Abstract
Some high-risk Avian Pathogenic Escherichia coli (APEC) clones have been associated with increased economic losses caused by avian colibacillosis. They may represent an additional food consumption concern due to the potential zoonotic role causing urinary tract infections mainly related to E. coli ST73 and ST95 lineages. This study aimed to characterize APEC isolated from slaughterhouse carcasses presenting lesions compatible with avian colibacillosis. We analyzed about 6500 broilers carcasses, and 48 showed lesions consistent with colibacillosis. Forty-four strains of E. coli were isolated, with 77.27% (n = 34/44) classified as APEC. The isolates belonged to the phylogenetic groups B2 (41.17%, n = 14/34), G (20.59%, n = 7/34), A (17.65%, n = 6/34), B1 (8.82%, n = 3/34), and E (5.88%, n = 2/34). Determining the phylogenetic group of 5.88% (n = 2/34) of the strains was impossible. Moreover, 20.59% (n = 7/34) were positive to the clonal groups ST117, 8.82% (n = 3/34) to ST95, and 8.82% (n = 3/34) were classified as belonging to serogroup O78 by PCR screening. Strains of APEC from O78 serogroup and ST117 are considered high-risk clones for poultry, and our data reinforced the need for surveillance of these pathogens in poultry farms and slaughterhouses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colibacillosis can be associated with opportunistic infection by commensal strains due to improper management of the poultry environment. Still, it can also occur as a primary disease when the birds are infected by strains of Avian pathogenic Escherichia coli (APEC). Avian colibacillosis leads to significant economic losses due to high carcass condemnation rates and the cost of treatment [1, 2]. The most common injuries related to carcass condemnation are pericarditis, perihepatitis, airsacculitis, and cellulitis [3].
APEC belongs to the extra-intestinal E. coli (ExPEC) pathotype presenting a population with a wide variety of genotypes [2]. There are no definitive specific virulence markers for diagnostic purposes to classify APEC, but some protocols have been proposed for differentiating between commensal strains and virulent lineages. Johnson and collaborators [4] described a quick tool for the classification of APEC related to the presence of minimum predictive virulence factors, namely, iroN, ompT, hlyF, iss, and iutA, encoding for salmochellin receptor, outer membrane protease, putative avian hemolysin, serum resistance, and presence of aerobactin, respectively. The determination of serogroups, based on the serologic or molecular detection of the somatic antigen O, can also be helpful for the identification of high-risk strains since most colibacillosis outbreaks are frequently related to serogroups O78, O2, and O1 [5,6,7].
Furthermore, E. coli can also be classified into phylogenetic groups and sequence types (ST). Clermont and collaborators [8] described seven different phylogenetic groups according to the presence of the genes arpA, chuA, yjaA, and TspE4.C2, which define the phylogenetic groups (A, B1, B2, C, D, E, and F). A new phylogenetic group, named G, was described by Clermont and collaborators [9], which includes both virulent and resistant lineages characterized by the presence of the ybgD gene, coding for a putative fimbrial protein.
Significant relationships exist between the phylogenetic groups and multi-locus sequence typing (MLST), a typing method for ST determination based on seven housekeeping genes and fumC, gyrB, icd, mdh, purA, and recA [10]. The G group is often related to ST117 and may, to a lesser extent, be related to ST657, ST454, ST738, and ST174 [9].
APEC can be classified as a high-risk clone or clonal complex, with the ability to colonize, persist, and spread antimicrobial resistance determinants and increase pathogenicity. APEC ST131, ST23, ST428, ST355, and ST117 are high-risk clones [11]. Some additional lineages of APEC can also be related to the zoonotic-related ExPEC clonal groups ST73 and ST95, suggesting that APEC could play a role in human urinary tract infection and sepsis [12, 13].
Although sequence type is considered the main predictor of bird or human infection risk, the MLST only be performed by partial or total genome sequencing. However, these tools still need to be available as a routine diagnostic at avian diseases laboratory in Brazil, resulting in a lack of epidemiologic data. Some protocols have been using specifics primers for screening the high-risk clones by the polymerase chain reaction (PCR), allowing the generation of diagnostic data quickly and with a lower cost, although with less precision compared to the standard gold techniques, such as the whole genome sequencing (WGS) [6, 14, 15].
This study aimed to characterize APEC isolated from carcasses with lesions compatible with colibacillosis in a slaughterhouse in São Paulo, Brazil, and make a screening of high-risk clones by PCR.
Material and methods
Sample collection and processing
This research was conducted in a poultry slaughterhouse under the authority of the Federal Inspection Service in São Paulo, Brazil. We analyzed about 6500 carcasses, and 48 samples from condemned carcasses due to lesions compatible with colibacillosis were collected in September 2019. As described in Table 1, the lesions included cellulitis, perihepatitis, airsacculitis, carcass condemnation triad (airsacculitis, perihepatitis, and pericarditis), salpingitis, fibrinous pneumonia, ascites, and abscess. The samples were collected with a sterile swab stored in a transport medium, sent to the Avian Medicine Laboratory of the School of Veterinary Medicine and Animal Sciences of the University of São Paulo (FMVZ-USP), streaked in MacConkey agar (Difco, Detroit, MI), and incubated at 37 °C for 24 h. Identification was performed by matrix-assisted laser ionization and desorption (MALDI-TOF MS). The DNA used for PCR was extracted by boiling at 115 °C for 20 min.
Determination of APEC
APEC identification was performed by the pentaplex PCR method, detecting minimal predictive factors, iroN, ompT, hlyF, iss, and iutA genes, according to the method described by Johnson and collaborators [4]. The primers used are described in Table 2. APEC was determined by a minimum of three or more virulence genes. Amplification conditions consisted of 95 °C for 15 min, followed by 34 cycles of denaturation at 95 °C for 30 s, an annealing cycle at 60 °C for 30 s, an extension cycle at 72 °C for 60 s, and a final extension cycle at 72 °C for 5 min. Amplicons were visualized under an ultraviolet light transilluminator in a 1.5% agarose gel electrophoresis.
Determination of the phylogenetic group
The determination of the phylogenetic group of the strains classified as APEC was performed according to Clermont and collaborators [8], consisting of a quadruplex PCR (chuA, TspE4.C2, yjaA, and arpA) classifying the strains into one of the seven phylogenetic groups (A, B1, B2, C, D, E, or F). The B2 and F strains were also subjected to PCR to detect the cfaB and ybgD genes, classifying the group G, as described by Clermont and collaborators [9]. Amplification was performed under the following conditions: 95 °C for 15 min, followed by 34 cycles of denaturation at 95 °C for 30 s, an annealing cycle at 59 °C for 30 s, an extension cycle at 72 °C for 60 s, and a final extension cycle at 72 °C for 5 min. Primers and visualization followed the previously described methods.
Determination of ST73, ST95, and ST117
ST117, ST95, and ST73 were determined using a uniplex PCR for each group. The primers used are described in Table 2. Amplification was performed under the following conditions: 95 °C for 15 min, followed by 34 cycles of denaturation at 95 °C for 30 s, an annealing cycle at 56 °C for 30 s, an extension cycle at 72 °C for 60 s, and a final extension cycle at 72 °C for 5min. Primers and visualization followed the previously described methods.
Serogroup O78
The serogroup O78 of APEC was determined using a uniplex PCR according to the methodology described by Liu and collaborators [15]. The primers used are described in Table 2. Amplification was performed under the following conditions: 95 °C for 15 min, followed by 34 cycles of denaturation at 95 °C for 30 s, annealing at 53 °C for 30 s, extension at 72 °C for 60 s, and a final extension cycle at 72 °C for 5 min. Primers and visualization followed the previously described methods.
Results
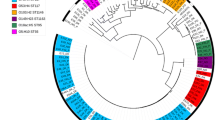
Forty-four strains of E. coli were isolated, and 77.27% (34/44) were classified as APEC. The phylogenetic analysis showed that 41.17% (14/34) belonged to the phylogenetic group B2, 20.55% (7/34) were classified as G, 17.65% (6/34) as A, 8.82% (3/34) as B1, and 5.88% (2/34) as E. It was not possible to determine the phylogenetic group of 5.88% (n = 2/34) of the strains by the Clermont typing [8, 9].
ST screening evaluation showed that 20.59% (n = 7/34) strains were classified as ST117, and 8.82% (n = 3/34) were ST95. No ST73 strains were found.
Regarding the serogroup, 8.82% (n = 3/34) were classified as O78, with 66.67% (n = 2/3) strains belonging to phylogroup G and 33.33% (n = 1/3) belonging to B2. The distribution of phylogenetic groups of different lineages and serogroups is described in Table 3.
Discussion
Colibacillosis is one of the most important bacterial diseases in the poultry industry because it is exceedingly difficult to prevent infections. For many decades, colibacillosis was controlled by adding antibiotics as a food additive. Nowadays, growth promoters are banned, and the challenges regarding husbandry in the industry are complex, particularly in Brazil, one of the biggest producers and exporters of poultry products in the world. Here, we evaluated the frequency of APEC in carcasses with common lesions related to colibacillosis in a Brazilian slaughterhouse and identified that 77.27% of strains were classified as APEC.
The frequency of APEC isolation described in this study (77.27%) was intermediate to the frequency (90%, n = 45/50) defined by Subedi and collaborators [16] in broilers with suspected colibacillosis in Nepal between 2016 and 2017, and the frequency (57.25%, n = 79/138) described by De Carli and collaborators [17] in breeders, broilers and turkeys with suspected colibacillosis in Brazil, between 2010 and 2011, suggesting regional and temporal variations of APEC isolation.
Although seasonal and geographic variations exist, the average carcass condemnation rate for colibacillosis in Brazil is usually less than 0.5%. However, between 2020 and 2021, the carcass condemnation increased by about forty times in Paraná, Brazil. Some hypotheses for this increase include the circulation of a new variant of the avian coronavirus in Brazil, for which commercial bronchitis infectious vaccine protection has not been proven, in addition to the local emergence of high-risk APEC strains detected by our group in 2018 [18]. So, the economic losses caused by the emergence of APEC and avian bronchitis infectious virus aroused great concern in other Brazilian states and highlighted the need to improve the diagnosis of high-risk APEC. We analyzed about 6500 carcasses, and 48 (±0,73%) were sampled due to macroscopic lesions. However, 77.2% of strains were considered virulent by the presence of minimal predictive virulence genes, as Johnson and collaborators (2008) described.
APEC is considered the more heterogeneous category of ExPEC pathotypes, and the characterization of agents involves a set of diagnostic tools ranging from evaluating virulence genes present in ColV plasmid, serogroup, and phylogroups [1]. Pires-dos-Santos and collaborators [19] reported a high frequency of phylogroup B2-APEC (52.9%, n = 36/68) in broiler breeders with salpingitis and peritonitis. The authors also demonstrate a relationship between APEC from the phylogenetic group B2 and ST95. The phylogroup B2 was the most predominant in our study, with a frequency of 41.17%. Here, we identified 3/34 ST95 APEC, positive for the virulent profile (ompT, iss, iroN, hlyF, iutA) (Table 3). Mehat and collaborators [20] mention that strains from lineages B2-ST95, serogroup O2 or O1, must be considered primary etiological agents of colibacillosis and have a distinct genotype compared with strains isolated from incidental or opportunistic infections in broilers. In addition, the authors highlighted the O78 serogroup found in the lineages ST23 (phylogroup C) and ST117 (phylogroup G).
Johnson and collaborators [6] described a relationship between the G group and ST117, also observed in this study. The phylogroup G is the second-most prevalent in our investigation, and ST117 represented 20.59% of APEC. However, only 2/34 isolates belonged to the O78 serogroup, suggesting the presence of other serogroups in São Paulo, Brazil. An outbreak of colibacillosis in 88 Nordic farms showed the high prevalence of ST117 O78:H4 strains. But some broilers are infected by other lineages, including ST117 of the serotype O53:H4 [2].
Here, the frequency of serogroup O78 was 8.82%. These results differ from those obtained by Barbieri and collaborators [21], which reported a 23% isolation frequency for the serogroup O78 in carcasses with colisepticemia in Brazil between 2007 and 2010. A similar frequency of O78 (26.2%) was reported by Newman and collaborators [22] in the USA. The high-risk clones of APEC included O78 but were also associated with other serogroups from lineages of ST117, ST95, ST131, ST23, ST428, ST428, and ST355 [6, 11, 20].
The next-generation sequencing and in silico analysis of APEC improved the knowledge of colibacillosis agents. But the use of more accessible screening PCR protocols is necessary and may contribute to the epidemiological studies because we currently have a lacune of information in Brazil. Here, the high frequency of APEC in broilers carcass in São Paulo and strains from B2-ST95 and G-ST117 present the current challenge for the poultry industry and reinforce the need to adopt new strategies for specific control and surveillance of these high-risk clones circulating in Brazil.
References
Kathayat D, Lokesh D, Ranjit S, Rajashekara G (2021) Avian pathogenic escherichia coli (Apec): an overview of virulence and pathogenesis factors, zoonotic potential, and control strategies. Pathogens 10(4). https://doi.org/10.3390/pathogens10040467
Ronco T, Stegger M, Olsen RH et al (2017) Spread of avian pathogenic Escherichia coli ST117 O78: H4 in Nordic broiler production. BMC Genomics 18(1). https://doi.org/10.1186/s12864-016-3415-6
Ferreira JAP, Knöbl T (2020) Colibacilose. In: Andreatti Filho RL, Berchieri Júnior Â, da Silva EN, Back A, di Fábio J, MAF Z (eds) Doença Das Aves, 3rd edn, pp 519–536
Johnson TJ, Wannemuehler Y, Doetkott C, Johnson SJ, Rosenberger SC, Nolan LK (2008) Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J Clin Microbiol 46(12):3987–3996. https://doi.org/10.1128/JCM.00816-08
Ewers C, Janßen T, Kießling S, Philipp HC, Wieler LH (2004) Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from colisepticemia in poultry. Vet Microbiol 104(1-2):91–101. https://doi.org/10.1016/j.vetmic.2004.09.008
Johnson TJ, Miller EA, Flores-Figueroa C et al (2022) Refining the definition of the avian pathogenic Escherichia coli (APEC) pathotype through inclusion of high-risk clonal groups. Poult Sci 101(10). https://doi.org/10.1016/j.psj.2022.102009
Christensen H, Bachmeier J, Bisgaard M (2021) New strategies to prevent and control avian pathogenic Escherichia coli (APEC). Avian Pathol 50(5):370–381. https://doi.org/10.1080/03079457.2020.1845300
Clermont O, Christenson JK, Denamur E, Gordon DM (2013) The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep 5(1):58–65. https://doi.org/10.1111/1758-2229.12019
Clermont O, Dixit OVA, Vangchhia B et al (2019) Characterization and rapid identification of phylogroup G in Escherichia coli, a lineage with high virulence and antibiotic resistance potential. Environ Microbiol 21(8):3107–3117. https://doi.org/10.1111/1462-2920.14713
Gordon DM, Clermont O, Tolley H, Denamur E (2008) Assigning Escherichia coli strains to phylogenetic groups: multi-locus sequence typing versus the PCR triplex method. Environ Microbiol 10(10):2484–2496. https://doi.org/10.1111/j.1462-2920.2008.01669.x
Baquero F, Tedim AP, Coque TM (2013) Antibiotic resistance shaping multi-level population biology of bacteria. Front Microbiol 4. https://doi.org/10.3389/fmicb.2013.00015
Cummins ML, Reid CJ, Djordjevic SP (2022) F Plasmid lineages in Escherichia coli ST95: implications for host range, antibiotic resistance, and zoonoses. mSystems 7:1–16. https://doi.org/10.1128/msystems.01212-21
Saidenberg ABS, van Vliet AHM, Stegger M et al (2022) Genomic analysis of the zoonotic ST73 lineage containing avian and human extraintestinal pathogenic Escherichia coli (ExPEC). Vet Microbiol 267. https://doi.org/10.1016/j.vetmic.2022.109372
Doumith M, Day M, Ciesielczuk H et al (2015) Rapid identification of major Escherichia coli sequence types causing urinary tract and bloodstream infections. J Clin Microbiol 53(1):160–166. https://doi.org/10.1128/JCM.02562-14
Liu B, Wu F, Li D et al (2010) Development of a serogroup-specific DNA microarray for identification of Escherichia coli strains associated with bovine septicemia and diarrhea. Vet Microbiol 142(3-4):373–378. https://doi.org/10.1016/j.vetmic.2009.10.019
Subedi M, Luitel H, Devkota B et al (2018) Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan Nepal. BMC Vet Res 14(1). https://doi.org/10.1186/s12917-018-1442-z
de Carli S, Ikuta N, Lehmann FKM et al (2015) Virulence gene content in Escherichia coli isolates from poultry flocks with clinical signs of colibacillosis in Brazil. Poult Sci 94(11):2635–2640. https://doi.org/10.3382/ps/pev256
Cunha MPV (2018) Determinantes emergentes de resistência antimicrobiana em Escherichia coli de origem clínica, fecal e de carne de aves e suínos. 196f. Tese (doutorado) – Universidade de São Paulo. Faculdade de Medicina Veterinária e Zootecnia, São Paulo
Pires-dos-Santos T, Bisgaard M, Christensen H (2013) Genetic diversity and virulence profiles of Escherichia coli causing salpingitis and peritonitis in broiler breeders. Vet Microbiol 162(2-4):873–880. https://doi.org/10.1016/j.vetmic.2012.11.008
Mehat JW, van Vliet AHM, la Ragione RM (2021) The Avian Pathogenic Escherichia coli (APEC) pathotype is comprised of multiple distinct, independent genotypes. Avian Pathol 50(5):402–416. https://doi.org/10.1080/03079457.2021.1915960
Barbieri NL, de OL, Tejkowski TM et al (2015) Molecular characterization and clonal relationships among Escherichia coli strains isolated from broiler chickens with colisepticemia. Foodborne Pathog Dis 12:74–83. https://doi.org/10.1089/fpd.2014.1815
Newman DM, Barbieri NL, de Oliveira AL, Willis D, Nolan LK, Logue CM (2021) Characterizing avian pathogenic Escherichia coli (APEC) from colibacillosis cases, 2018. PeerJ 9. https://doi.org/10.7717/peerj.11025
Acknowledgements
Coordination for the Improvement of Higher Education Personnel (CAPES – Finance code 001) and National Council for Scientific and Technological Development (CNPq – 306396/2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mariana X Byndloss
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barbosa, F., Santos, B., Rocha, V. et al. Detection of high-risk Avian Pathogenic Escherichia coli (APEC) isolated from broilers in São Paulo, Brazil. Braz J Microbiol 54, 2471–2475 (2023). https://doi.org/10.1007/s42770-023-01023-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01023-0