Abstract
Background
Cyclic nucleotide-gated ion channels (CNGCs) are nonselective cation channels that are ubiquitous in eukaryotic organisms. As Ca2+ channels, some CNGCs have also proven to be K+-permeable and involved in plant development and responses to environmental stimuli. Sugarcane is an important sugar and energy crop worldwide. However, reports on CNGC genes in sugarcane are limited.
Results
In this study, 16 CNGC genes and their alleles were identified from Saccharum spontaneum and classified into 5 groups based on phylogenetic analysis. Investigation of gene duplication and syntenic relationships between S. spontaneum and both rice and Arabidopsis demonstrated that the CNGC gene family in S. spontaneum expanded primarily by segmental duplication events. Many SsCNGCs showed variable expression during growth and development as well as in tissues, suggesting functional divergence. Light-responsive cis-acting elements were discovered in the promoters of all the identified SsCNGCs, and the expression of most of the SsCNGCs showed a diurnal rhythm. In sugarcane, the expression of some SsCNGCs was regulated by low-K+ treatment. Notably, SsCNGC13 may be involved in both sugarcane development and its response to environmental stimuli, including response to low-K+ stress.
Conclusion
This study identified the CNGC genes in S. spontaneum and provided insights into the transcriptional regulation of these SsCNGCs during development, circadian rhythm and under low-K+ stress. These findings lay a theoretical foundation for future investigations of the CNGC gene family in sugarcane.
Similar content being viewed by others
Introduction
Calcium ions (Ca2+) are ubiquitous and important second messengers in all eukaryotes [1] and participate in a variety of physiological, biochemical, and metabolic processes. In plants, Ca2+ is involved in plant growth regulation; development; responses to abiotic [2] and biotic [3] factors [4, 5]; and processes such as pollen tube and root hair growth [6], senescence programming [7], responses to low-potassium (K+) stress [8] and pathogen-associated molecular pattern (PAMP)-triggered immunity [9,10,11]. After a stimulus is detected, a specific Ca2+ influx occurs immediately and serves as a specific Ca2+ signal. The occurrence of Ca2+ influx in plant cells is based on Ca2+-permeable channels that are located in the plasma membrane and that can deliver Ca2+ into the cytoplasm from the extracellular matrix or from intracellular stores [12, 13]. In plants, several putative Ca2+-permeable channels have been identified, including cyclic nucleotide-gated channels (CNGCs) and glutamate receptors (GLRs); annexins and several types of mechanosensitive channels [14]; mid-complementing activity channels (MCA) [15, 16]; and hyperosmolality-gated Ca2+ permeable channel 1.3 (OSCA1.3) [17, 18]. Notably, the members of CNGC family have proven to be broadly involved in and critical to both development and stress resistance in plants [19].
CNGCs are evolutionarily conserved 3’,5’-cyclic adenosine/guanosine monophosphate (cAMP/cGMP)-gated ion channels that exist widely in animals and plants [20]. All CNGC proteins are mainly composed of six transmembrane domains (TM1-TM6) and a pore region (P) located between TM5 and TM6. Moreover, CNGCs also contain a calmodulin-binding domain (CaMB) and a cyclic nucleotide-binding domain (CNBD). There is a phosphate binding cassette (PBC) and a hinge region adjacent to the PBC in the CNBD [19]. However, there is a difference in CNGC structure between plants and animals. In plants, both CNBD and CaMBD are located in the cytosolic CNGC C-terminal, and there is an overlap at the C-terminal side of CNBD [21]. However, in animals, the two domains are located in the N-terminal and C-terminal, respectively [16]. Interestingly, an N-terminal CaMBD has been identified in AtCNGC12 [22].
To date, the CNGC gene family in many plants has been identified, and the members in various plant species vary in quantity from 9 [23] to 47 [19, 24]. Generally, plant CNGCs have been classified into 5 groups: Groups I, II, III, IVa, and IVb. According to previous reports, CNGC members are involved in responses to a wide range of developmental and environmental stimuli [20].
In Arabidopsis thaliana, AtCNGC6 and AtCNGC9, together with the leucine-rich repeat (LRR) RLK CLAVATA1 (CLV1), are essential for the elevation of [Ca2+]cyt and for stem cell fate in roots [25]. AtCNGC16 and AtCNGC18 were found to primarily be expressed in pollen. Loss of AtCNGC18 function leads to defects in pollen tube growth and growth into the transmitting tract and results in male sterility [26]. AtCNGC16 is crucial for pollen tolerance to heat, drought and external calcium chloride during germination and the initiation of pollen tube tip growth. Disruptions of Atcngc16 have been found to result in a more than 10-fold stress-dependent reduction in seed set [27].
Arabidopsis CNGC2 and CNGCb from Physcomitrella patens are reported to control land plant thermal sensing and to have been acquired for thermotolerance. Atcngc2 and Ppcngcb mutant plants show growth retardation and a hyperthermosensitive phenotype [28]. In addition, AtCNGC6 also mediates heat-induced Ca2+ influx, which promotes the expression of heat shock protein (HSP) genes and increases thermotolerance [29]. In rice (Oryza sativa), OsCNGC14 and OsCNGC16 are crucial for Ca2+ signals induced by temperature stresses. The null mutant of Oscngc14 or Oscngc16 has been shown to display higher accumulation levels of hydrogen peroxide, increased cell death, and reduced survival rates under heat or chilling stress [30]. Moreover, overexpression of AtCNGC19 and AtCNGC20 can enhance plant tolerance to salt stress [31].
CNGCs have also been confirmed to contribute to plant immunity by increasing cytosolic Ca2+ [3]. First, AtCNGC2 was found to be involved in plant immunity. The atcngc2 null mutant was characterized as “defense, no death 1” (dnd1), which showed a deficient autoimmune phenotype with high salicylic acid (SA) accumulation and a constitutive PATHOGENESIS-RELATED (PR) gene [32]. Another Arabidopsis “defense, no death” mutant was characterized as yet another CNGC mutant, the atcngc4 mutant [33]. AtCNGC2 and AtCNGC4 were also confirmed to work together and assemble into a functional Ca2+ channel that mediated Ca2+ influx after flg22 (a bacterial flagellin peptide that is always used as a PAMP) was recognized by the receptor complex [34]. The rice OsCNGC9 was also labeled CELL DEATH and SUSCEPTIBLE to BLAST 1 (CDS1), and its deletion was found to impair plant blast resistance. Moreover, overexpression of OsCNGC9 can enhance rice pattern-triggered immunity (PTI) and resistance to blast [35].
In tomato, SlCNGC16 is member of group IVb, and silencing of one of these genes enhances resistance to Pythium aphanidermatum and Sclerotinia sclerotiorum while reducing resistance to tobacco rattle virus [36]. Moreover, the members of group IVb in wheat (Triticum aestivum L.), TaCNGC14 and TaCNGC16, contribute to plant resistance against Puccinia striiformis f. sp. tritici (Pst) [24]. CNGC genes in cotton (Gossypium hirsutum L.) were also thought to contribute to resistance against Verticillium dahliae [37]. AtCNGC20 has proven to be important for regulated immunity, and the gain-of-function mutant Atcngc20-4 (AtCNGC20L371F) with misregulation of Ca2+-permeability exhibits autoimmunity and leads to an increased plant defense response [38]. AtCNGC19 in the same subfamily as AtCNGC20 also mediates basal defense signaling to regulate Pirformospora indica colonization of Arabidopsis roots [39]. In addition, AtCNGC19 has proven to activate herbivory-induced Ca2+ influx and plant defense against Spodoptera litura. Loss of AtCNGC19 function results in decreased defense against S. litura [40].
Sugarcane (Saccharum spp.) is an important C4 graminoid crop worldwide that can be used for renewable fuels and sucrose production. Sugarcane is a polyploid interspecific hybrid with singularly complex genomes. Therefore, studies on functional genes in sugarcane are slow to develop. To the best of our knowledge, there have been few studies on the CNGC gene family in sugarcane. In 2018, the allele-defined genome of Saccharum spontaneum L. (AP85-441), one ancestor of modern sugarcane, was published and now serves as a resource to accelerate sugarcane functional gene studies [41]. In this study, we identified the members of the CNGC gene family in S. spontaneum based on genome-wide sequence information. Moreover, a series of bioinformatics analyses and expression profiles of these CNGC genes during plant growth and in response to low K+ conditions were performed. The results of this study could provide important information and lay a theoretical foundation for further functional characterization of CNGC genes in sugarcane.
Materials and methods
Plant materials and growth conditions
The sugarcane commercial hybrid YT99-66 (bred by Institute of Bioengineering, Guangdong Academy of Sciences) was used as the experimental material in this study for identification of CNGC genes involved in sugarcane response to low-K+ stress. Healthy single-bud sets of YT 99–66 were buried in the sand and cultured in a greenhouse. Forty-five-day after budding, sugarcane seedlings were hydroponically cultured for 1 month and then treated with low-K+. The culture medium was replaced every week, and the roots were collected at 0, 6, 12, 24, 48 and 72 h after treatment. All the materials were frozen in liquid nitrogen immediately after collection and stored at − 80 °C. There were three independent replicates in each treatment, and there were 15 seedlings in each group. All the samples were divided into two aliquots, one part for transcriptome sequencing and the other for validating gene expression.
Identification and sequence analysis of CNGC gene family members in S. spontaneum
The S. spontaneum L. (AP85-441) genome [41] was used as the reference genome in this study. All the data for S. spontaneum used in this study were downloaded from the SGD (Saccharum Genome Database, http://sugarcane.zhangjisenlab.cn/sgd/html/download.html).
The identification of CNGC gene family members in S. spontaneum was carried out in three steps. First, the protein sequences of CNGC genes from Arabidopsis [42], rice [43] and maize [44] were retrieved from the Phytozome 12 database (https://phytozome.jgi.doe.gov/pz/portal.html) [45] and used as reference sequences for potential S. spontaneum CNGC identification. These reference CNGC sequences were searched against all the S. spontaneum protein sequences using National Center for Biotechnology Information (NCBI) BLASTp searches (http://www.ncbi.nlm.nih.gov/) with a threshold e-value < e−5. Proteins from S. spontaneum that were homologous with one of the reference sequences were considered candidate CNGC members. Second, CNGC candidates that contain both the cNMP binding domain (CNBD, Pfam No. PF00027) and ion trans domain (Pfam No. PF00520) were screened using HMMER v5.0.1 software (domE = e–5) with the Pfam database (https://www.ebi.ac.uk/interpro/). Finally, protein domains and domain structure analysis of CNGC candidates were performed using the Simple Modular Architecture Research Tool (SMART) database (http://smart.embl-heidelberg.de/) and the InterProScan database (http://www.ebi.ac.uk/Tools/pfa/iprscan5/). Proteins with more than 200 amino acids and a CNBD that contained the PBC and hinge regions were recognized as members of the CNGC gene family in S. spontaneum and named SsCNGCs.
The ExPASy Proteomics Server (https://web.expasy.org/protparam/) was employed for protein length, molecular weight, theoretical pI and instability index analysis of SsCNGC proteins. The online tool Softberry (http://linux1.softberry.com/berry.phtml) was used to predict the subcellular location of SsCNGC proteins.
Multiple sequence alignment and phylogeny analysis
Multiple sequence alignment and phylogenetic analysis of SsCNGCs with all CNGC proteins from Arabidopsis, rice and maize were performed using the MUSCLE program [46]. The conserved domains of CNGCs were checked manually. Phylogenetic analysis was performed using MEGA 7.0 software under the MUSCLE model [47]. The bootstrap test was set as 1000 replicates. Scale bars correspond to 0.1 amino acid substitutions.
Chromosome location, gene structure and protein conserved motif analysis
The exon‒intron structure of SsCNGCs was analyzed using the online tool Gene Structure Display Server (GSDS, http://gsds.cbi.pku.edu.cn/) [48] based on genome annotation data downloaded from the SGD database (http://sugarcane.zhangjisenlab.cn/sgd/html/index.html).
The conserved motif analysis of SsCNGCs was performed with Multiple Em for Motif Elicitation (MEME) online software (http://meme-suite.org/tools/meme) [49]. The maximum motif search value was set at 15, and the optimum motif width was 10–100 aa. Other parameters are default.
Chromosome location, duplication, and syntenic analyses
The chromosomal locations of the SsCNGCs were determined by the genome annotation files and mapped using the SVG package of the Perl programming language.
Homology between protein sequences encoded by SsCNGCs was analyzed by the BLASTp program. These results were submitted to the duplicate gene classifier script in MCScanXv8.0 software for potential gene duplication events identified with a cutoff E-value ≤ 1e−5. The collinearity of multiple species was constructed by using McScanXv8.0 software, and the SVG model was drawn using Perl.
Cis-acting element analysis
According to the genome sequence from the SGD database, 2 kb DNA sequences upstream of the start codon of each SsCNGC were obtained and submitted to the online tool PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) server [50] for putative cis-acting element prediction.
Expression pattern analysis
Transcription data for different S. spontaneum leaf sections and growth and development periods were downloaded from the SGD database. Transcriptome data of sugarcane hybrid YT99-66 root samples treated with low-K+ were used for SsCNGC gene transcriptional expression under low-K+ treatment. Fragments per kilobase per million (FPKM) values of SsCNGCs extracted from these transcriptome data were normalized by z score and hierarchically clustered by Pheatmap v1.0.8 R package.
To validate the expression of SsCNGCs under low-K+ stress, total RNA was extracted from the root samples of YT99-66 after low-K+ treatment using RNAiso Plus (TaKaRa, Japan). The cDNA was obtained using the PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time, Takara, Japan) according to the instruction. RT-qPCR was preformed using cDNA and TB Green® Premix Ex Taq™ II (Tli RNaseH Plus, Takara, Japan) on the LightCycler 96 (Roch, USA) with primers listed in Supplementary Table 1. The 2−∆∆CT approach was used for quantifying relative gene expression levels. SsAPRT was as used as normalization controls.
Results
Identification of CNGC genes in S. spontaneum
To identify CNGC genes in S. spontaneum, the homologous genes in Arabidopsis, rice and maize (Zea mays) were obtained using the Protein–Protein Basic Local Alignment Search Tool (BLASTp) algorithm. Homologous genes containing CNGC-specific domains, CNBD, CaMBD and IQ motifs as well as a most conserved phosphate binding cassette (PBC) and a “hinge” region in the CNBD were identified as SsCNGC genes (Fig. 1a and b). In this study, a total of 16 SsCNGC genes with 27 alleles were identified and named SsCNGC1-16 according to their phylogenetic relationships with CNGC genes in rice and based on allelic annotation of the sugarcane genome [41].
Amino acid sequence alignment, phylogenetic tree, gene structures, and conserved motif analysis of the CNGC gene family members in S. spontaneum. a Amino acid sequence alignment of SsCNGCs. CNBD is highlighted by blue box, Phosphate Binding Cassette, Hinge, CaMBD and IQ motif are highlighted by black underline. b The phylogenetic tree was constructed based on the aa sequence of SsCNGCs using MEGA 7.0 and the Multiple Sequence Comparison by Log-Expectation (MUSCLE) method. c Gene structures of SsCNGCs. Yellow and blue boxes indicate exons of coding and noncoding regions, respectively; black lines indicate introns. d Conserved motifs of SsCNGC proteins were discovered using MEME tools. The order of the motifs is consistent with their position in the protein sequence. Different colored boxes represent different conserved motifs
Detailed physiological and biochemical information of these 16 SsCNGC genes is listed in Table 1. Most of the SsCNGC genes identified have 2 ~ 4 alleles except for SsCNGC14 and SsCNGC15, which have only 1 allele (Supplementary Table 2). However, alleles of some SsCNGC genes were truncated, such as those of SsCNGC1-2C, SsCNGC2-1A/2B, and SsCNGC3-2B. The nucleic and amino acid sequences of SsCNGC genes and their alleles are shown in Supplementary Files 1 and 2, respectively. The coding sequence (CDS) lengths of SsCNGC genes and their alleles ranged from 378 bp (SsCNGC7-1 T) to 2478 bp (SsCNGC2-1P), with an average length of 1851 bp. The SsCNGC protein length ranged from 126 to 826 amino acids (aa), with an average of length of 633 aa. The predicted molecular weight (Mw) of these SsCNGC proteins ranged from 14.1 to 102.30 kDa, and the theoretical isoelectric point (pI) ranged from 8.65 (SsCNGC3-2B) to 10.34 (SsCNGC15-1B).
The cluster analysis, gene structures and conserved protein motifs of all SsCNGCs and alleles were also investigated. Few common features were found between the SsCNGCs within the same group (Fig. 1). Except for SsCNGC6 and its allele SsCNGC6-2D, as well as SsCNGC1-2C, SsCNGC3-2B, and SsCNGC5-3C/4D, all the other SsCNGCs and their alleles have introns, with exon numbers ranging from 2 to 13 (Fig. 1c). The conserved motifs of SsCNGC proteins were identified with the online Multiple Em for Motif Elicitation (MEME) program. The details of the sequence logo of motifs are shown in Supplementary Fig. 1. Notably, 93% of SsCNGC proteins contain motif 2 and motif 4, indicating that these two motifs were most common among the various CNGC gene family members. In addition, motif 2 represents the most conserved sequence in the CNBD domain, and the ion trans domain might be composed of motifs 7, 6, 12, 5, 11, and 13 (Fig. 1d).
Phylogenetic and syntenic analysis of SsCNGCs
To explore the phylogenetic relationship of SsCNGC proteins, an unrooted phylogenetic tree was constructed based on the alignment results of the available full-length amino acid sequences of Arabidopsis, rice, maize and S. spontaneum CNGCs (Supplementary file 3). As shown in Fig. 2, all the CNGC proteins could be clustered into four groups as described by Jarratt-Barnham et al. (2021) [19]. Group IV was divided into two subgroups (groups IVa and IVb). Similar to the CNGCs in rice and maize, the SsCNGCs in each group exhibited a great diversity in number. For example, groups III and IVa contained the most and the fewest members, 5 and 1, respectively. This is basically similar to the quantities in these groups in rice and maize but not to the quantities in Arabidopsis, which had the highest CNGC number in group I. Arabidopsis and rice are the model plants of dicots and monocots, respectively. In general, all CNGCs and their subgroups are present in dicots and monocots. It is speculated that the appearance of most CNGCs in plants predated monocot-dicot divergence.
Phylogenetic analysis of CNGCs from S. spontaneum and A. thaliana, rice and maize. Multiple sequence alignment of 16 putative SsCNGCs with 20 AtCNGCs, 16 OsCNGCs and 12 ZmCNGCs was performed by using MEGA 7.0, which was also used to create the unrooted maximum likelihood tree under the MUSCLE model. The bootstrap test was carried out with 1,000 replicates
To further investigate the origin and evolution of CNGCs in S. spontaneum, the syntenic relationships between S. spontaneum and both rice and Arabidopsis were examined using McScanXv8.0 [51]. The results indicated that a great number of syntenic relationship events existed between rice and S. spontaneum, including many CNGC gene pairs. This means that many consensuses in SsCNGCs may have existed before the species divergence between rice and S. spontaneum (Fig. 3a). However, there was only one collinear gene pair between the Arabidopsis and S. spontaneum CNGC genes, suggesting that the origin of this gene pair was very old (Fig. 3b).
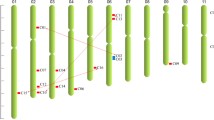
Syntenic analysis of CNGC genes between S. spontaneum and both rice (a) and Arabidopsis (b). The S. spontaneum, rice and Arabidopsis chromosomes are represented by red, green and blue bars, respectively. Gray lines in the background indicate the collinear blocks within two different genomes, while the red lines highlight the syntenic CNGC gene pairs. Schematic representations were displayed by using the SVG Perl package
Chromosome location and duplication events of CNGC family members in S. spontaneum
The chromosome location information for CNGC gene family members showed that they were unevenly distributed on the 23 S. spontaneum chromosomes (Fig. 4). The number of SsCNGC genes mapped on each chromosome varied widely and ranged from 1 to 5. Among the 23 chromosomes, Chr4D had 5 SsCNGCs, Chr1B and Chr4A/B/C/D each had 4 SsCNGCs, and Chr8A and Chr2D had 3 SsCNGCs, while only one SsCNGC was found to be located on the other chromosomes. Almost all SsCNGC genes and their alleles were located on homologous chromosomes, except for SsCNGC2, which is located on Chr8A, with two alleles located on Chr8A (SsCNGC2-1P) and Chr1B (SsCNGC2-2B) respectively.
Chromosome location and duplication of SsCNGCs in S. spontaneum. All SsCNGCs and alleles were mapped onto the 23 S. spontaneum chromosomes. The chromosome number is shown at the top of each chromosome. The scale is in megabases (Mb). The seven dispersed duplication genes are in magenta; the four tandem duplication genes are in green; the three proximal duplication genes are in blue; the twenty-nine segmental duplication genes are in black
Gene duplication is responsible for gene family evolution and differentiation and even participates in the occurrence of both evolutionary novelties and increases in biological complexity (including adaptation to stresses and resistance to diseases) as well as in speciation [52,53,54]. Genome-wide duplication events of SsCNGCs were analyzed in this study (Fig. 4). The results indicated that 3 (7%, marked in blue), 4 (9.3%, marked in green) and 7 (16.3%, marked in magenta) SsCNGCs were duplicated from proximal, tandem and dispersed duplication events, respectively, and that the other 29 (67.4%) SsCNGCs originated from segmental duplication.
Prediction of cis-acting acting regulatory elements in the promoter of SsCNGCs
Investigation of cis-acting elements in the promoter region was conducted to better elucidate the functions of the SsCNGCs. In this study, 2.0 kb sequences upstream from the transcriptional start site of the SsCNGCs were extracted from the gff3 file and submitted to the Plant Cis-Acting Regulatory Element (PlantCARE) database for cis-element identification. According to the functional annotation, these cis-acting elements can be divided into three categories: those involved in development processes, hormone signaling and environmental responses (Supplementary Table 3 and Fig. 5). All the promoter sequences of SsCNGCs contained several light-responsive elements such as Sp1, G-box, and ATCT-motif, suggesting that SsCNGCs may be involved in the light responses of S. spontaneum. Moreover, the promoters of SsCNGCs also contained phytohormone responsiveness elements that are always involved in plant development as well as responses to biotic and abiotic stresses. The promoters of all the SsCNGCs except for SsCNGC9-1 T contained the CGTCA motif, a cis-acting acting regulatory element involved in methyl jasmonate (MeJA) responsiveness. In addition, the abscisic acid (ABA) responsiveness element ABRE was discovered in 40 SsCNGCs, although it was absent from SsCNGC1-1P, SsCNGC6-2D and SsCNGC9-1 T. In addition, the promoters of all the SsCNGCs contained several ABRE elements, with an average of 4.4. Additionally, all the SsCNGCs contained cis-acting acting elements that participate in defense and/or stress responsiveness, including low-temperature response (LTR) cis-acting elements involved in low-temperature responsiveness, MYB binding site (MBS) cis-acting elements involved in drought inducibility, TC-rich repeat enhancer cis-acting elements involved in defense and stress responsiveness, and so on. These results indicate that SsCNGCs may perform diverse functions to regulate S. spontaneum development and to respond to environmental stresses.
Expression profiles of SsCNGCs across development and leaf segment gradients
Tissue-specific expression patterns are interrelated with the functions of genes. In this study, transcriptome profiles of SsCNGCs in different tissues at different developmental stages of S. spontaneum were analyzed based on the RNA-seq data from the Saccharum Genome Database (SGD) (http://sugarcane.zhangjisenlab.cn/sgd/html/index.html, Fig. 6a, Supplementary Table 4). The SsCNGCs showed similar transcriptional profiles, and most of them showed tissue-specific expression patterns (Fig. 6a). SsCNGC1, 2, 4, 9, and 13 were highly expressed in most tissues tested at various expression levels. SsCNGC1 and 7, together with their alleles, showed higher expression levels in stem tissues. SsCNGC2, 9 and 13·, together with their alleles, showed significantly higher expression levels in maturing and mature stalk tissues (stem6 and stem9 tissues, the 6th and 9th internodes from the terminal bud) at the premature stage. Interestingly, several SsCNGCs, such as SsCNGC16 and its alleles, showed higher expression levels in leaf roll and leaves at the seedling and mature stages but not at the premature stage. In different sections of mature leaves, SsCNGCs showed various expression levels (Fig. 6b). SsCNGC1 and 7 showed a decreasing trend in expression from the basal zone to the mature zone, while SsCNGC10, 11, 12, and 16 showed an upward tendency. SsCNGC2 and 8 were highly expressed in the transition and maturing zones. Transcripts of SsCNGC14 and 15 showed high accumulation in the tender stems. The expression of SsCNGC3, 5 and 6 in the aerial tissues was low and was barely detected (Supplementary Table 4). Therefore, it can be speculated that their effects on growth and development were limited.
Expression pattern of SsCNGCs in S. spontaneum in different tissues at three developmental stages (a) and across leaf gradients (b) based on FPKM. Tissues are indicated at the top of each column. Stems 3, 6 and 9 are the 3rd (immature stem), 6th (maturing stem) and 9th (mature stem) internodes from the terminal bud. b) The mature leaves of S. spontaneum were divided into 15 segments (1–15) and four regions: the basal zone (sink tissue), transitional zone (sink‒source transition), maturing zone and mature zone (fully differentiated, active photosynthetic zone)
Expression pattern of SsCNGCs in relation to the circadian rhythm
According to the results of the previous analysis, SsCNGCs should participate in responses to light intensity changes in S. spontaneum. To investigate the roles of SsCNGCs in relation to the circadian rhythm, the expression profiles of SsCNGCs in mature leaves were analyzed at 2-h intervals (Fig. 7, Supplementary Table 5). The results showed that the expression of most detected SsCNGCs was regulated by the circadian rhythm. These SsCNGCs could be categorized into Groups 1, 2, and 3 based on their expression patterns, which had higher expression at dawn, afternoon and night, respectively (Fig. 7). Many SsCNGCs showed high expression at night, from 20:00 to 0:00 the next day, including SsCNGC2, 4, 8, 12 and 16. The expression of SsCNGC1 and 7 reached their peak values at dusk. Relatively high expression of SsCNGC10 and 11 began at dawn and persisted from 4:00 to 8:00. Only SsCNGC9 showed a high expression level in the afternoon. These results can be explained by the effects of light-responsive cis-acting elements at the promoters.
Expression of SsCNGCs was regulated by K+-deficient stress
As Ca2+ channels, CNGCs are important for channeling extracellular stimuli, including those related to many biotic and abiotic stresses, to the cytoplasm. Accordingly, we investigated the expression profiles of SsCNGCs in the sugarcane cultivar YT 99–66 roots under K+-deficient stress. Generally, low-K+ stimuli altered the expression of many SsCNGCs (Fig. 8a). The expression patterns of SsCNGC genes and their alleles always showed slight differences, such as SsCNGC1 and 9. Under low-K+ stress, the expression of SsCNGC1, 3, 9 and 9-2D was inhibited, while the expression of SsCNGC1-1P, 1-2C, 3-3B and 9-1 T was upregulated at different time points. The expression of SsCNGC2 and 2-1P was upregulated under K+ starvation except at 72 h after treatment, while SsCNGC16 and its alleles presented the opposite expression trend. According to these results, a hypothesis is that CNGCs are involved in the sugarcane cultivar YT 99-66 response to low-K+ stress. In addition, SsCNGC3, 5 and 6, as well as their alleles, were also rarely expressed in roots and were probably not regulated by K+ starvation (Supplementary Table 6). To validate the transcriptome data, RT-qPCR were performed to evaluate the expression patterns of 6 of these SsCNGCs with relative high-level of transcription (Supplementary Table 6). The results of RT-qPCR were largely consistent with the transcriptome data. For example, SsCNGC7 exhibited lowest expression at 24 h under low-K+ treatment. Expression of SsCNGC1 and 12 were significantly reduced at 72 h. SsCNGC8 were down-regulated by low-K+ treatment with a restore at 48 h (Fig. 8b). We hypothesized that these three SsCNGCs might respond to other specific spatiotemporal conditions. The SsCNGCs exhibited varying expression patterns and likely play different roles in the sugarcane cultivar YT 99-66 response to low-K+ stress.
Discussion
Sugarcane is an important sugar crop and a bioenergy source. It is therefore critical to understand the development and responses of sugarcane to environmental stimuli. Ca2+ is an essential second messenger that participates in plant responses to environmental stimuli and developmental cues. Stimulus-specific Ca2+ signaling is produced based on activating Ca2+-permeable channels [55, 56]. As a nonselective, ligand-gated cation channel, CNGCs have been identified across the plant kingdom [20]. CNGCs are permeable to Ca2+ and K+ and have been confirmed to be involved in plant development and responses to a variety of stresses [57]. However, genome-wide analysis of the CNGC gene family has not been conducted in Saccharum due to its complex genetic background. In this study, a total of 16 CNGC genes and 27 alleles were initially identified in the genome of S. spontaneum (Table 1). All these members of the CNGC family contained typical CNBD, CaMBD and IQ motifs (Fig. 1a). Similar to CNGCs in other plant species, members of the CNGC family in S. spontaneum were able to be categorized into 4 groups with divergence in distribution [20]. SsCNGCs in Groups II, III and IVa shared similar gene structures and patterns of conserved motifs but the same was not true for members of Groups I and IVb (Figs. 1 and 2). The conserved motifs in SsCNGCs may imply similar modes of interaction with their target proteins.
Membranes of CNGC gene family from A. thaliana and S. spontaneum share a relatively low amino acid identify (data not shown), and close alignment of most AtCNGCs was not identified in S. spontaneum, including AtCNGC16 and AtCNGC18 which were proved to be important for pollen development. However, based on the phylogenic tree, the SsCNGC1, 5, 6, and 12 were identified as the close alignments of ZmCNGC1, ZmCNGC5, OsCNGC4, OsCNGC5 and OsCNGC13 respectively (Fig. 2), which were predominantly involved in pollen development [44, 58]. What’s more, the probable pollen-preferred cis-acting regulatory, TCTTYCTCC and GCGGMGGCG [58], were identified in the promoter of SsCNGC5 and 6 (Supplementary File 4). Accordingly, the SsCNGC5 and 6 are possible to form a homomeric complex like their close alignment, OsCNGC4 and OsCNGC5 [58], and involved in pollen development of sugarcane.SsCNGCs were unevenly dispersed across the 23 S. spontaneum chromosomes, and the number of genes on each chromosome ranged from 1 to 5 (Fig. 4). Gene duplication contributes to the expression of the gene family [59], and most SsCNGCs originated from segmental (67.4%) and dispersed (16.3%) duplication events (Fig. 4). It seems that the expansion of the CNGC family is closely related to the genome duplication of S. spontaneum. Synteny analysis also revealed that many SsCNGC genes are located in conserved syntenic blocks between rice and S. spontaneum. It is speculated that these SsCNGC genes are crucial for plant development [60, 61].Unlike CNGC genes in other species that arising from tandem duplications are mostly in Groups I and IVa [62], and the 4 SsCNGCs with tandem duplication events identified in this study are in Groups I and III (Figs. 2 and 4). Among the 4 tandem duplication SsCNGC gene pairs, SsCNGC9-2D/SsCNGC9-1 T and SsCNGC10-3D/SsCNGC10-1 T showed similar gene structures (Fig. 1) and expression patterns under normal conditions (Figs. 4, 6 and 7). For the other two pairs (SsCNGC7-3D/SsCNGC7-1 T, SsCNGC1/SsCNGC3), SsCNGC7-1 T and SsCNGC3 were truncated (Fig. 1) and were rarely expressed in leaves, stems and roots (Supplementary Tables 4, 5 and 6). Genovariation always leads to the functional expansion of genes. This study revealed that the expression patterns of the 4 tandem duplication gene pairs under low-K+ conditions were different. This suggests that these genes may exercise different functions in the sugarcane response to low-K+ stress.
SsCNGC1 showed higher expression levels in the stems and basal zone of leaves (Fig. 6 and Supplementary Table 4). The closest homologous genes of SsCNGC1 in Arabidopsis, AtCNGC3 and 10, are involved in germination, hypocotyl elongation, Na+ and K+ uptake and homeostasis [63, 64]. It is speculated that SsCNGC1 is critical for the regulation of stem elongation and Na+ and K+ homeostasis. As the homolog gene of AtCNGC2 and OsCNGC14, SsCNGC13 exhibited higher expression levels in maturing and mature stem tissues and the sink tissue of leaves and has been suggested to impact plant responses to thermal stress, chilling, and pathogens [28, 30, 64].
Researchers have identified the circadian regulation of the CNGC in chicken cone photoreceptors [65, 66]. Zia et al. also identified light-responsive cis-regulatory elements in almost CNGC genes in Citrus recticulata [67]. However, there have been few studies on the role of CNGCs in the plant circadian rhythm or in response to light intensity changes. In this study, light-responsive elements were found in protomers of all the SsCNGCs, and the expression of most of the SsCNGCs was regulated by circadian rhythm.
As cation channels, some CNGCs in plants have been confirmed to be K+-permeable channels, such as AtCNGC2, AtCNGC3, AtCNGC4, AtCNGC10, and so forth [19]. AtCNGC3 and AtCNGC10, which have strong K+ permeation, are likely to be important for root K+ uptake [57, 68]. In this study, we found that the expression of SsCNGC1, 1-2C, 8, 8-2B, 8-3C, 8-4D, 9-2D, 12-2D and 13-1P was downregulated by low-K+ treatment at different levels. Among them, the downregulation of SsCNGC1-2C expression was just 6 h after low-K+ treatment (Fig. 8 and Supplementary Table 6). Regarding the diversity of gene structure and expression patterns (Figs. 1 and 8), it is speculated that SsCNGC1 and 1-2C play divergent roles in the sugarcane response to low-K+ stress. After treatment for 72 h, the expression of SsCNGC13-1P was upregulated to over threefold the normal level. SsCNGC13-1P is a homologous gene of AtCNGC2 and may be another K+-permeable channel in sugarcane. Under low-K+ stress, SsCNGC9-1 T shared a similar gene structure and pattern of motifs but not a similar expression pattern. SsCNGC9-1 T showed increased expression after low-K+ treatment. SsCNGC9 is the homologous gene of OsCNGC9 that does not have obvious K+ conductance. Further studies need to be carried out to explore the roles of SsCNGCs in the sugarcane response to low-K+ stress. For Saccharum hybrid, S. officinarum was assumed to contribute to genetic background of high sugar content, and S. spontaneum contributed to the stress tolerance and pest and disease resistance [69]. It is possible that roles of SsCNGC genes in sugarcane response to low-K+ stress is universal in other hybrid cultivars. The results should provide some theoretical guidance to breeding of K+ high-efficiency sugarcane cultivars.
Conclusions
Altogether, identify and systematic informatics analyses of 16 CNGCs and their alleles in S. spontaneum were carried out firstly, including phylogenetic, chromosome location, gene structure, pattern of conserved motifs, duplication, syntenic analyses, and cis-elements in promoter. Moreover, the expression profiles of SsCNGCs during development, circadian rhythm and under low-K+ stress were investigated. Many SsCNGCs were highly tissue-specific expression during S. spontaneum development, such as SsCNGC1 and 13. And light-responsive elements were found in the promoters of the expression of most SsCNGCs could be regulated by circadian rhythm. What’s more, the expression of SsCNGC13 was also regulated by low-K+ treatment, it may participate in S. spontaneum development and response to low-K+ stress.
Availability of data and materials
All RNA-seq data of S. spontaneum were downloaded from the sugarcane database website (http://sugarcane.zhangjisenlab.cn/sgd/html/index.html). The S. spontaneum genome project was deposited into Genbank with accession numbers: QVOL00000000. The RNA-seq data of YT 99–66 is the original data in this study.
References
Clapham DE. Calcium Signaling. Cell. 2007;131(6):1047–58.
Gong Z, Xiong L, Shi H, Yang S, Herrera-Estrella LR, Xu G, Chao DY, Li J, Wang PY, Qin F, et al. Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci. 2020;63(5):635–74.
Moeder W, Phan V, Yoshioka K. Ca2+ to the rescue - Ca2+ channels and signaling in plant immunity. Plant Sci. 2019;279:19–26.
Lecourieux D, Ranjeva R, Pugin A. Calcium in plant defence-signalling pathways. New Phytol. 2006;171(2):249–69.
Hepler PK. Calcium: a central regulator of plant growth and development. Plant Cell. 2005;17(8):2142–55.
Yang Z. Cell polarity signaling in Arabidopsis. Annu Rev Cell Dev Bi. 2008;24:551–75.
Ma W, Berkowitz GA. Cyclic nucleotide gated channel and Ca(2)(+)-mediated signal transduction during plant senescence signaling. Plant Signal Behav. 2011;6(3):413–5.
Wang X, Hao L, Zhu B, Jiang Z. Plant calcium signaling in response to potassium deficiency. Int J Mol Sci. 2018;19(11):3456.
Yuan P, Jauregui E, Du L, Tanaka K, Poovaiah BW. Calcium signatures and signaling events orchestrate plant–microbe interactions. Curr Opin Plant Biol. 2017;38:173–83.
Zipfel C, Oldroyd GED. Plant signalling in symbiosis and immunity. Nature. 2017;543(7645):328–36.
Zhang X, Dong W, Sun J, Feng F, Deng Y, He Z, Oldroyd GED, Wang E. The receptor kinase CERK1 has dual functions in symbiosis and immunity signalling. Plant J. 2015;81(2):258–67.
Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol. 2010;61(1):593–620.
Krebs M, Held K, Binder A, Hashimoto K, Den Herder G, Parniske M, Kudla J, Schumacher K. FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J. 2012;69(1):181–92.
Demidchik V, Shabala S, Isayenkov S, Cuin TA, Pottosin I. Calcium transport across plant membranes: mechanisms and functions. New Phytol. 2018;220(1):49–69.
Yamanaka T, Nakagawa Y, Mori K, Nakano M, Imamura T, Kataoka H, Terashima A, Iida K, Kojima I, Katagiri T, et al. MCA1 and MCA2 That Mediate Ca2+ Uptake Have Distinct and Overlapping Roles in Arabidopsis. Plant Physiol. 2010;152(3):1284–96.
Jammes F, Hu H, Villiers F, Bouten R, Kwak JM. Calcium-permeable channels in plant cells. Febs J. 2011;278(22):4262–76.
Thor K, Jiang S, Michard E, George J, Scherzer S, Huang S, Dindas J, Derbyshire P, Leitao N, DeFalco TA, et al. The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature. 2020;585(7826):569–73.
Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B, et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature. 2014;514(7522):367–71.
Jarratt-Barnham E, Wang L, Ning Y, Davies JM. The complex story of plant cyclic nucleotide-gated channels. Int J Mol Sci. 2021;22(2):874.
Baloch AA, Kakar KU, Nawaz Z, Mushtaq M, Abro A, Khan S, Latif A. Comparative genomics and evolutionary analysis of plant CNGCs. Biol Methods Protoc. 2022;7(1):bpac018.
Fischer C, Kugler A, Hoth S, Dietrich P. An IQ Domain Mediates the Interaction with Calmodulin in a Plant Cyclic Nucleotide-Gated Channel. Plant Cell Physiol. 2013;54(4):573–84.
DeFalco TA, Marshall CB, Munro K, Kang H, Moeder W, Ikura M, Snedden WA, Yoshioka K: Multiple calmodulin-binding sites positively and negatively regulate Arabidopsis CYCLIC NUCLEOTIDE-GATED CHANNEL12. The Plant Cell 2016:870–2015.
Mori I, Nobukiyo Y, Nakahara Y, Shibasaka M, Furuichi T, Katsuhara M. A cyclic nucleotide-gated channel, HvCNGC2-3, is activated by the co-Presence of Na+ and K+ and permeable to Na+ and K+ non-selectively. Plants. 2018;7(3):61.
Guo J, Islam MA, Lin H, Ji C, Duan Y, Liu P, Zeng Q, Day B, Kang Z, Guo J. Genome-wide identification of cyclic nucleotide-gated ion channel gene family in wheat and functional analyses of TaCNGC14 and TaCNGC16. Front Plant Sci. 2018;9:18.
Breiden M, Olsson V, Blümke P, Schlegel J, Gustavo-Pinto K, Dietrich P, Butenko MA, Simon R. The cell fate controlling CLE40 peptide requires CNGCs to trigger highly localized Ca2+ transients in Arabidopsis thaliana root meristems. Plant Cell Physiol. 2021;62(8):1290–301.
Frietsch S, Wang Y, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF. Cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci. 2007;104(36):14531–6.
Tunc-Ozdemir M, Tang C, Ishka MR, Brown E, Groves NR, Myers CT, Rato C, Poulsen LR, McDowell S, Miller G, et al. A cyclic nucleotide-gated channel (CNGC16) in pollen Is critical for stress tolerance in pollen reproductive development. Plant Physiol. 2013;161(2):1010–20.
Finka A, Cuendet AFH, Maathuis FJM, Saidi Y, Goloubinoff P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell. 2012;24(8):3333–48.
Gao F, Han X, Wu J, Zheng S, Shang Z, Sun D, Zhou R, Li B. A heat-activated calcium-permeable channel - Arabidopsis cyclic nucleotide-gated ion channel 6 - is involved in heat shock responses. Plant J. 2012;70(6):1056–69.
Cui Y, Lu S, Li Z, Cheng J, Hu P, Zhu T, Wang X, Jin M, Wang X, Li L, et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 2020;183(4):1794–808.
Oranab S, Ghaffar A, Kiran S, Yameen M, Munir B, Zulfiqar S, Abbas S, Batool F, Umar Farooq M, Ahmad B, et al. Molecular characterization and expression of cyclic nucleotide gated ion channels 19 and 20 in Arabidopsis thaliana for their potential role in salt stress. Saudi J Biol Sci. 2021;28(10):5800–7.
Yu I, Parker J, Bent AF. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci. 1998;95(13):7819–24.
Jurkowski GI, Smith RK, Yu I, Ham JH, Sharma SB, Klessig DF, Fengler KA, Bent AF. Arabidopsis DND2, a second cyclic nucleotide-gated ion channel gene for which mutation causes the “Defense, No Death” phenotype. Mol Plant Microbe In. 2004;17(5):511–20.
Tian W, Hou C, Ren Z, Wang C, Zhao F, Dahlbeck D, Hu S, Zhang L, Niu Q, Li L, et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature. 2019;572(7767):131–5.
Wang J, Liu X, Zhang A, Ren Y, Wu F, Wang G, Xu Y, Lei C, Zhu S, Pan T, et al. A cyclic nucleotide-gated channel mediates cytoplasmic calcium elevation and disease resistance in rice. CELL RES. 2019;29(10):820–31.
Saand MA, Xu Y, Li W, Wang J, Cai X. Cyclic nucleotide gated channel gene family in tomato: genome-wide identification and functional analyses in disease resistance. FRONT PLANT SCI. 2015;6:303.
Zhang Y, Yang N, Zhao L, Zhu H, Tang C. Transcriptome analysis reveals the defense mechanism of cotton against Verticillium dahliae in the presence of the biocontrol fungus Chaetomium globosum CEF-082. Bmc Plant Biol. 2020;20(1):89.
Zhao C, Tang Y, Wang J, Zeng Y, Sun H, Zheng Z, Su R, Schneeberger K, Parker JE, Cui H. A mis-regulated cyclic nucleotide-gated channel mediates cytosolic calcium elevation and activates immunity in Arabidopsis. New Phytol. 2021;230(3):1078–94.
Jogawat A, Meena MK, Kundu A, Varma M, Vadassery J. Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. J Exp Bot. 2020;71(9):2752–68.
Meena MK, Prajapati R, Krishna D, Divakaran K, Pandey Y, Reichelt M, Mathew MK, Boland W, Mithöfer A, Vadassery J. The Ca2+ channel CNGC19 regulates Arabidopsis defense against spodoptera herbivory. Plant Cell. 2019;31(7):1539–62.
Zhang J, Zhang X, Tang H, Zhang Q, Hua X, Ma X, Zhu F, Jones T, Zhu X, Bowers J, et al. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L. Nat Genet. 2018;50(11):1565–73.
Talke I. CNGCs: prime targets of plant cyclic nucleotide signalling? Trends Plant Sci. 2003;8(6):286–93.
Nawaz Z, Kakar KU, Saand MA, Shu QY. Cyclic nucleotide-gated ion channel gene family in rice, identification, characterization and experimental analysis of expression response to plant hormones, biotic and abiotic stresses. BMC Genomics. 2014;15:853.
Hao L, Qiao X. Genome-wide identification and analysis of the CNGC gene family in maize. Peer J. 2018;6:e5816.
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40(D1):D1178–86.
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 20. Bioinformatics. 2007;23(21):2947–8.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4.
Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–7.
Bailey TL, Williams N, Misleh C, Li WW. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006;34(Web Server):W34369–73.
Lescot M. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7.
Wang Y, Tang H, DeBarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49.
De Smet R, Van de Peer Y. Redundancy and rewiring of genetic networks following genome-wide duplication events. Curr Opin Plant Biol. 2012;15(2):168–76.
Magadum S, Banerjee U, Murugan P, Gangapur D. RAVIKESAVAN R: Gene duplication as a major force in evolution. J Genet. 2013;92(1):155–61.
Porturas LD, Anneberg TJ, Curé AE, Wang S, Althoff DM, Segraves KA. A meta-analysis of whole genome duplication and the effects on flowering traits in plants. AM J BOT. 2019;106(3):469–76.
Tian W, Wang C, Gao Q, Li L, Luan S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat Plants. 2020;6(7):750–9.
Tang R, Luan S. Regulation of calcium and magnesium homeostasis in plants: from transporters to signaling network. Curr Opin Plant Biol. 2017;39:97–105.
Kaplan B, Sherman T, Fromm H. Cyclic nucleotide-gated channels in plants. FEBS LETT. 2007;581(12):2237–46.
Lee SK, Lee SM, Kim MH, Park SK, Jung KH. Genome-wide analysis of cyclic nucleotide-gated channel genes related to pollen development in rice. PLANTS. 2022;11(22):3145.
Fischer I, Diévart A, Droc G, Dufayard J, Chantret N. Evolutionary dynamics of the leucine-rich repeat receptor-like kinase (LRR-RLK) subfamily in angiosperms. PLANT PHYSIOL. 2016;170(3):1595–610.
Kakar KU, Nawaz Z, Kakar K, Ali E, Almoneafy AA, Ullah R, Ren X, Shu Q. Comprehensive genomic analysis of the CNGC gene family in Brassica oleracea: novel insights into synteny, structures, and transcript profiles. Bmc Genomics. 2017;18(1):869.
Cheng F, Mandáková T, Wu J, Xie Q, Lysak MA, Wang X. Deciphering the diploid ancestral genome of the mesohexaploid Brassica rapa. Plant Cell. 2013;25(5):1541–54.
Mao X, Wang C, Lv Q, Tian Y, Wang D, Chen B, Mao J, Li W, Chu M, Zuo C. Cyclic nucleotide gated channel genes (CNGCs) in Rosaceae: genome-wide annotation, evolution and the roles on Valsa canker resistance. Plant Cell Rep. 2021;40(12):2369–82.
Gobert A, Park G, Amtmann A, Sanders D, Maathuis FJM. Arabidopsis thaliana Cyclic Nucleotide Gated Channel 3 forms a non-selective ion transporter involved in germination and cation transport. J Exp Bot. 2006;57(4):791–800.
Jin Y, Jing W, Zhang Q, Zhang W. Cyclic nucleotide gated channel 10 negatively regulates salt tolerance by mediating Na+ transport in Arabidopsis. J Plant Res. 2015;128(1):211–20.
Chae K, Ko GYP, Dryer SE. Tyrosine phosphorylation of cGMP-gated ion channels is under circadian control in chick retina photoreceptors. Invest Ophthalmol Vis Sci. 2007;48(2):901.
Ko GYP, Ko ML, Dryer S. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: role of cAMP and ras. J NEUROSCI. 2004;24(6):1296–304.
Zia K, Rao MJ, Sadaqat M, Azeem F, Fatima K. Tahir Ul Qamar M, Alshammari A, Alharbi M: Pangenome-wide analysis of cyclic nucleotide-gated channel (CNGC) gene family in citrus Spp. Revealed their intraspecies diversity and potential roles in abiotic stress tolerance. Front Gent. 2022;13:1034921.
Borsics T, Webb D, Andeme-Ondzighi C, Staehelin LA, Christopher DA. The cyclic nucleotide-gated calmodulin-binding channel AtCNGC10 localizes to the plasma membrane and influences numerous growth responses and starch accumulation in Arabidopsis thaliana. Planta. 2007;225(3):563–73.
Roach B. Nobilisation of sugarcane. Proc Int Soc Sugar Cane Technol. 1972;1972:206–16.
Acknowledgements
The authors are grateful to the Center for Genomics and Biotechnology, Haixia Institute of Science and Technology, Fujian Agriculture and Forestry University for providing open access to S. spontaneum data and the sugarcane leaf picture which is presented in Fig. 6b (SGD database).
Funding
This research was funded by GDAS’ Project of Science and Technology Development (2020GDASYL-20200103057), National Natural Science Foundation of China (31901512), Guangdong Basic and Applied Basic Research Foundation (2021A1515011321, 2021A1515110917), CARS (CARS-170112) and Guangzhou Science and Technology Plan Project (202102020391).
Author information
Authors and Affiliations
Contributions
N.Z., Q.L. and Z.W. conceived the study and designed the experiments. N.Z., H.L., Q.Z., D.F., X.F. and Q.W. carried out the experiments and analyzed the data. N.Z. and Z.W. wrote the manuscript. X.G., J.W. and Q.L. revised and improved the manuscript. All authors reviewed and approved this submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study complies with local and national regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 2:
Supplementary file 1. Nucleotide sequences of the coding region of SsCNGCs and alleles.
Additional file 3:
Supplementary file 2. Amino acid sequences of SsCNGCs and alleles.
Additional file 4:
Supplementary file 3. Amino acid sequences of CNGCs from Arabidopsis thaliana, Oryza sativa and Zea mays.
Additional file 5: Supplementary file 4.
Promoter sequences of SsCNGCs and alleles.
Additional file 6:
Supplementary Table 1. Sequence of primers for RT-qPCR.
Additional file 7:
Supplementary Table 2. Detailed physiological and biochemical information of SsCNGC genes and their alleles.
Additional file 8:
Supplementary Table 3. The detailed distribution of cis-regulatory elements in the promoters of SsCNGCs.
Additional file 9:
Supplemental Table 4. The expression pattern of SsCNGCs based on FPKM in different tissues at different stages.
Additional file 10:
Supplementary Table 5. The expression pattern of SsCNGCs based on FPKM during the circadian rhythms.
Additional file 11:
Supplementary Table 6. The expression pattern of SsCNGCs based on FPKM under low-K+ stress.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, N., Lin, H., Zeng, Q. et al. Genome-wide identification and expression analysis of the cyclic nucleotide-gated ion channel (CNGC) gene family in Saccharum spontaneum. BMC Genomics 24, 281 (2023). https://doi.org/10.1186/s12864-023-09307-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09307-3