeks.
Results had been very scarce about the prevalence of aEPEC in ducks [26], and no data were available about aEPEC in geese yet. Atypical EPEC were not carried by ducks (n = 87 from 9 farms) according to our findings, which were in harmony with the results of another research group [27]. However, our samples cannot be compared properly with the results of others, because the other studies did not focus on or record the ducks’ age. Our E. coli strains came from young (0–1 week old, n = 31), middle-aged (14–15 weeks old, n = 55) groups, and in one case the age was over one year. Furthermore, our two aEPEC strains isolated from geese represented the first detection of aEPEC in this species. Interestingly, they were isolated from the middle-aged group, from force-fed geese used for foie gras production.
Atypical EPEC were carried by 3 pigeons (n = 1 from the DD, n = 2 from BY), one of which originated from a 6-month-old pigeon and two from nestling pigeons. We could not detect atypical EPEC in older and adult pigeons. Our findings in pigeons are in harmony with the results of other scientist in that pigeons can carry aEPEC. However, the comparison with the findings of other researchers was very limited because they focused on searching antibiotic resistance and virulence genes of E. coli and did not record the age of sampled pigeons which may influence E. coli pathogroups distribution [26, 28,29,30].
In summary, according to our own findings and data of the literature about the distribution of aEPEC in the main poultry species, we suppose that all poultry have the capability to carry aEPEC. However, we suppose that the age of the birds and certain environmental factors (e.g.: force-feeding) or diseases (causing mortality in our cases) can influence the prevalence of carriage. We assume that poultry do not carry aEPEC in a considerable degree in the first weeks of life, and only in the later phases, around 4–6 weeks of age, can aEPEC propagate in high numbers in the intestines of healthy [9, 19] and sick birds [14]. Later on aEPEC will disappear from poultry as recorded by others in sheep [13].
By studying the antimicrobial resistance of aEPEC, we found significant differences between turkeys, geese and pigeons. Turkeys and geese as intensively kept birds had more opportunity to get medical treatment from time to time. This fact could be behind the very high levels of antimicrobial resistance found in turkey and goose. However, pigeons, especially as nestlings, have a lower chance to receive antimicrobial treatment, and thus the members of their microbiota have lower resistance to antimicrobials. However, the evidence that aEPEC strains are frequently MDR, especially against widely used antimicrobials, can suggest a possible horizontal gene transfer of resistance genes to humans as well.
Three out of the 7 aEPEC strains belonged to phylogenetic groups F and B2, which contain potential ExPEC strains and, therefore, could pose a higher risk of zoonotic infection. Furthermore, groups F and B2 (both of which had belonged to the B2 phylogenetic group earlier) are common among aEPEC strains as we detected earlier [19]. The serotypes of aEPEC strains were uniform from same farms and showed diversity (O76, O145, O109) comparing their origins.
Conclusions
In summary, our main result is to report the presence of aEPEC in goose (Anser anser domesticus) for the first time in the literature. Furthermore, we first isolated aEPEC from turkeys and pigeons in Hungary. From the uneven distribution of aEPEC in the different age groups of poultry we conclude that aEPEC disappears with the advancement of age.
Methods
Sample collection
Samples were collected from poultry carcasses at the Veterinary Diagnostic Directorate of the National Food Chain Safety Office (DD; Budapest, Hungary) from sick birds (animals originated from 8 chicken, 8 duck, 10 goose, 1 turkey farm) and from healthy poultry at two slaughterhouses (SH) (one waterfowl and one turkey), at one backyard chicken farm (BY) and at two pigeon keepers (PK) in 2020.
We collected samples from birds of diverse ages (from day-old to 3 years) in order to identify possible differences in the distribution of the E. coli pathotypes. Birds were classified into age groups for better visualization of the age distribution in each poultry species.
Samples were aseptically collected from the cecum of dead or slaughtered birds and from the cloaca of live chickens and pigeons with a sterile cotton swab, and they were stored at 4 °C at most for 2 h before further processing.
Bacteriological identification
All cotton swabs were smeared on MacConkey agar, and one lactose-positive colony from each sample was inoculated further until they seemed to be uniform. Then, bacterial colonies were examined by primary (catalase, oxidase) and secondary biochemical tests (indol, methyl red, Voges–Proskauer, citrate utilization tests) to confirm them as E. coli. Their pure cultures were kept at – 80 °C for long-term storage.
Antimicrobial resistance
Antimicrobial resistance of the bacteria was determined using the disc diffusion method performed according to the recommendations of the Clinical and Laboratory Standards Institute (M100-S25, 2020) [31]. Briefly, the procedure was as follows: 0.5 McFarland even solutions were made from pure bacterial cultures and were streaked evenly on Mueller–Hinton agar. Then, the antimicrobial discs were evenly placed on it and the plates were incubated overnight at 37 °C until their evaluation. Based on the appearing inhibition zones, the bacteria were categorized into a resistant or a sensitive group (the intermediate group was regarded as sensitive) according to the CLSI recommendation for the Enterobacteriaceae family [31, 32].
The following antimicrobials were used: penicillins [ampicillin (10 µg)]; ß-lactam/ß-lactam inhibitor combination [amoxicillin-clavulanate (20 µg/10 µg)]; cephems [cefoxitin (30 µg)]; aminoglycosides [gentamicin (10 µg), kanamycin (30 µg), streptomycin (10 µg)]; tetracyclines [oxytetracycline (30 µg)]; fluoroquinolones [ciprofloxacin (5 µg), enrofloxacin (5 µg)]; quinolones [nalidixic acid (30 µg)]; folate pathway inhibitors [trimethoprim (30 µg), sulfonamide (300 µg), trimethoprim + sulfonamide (1.25 µg/23.75 µg)]; phenicols [chloramphenicol (30 µg)]; nitrofurans [nitrofurantoin (300 µg)]. If an E. coli strain showed resistance to more than four groups of antimicrobials, we considered it a multidrug-resistant strain (MDR).
Genotypic evaluation of Escherichia coli
DNA templates were made from E. coli by the boiling method. In this procedure we inoculated 2 ml LB (Luria–Bertani) medium with the pure culture of isolated E. coli and incubated the culture overnight at 37 °C. In the next step five hundred microliters bacterial broth was measured and centrifuged at 9000 rpm for 2 min, then the supernatant was discarded. The remaining pellets were covered with bi-distilled water and boiled for 10 min at 96 °C, then they were centrifuged for 10 s. The supernatants were removed as template and were used for further procedures.
The PCR master mix was made from DreamTaq Green© and its buffer according to the manufacturer’s recommendations (Invitrogen) with 0.5 µM specific primer for each reaction. The details of the primers used are summarized in Table 3.
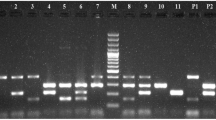
Amplicons were separated by gel electrophoresis in 1.5 % gel at constant 110 V for approximately 30 min by the use of positive (amplicon of strain which carrying the appropriate gene) and negative control (empty PCR master mix) beside a 100-bp marker (Invitrogen©) for each run. The gels were recorded by the use of UV light with a camera.
Phylogenetic classification
Phylogenetic groups of E. coli were determined by multiplex PCR (ChuA, YjaA, TspE4C2, arpA) described by Clermont et al. [39].
Serotyping the eae positive E. coli strains
Determination of O and H antigens was performed with agglutination test described by Ørskov et al. [41] at the National Public Health Center, Budapest, Hungary.
Statistical analysis
The comparison of frequency of antimicrobial resistance between eae positive strains was made with ANOVA (with 95 % confidence intervals) using the R statistical program (R Core Team, 2020) [42]. The other results were not as comprehensive as to require statistical tests for their comparison and interpretation.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- aEPEC:
-
Atypical enteropathogenic Escherichia coli
- BFP:
-
Bundle-forming pilus
- BY:
-
Backyard chicken farm
- DAEC:
-
Diffusely adherent Escherichia coli
- DD:
-
Veterinary Diagnostic Directorate of the National Food Chain Safety Office
- DEC:
-
Intestinal pathogenic Escherichia coli
- EAEC:
-
Enteroaggregative Escherichia coli
- EAF:
-
EPEC adherence factor
- EIEC:
-
Enteroinvasive Escherichia coli
- EPEC:
-
Enteropathogenic Escherichia coli
- ETEC:
-
Enterotoxigenic Escherichia coli
- ExPEC:
-
Extraintestinal pathogenic Escherichia coli
- MDR:
-
Multidrug-resistant
- MNEC:
-
Meningitis-associated Escherichia coli
- NT:
-
Not typable
- PCR:
-
Polymerase chain reaction
- SP:
-
Spontaneous agglutination
- SH:
-
Slaughterhouse
- STEC:
-
Shigatoxigenic Escherichia coli
- tEPEC:
-
Typical enteropathogenic Escherichia coli
- UPEC:
-
Uropathogenic Escherichia coli
- VTEC:
-
Verotoxigenic Escherichia coli
References
Kaper JB. Pathogenic Escherichia coli. Int J Med Microbiol. 2005;295(6–7):355–6.
Lopes LM, Fabbricotti SH, Ferreira AJ, Kato MA, Michalski J, Scaletsky IC. Heterogeneity among strains of diffusely adherent Escherichia coli isolated in Brazil. J Clin Microbiol. 2005;43(4):1968–72.
Hernandes RT, Elias WP, Vieira MA, Gomes TA. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol Lett. 2009;297(2):137–49.
Hu J, Torres AG. Enteropathogenic Escherichia coli: foe or innocent bystander? Clin Microbiol Infect. 2015;21(8):729–34.
Doregiraee F, Alebouyeh M, Fasaei BN, Charkhkar S, Tajedin E, Zali MR. Isolation of atypical enteropathogenic and shiga toxin encoding Escherichia coli strains from poultry in Tehran, Iran. Gastroenterol Hepatol from Bed to Bench 2016;9(1):53–7.
Wang L, Nakamura H, Kage-Nakadai E, Hara-Kudo Y, Nishikawa Y. Prevalence, antimicrobial resistance and multiple-locus variable-number tandem-repeat analysis profiles of diarrheagenic Escherichia coli isolated from different retail foods. Int J Food Microbiol. 2017;249:44–52.
Lee GY, Jang HI, Hwang IG, Rhee MS. Prevalence and classification of pathogenic Escherichia coli isolated from fresh beef, poultry, and pork in Korea. Int J Food Microbiol. 2009;134(3):196–200.
Kariuki S, Gilks C, Kimari J, Muyodi J, Getty B, Hart CA. Carriage of potentially pathogenic Escherichia coli in chickens. Avian Dis. 2002;46:721–4.
Alonso MZ, Padola NL, Parma AE, Lucchesi PMA. Enteropathogenic Escherichia coli contamination at different stages of the chicken slaughtering process. Poult Sci. 2011;90(11):2638–41.
Møller-Stray J, Eriksen HM, Bruheim T, Kapperud G, Lindstedt BA, Skeie Å, et al. Two outbreaks of diarrhoea in nurseries in Norway after farm visits, April to May 2009. Euro Surveill. 2012;17(47):20321.
Trabulsi LR, Keller R, Tardelli Gomes TA. Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis. 2002;8(5):508–13.
Kjaergaard AB, Carr AP, Gaunt MC. Enteropathogenic Escherichia coli (EPEC) infection in association with acute gastroenteritis in 7 dogs from Saskatchewan. Can Vet J. 2016;57(9):964–8.
Martins FH, Guth BE, Piazza RM, Elias WP, Leão SC, Marzoa J, et al. Lambs are an important source of atypical enteropathogenic Escherichia coli in southern Brazil. Vet Microbiol. 2016;196:72–7.
Pakpinyo S, Ley DH, Barnes HJ, Vaillancourt JP, Guy JS. Enhancement of enteropathogenic Escherichia coli pathogenicity in young turkeys by concurrent turkey coronavirus infection. Avian Dis. 2003;47(2):396–405.
Puño-Sarmiento J, Medeiros L, Chiconi C, Martins F, Pelayo J, Rocha S, et al. Detection of diarrheagenic Escherichia coli strains isolated from dogs and cats in Brazil. Vet Microbiol. 2013;166(3–4):676–80.
Watson VE, Jacob ME, Flowers JR, Strong SJ, DebRoy C, Gookin JL. Association of atypical enteropathogenic Escherichia coli with diarrhea and related mortality in kittens. J Clin Microbiol. 2017;55(9):2719–35.
Arais LR, Barbosa AV, Andrade JRC, Gomes TAT, Asensi MD, Aires CAM et al. Zoonotic potential of atypical enteropathogenic Escherichia coli (aEPEC) isolated from puppies with diarrhoea in Brazil. Vet Microbiol. 2018;227:45–51.
Watson VE, Hazen TH, Rasko DA, Jacob ME, Elfenbein JR, Stauffer SH et al. Comparative genomics of atypical enteropathogenic Escherichia coli from kittens and children identifies bacterial factors associated with virulence in kittens. Infect Immun. 2020;IAI.00619 – 20.
Adorján A, Makrai L, Mag T, Jánosi S, Könyves L, Tóth I. High frequency of multidrug-resistant (MDR) atypical enteropathogenic Escherichia coli (aEPEC) in broilers in Hungary. Front Vet Sci. 2020;7:511.
Comery R, Thanabalasuriar A, Garneau P, Portt A, Boerlin P, Reid-Smith RJ, et al. Identification of potentially diarrheagenic atypical enteropathogenic Escherichia coli strains present in Canadian food animals at slaughter and in retail meats. Appl Environ Microbiol. 2013;79(12):3892–6.
Bélanger L, Garenaux A, Harel J, Boulianne M, Nadeau E, Dozois CM. Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol Med Microbiol. 2011;62(1):1–10.
Manges AR. Escherichia coli and urinary tract infections: the role of poultry-meat. Clin Microbiol Infect. 2016;22(2):122–9.
Stromberg ZR, Johnson JR, Fairbrother JM, Kilbourne J, Van Goor A, Curtiss R, et al. Evaluation of Escherichia coli isolates from healthy chickens to determine their potential risk to poultry and human health. PLoS One. 2017;12(7):e0180599.
Pakpinyo S, Ley DH, Barnes HJ, Vaillancourt JP, Guy JS. Prevalence of enteropathogenic Escherichia coli in naturally occurring cases of poult enteritis-mortality syndrome. Avian Dis. 2002;46(2):360–9.
Guy JS, Smith LG, Breslin JJ, Vaillancourt JP, Barnes HJ. High mortality and growth depression experimentally produced in young turkeys by dual infection with enteropathogenic Escherichia coli and turkey coronavirus. Avian Dis. 2000;44(1):105–13.
Farooq S, Hussain I, Mir MA, Bhat MA, Wani SA. Isolation of atypical enteropathogenic Escherichia coli and Shiga toxin 1 and 2f-producing Escherichia coli from avian species in India. Lett Appl Microbiol. 2009;48(6):692–7.
Sacristán C, Esperón F, Herrera-León S, Iglesias I, Neves E, Nogal V, et al. Virulence genes, antibiotic resistance and integrons in Escherichia coli strains isolated from synanthropic birds from Spain. Avian Pathol. 2014;43(2):172–5.
Borges CA, Cardozo MV, Beraldo LG, Oliveira ES, Maluta RP, Barboza KB, et al. Wild birds and urban pigeons as reservoirs for diarrheagenic Escherichia coli with zoonotic potential. J Microbiol. 2017;55(5):344–8.
Torres-Mejía AM, Blanco-Peña K, Rodríguez C, Duarte F, Jiménez-Soto M, Esperón F. Zoonotic agents in feral pigeons (Columba livia) from Costa Rica: possible improvements to diminish contagion risks. Vector Borne Zoonotic Dis. 2018;18(1):49–54.
van Hoek AHAM, van Veldhuizen JNJ, Friesema I, Coipan C, Rossen JWA, Bergval IL, et al. Comparative genomics reveals a lack of evidence for pigeons as a main source of stx2f-carrying Escherichia coli causing disease in humans and the common existence of hybrid Shiga toxin-producing and enteropathogenic E. coli pathotypes. BMC Genomics. 2019;20(1):271.
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 30th ed. Wayne: CLSI supplement M100 Clinical and Laboratory Standards Institute. 2020.
CLSI. Performance Standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. 4th ed. Wayne: CLSI supplement VET08. 2018.
China B, Pirson V, Mainil J. Typing of bovine attaching and effacing Escherichia coli by multiplex in vitro amplification of virulence-associated genes. Appl Environ Microbiol. 1996;62:3462–5.
Rajkhowa S, Hussain I, Rajkhowa C. Detection of heat-stable and heat-labile enterotoxin genes of Escherichia coli in diarrhoeic faecal samples of mithun (Bos frontalis) calves by polymerase chain reaction. J Appl Microbiol. 2009;106(2):455–8.
Echeverria P, Sethabutr O, Venkatesan M, Murphy GS, Eampokalap B, Hoge CW. Detection of Shigellae and enteroinvasive Escherichia coli by amplification of the invasion plasmid antigen H DNA sequence in patients with dysentery. J Infect Dis. 1993;167(2):458–61.
Kimata K, Shima T, Shimizu M, Tanaka D, Isobe J, Gyobu Y, et al. Rapid categorization of pathogenic Escherichia coli by multiplex PCR. Microbiol Immunol. 2005;49:485–92.
Gunzburg ST, Tornieporth NG, Riley LW. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J Clin Microbiol. 1995;33:1375–7.
Franke J, Franke S, Schmidt H, Schwarzkopf A, Wieler LH, Baljer G, et al. Nucleotide sequence analysis of enteropathogenic Escherichia coli (EPEC) adherence factor probe and development of PCR for rapid detection of EPEC harboring virulence plasmids. J Clin Microbiol. 1994;32:2460–3.
Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013;5(1):58–65.
Ogura Y, Ooka T, Whale A, Garmendia J, Beutin L, Tennant S, Krause G et al. TccP2 of O157:H7 and non-O157 enterohemorrhagic Escherichia coli (EHEC): challenging the dogma of EHEC-induced actin polymerization. Infect Immun. 2007;(2):604–12. https://doi.org/10.1128/IAI.01491-06.
Ørskov F, Ørskov I. Serotyping of Escherichia coli. Methods Microbiol. 1984;(14):43–112. https://doi.org/10.1016/S0580-9517(08)70447-1
R Core Team, R Foundation for Statistical Computing, Vienna, Austria. 2020, R: A language and environment for statistical computing. https://www.R-project.org/.
Acknowledgements
The first author would like to thank László Fodor for his valuable help and guidance in the laboratory work which facilitated the completion of this research. We also thank for Szilárd Tóth (National Public Health Center, Budapest, Hungary) for serotyping of EPEC strains.
Funding
This work was supported by the European Union and co-financed by the European Social Fund (Grant agreement nos.: EFOP-3.6.1-16-2016-00024 and EFOP-3.6.3-VEKOP-16-2017-00005).
Author information
Authors and Affiliations
Contributions
AA performed most of the steps of the experimental work, ÁT took part in the sample collection and the isolation of bacteria, LK and IT took part in the coordination of the experimental work, and all of the authors participated in the writing of this scientific paper. The author(s) read and approved the final manuscript.
Authors' information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Adorján, A., Thuma, Á., Könyves, L. et al. First isolation of atypical enteropathogenic Escherichia coli from geese (Anser anser domestica) and first description of atypical EPEC from turkeys and pigeons in Hungary. BMC Vet Res 17, 263 (2021). https://doi.org/10.1186/s12917-021-02968-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-021-02968-w