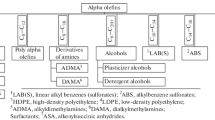
Abstract
This review continues Part 1, which considered current trends in the processing of linear alpha olefins to ethylene copolymers, and anionic and nonionic surfactants. This review also briefly outlines such importnant trends in the processing of LAOs as alkylation to linear alkylbenzenes, the oligomerization of alpha olefins into poly alpha olefins, sulfonation to alpha olefin sulfonates, and other large-scale processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
CONTENTS
Introduction
1. Processing Alpha Olefins
1.1. Producing Alpha Olefin Sulfonates from LAOs
1.2. Producing Linear Alkylbenzenes and Linear Alkylbenzene Sulfonates from LAOs
1.3. Producing Industrially Important Derivatives of Amines from LAOs
1.4. Producing Poly Alpha Olefins from LAOs
1.5. Producing Industrially Less Important Derivatives of LAOs
Conclusions
References
INTRODUCTION
Surfactants are products of the chemical industry that are in high demand. Surfactants of different natures are used to manufacture a wide range of consumer goods. Anionic surfactants are components of shampoos and detergents (both liquid and powder). Cationic surfactants are widely used in the cosmetic industry, and nonionic surfactants are effective emulsifiers and conditioners.
Being effective synthetic lubricants, C20+ poly alpha olefins are of great importance to the automotive and aviation industries. Surfactants are also important components of dielectric liquids and drilling muds.
Large-scale surfactant production uses processes of the sulfonation, alkylation, and amination of LAO. The synthesis of poly alpha olefins is based on the oligomerization of long-chain LAO.
1 PROCESSING ALPHA OLEFINS
1.1 Producing Alpha Olefin Sulfonates from LAOs
Linear alpha olefins are used to obtain anionic surfactants known as alpha olefin sulfonates (AOSes). These surfactants have high detergency (in both soft and very hard water) along with good wetting properties and low foaming characteristics. AOSes are neither irritants nor allergens, and rapidly decompose in the environment. They are therefore used in premium shampoos and in liquid and some powder detergents. In industry, AOSes are used in emulsion polymerization [1].
Alpha olefin sulfonates are synthesized via the sulfonation of AOs with subsequent neutralization of the reaction products. Suitable sulfonating agents were described in Part 1. The most widely used of these are sulfur trioxide (used only in industry) and complexes of it, along with chlorosulfonic acid. Alpha olefins are virtually the only reactants used to produce alpha sulfonates, due to the possibility of industrially producing C12–C18 AOs with the required purity. Sulfonates with SO3H groups in the central parts of their carbon chains are not widely used in industry.
Upon dilution with an inert gas, the sulfonation of AOs with, e.g., sulfur trioxide (Fig. 1), initially yields a mixture of products that includes (β, γ, δ)-sultones and alkene sulfo acids. The obtained β-sultones isomerize quite rapidly into γ-sultones, and then less rapidly into the δ-form. Sultones almost exclusively form at temperatures of up to 40°C, and the conversion of olefines can be as high as 60%. At higher temperatures, sultones partially isomerize to alkene sulfonic acids, and the conversion of olefines increases [2]. The resulting mixture must be hydrolyzed to completely convert sultones into alkene sulfonic acids or sulfonates. Such hydrolysis is done either in an acidic medium to produce mainly alkene sulfonic acids with double bonds in positions 2 and 3, or in an alkaline medium to form mixtures of salts of hydroxyalkane sulfo acids and alkene sulfo acids in a ratio of approximately 2 : 1.
To minimize the content of sultones in the resulting mixture of sulfonates, industrial hydrolysis is performed under severe alkaline conditions at elevated temperatures [2].
Because the produced AOSes have hydrophobic long-chain tails and hydrophilic ends, they are effective surfactants with high detergency. C10–C18 sulfonates are widely used in different detergents, cosmetics, and shampoos.
In the production of high-quality AOSes, particular attention must be given to the conditions of sulfonation. The reaction between sulfur trioxide and olefins proceeds at a high rate and is highly exothermic (ΔrH298°C = −180 kJ/mol). In conventional sulfonation reactors, it is impossible to avoid local overheatings that carbonize products and reduce their quality. Ways of controlling the temperature of the reaction and the AO feed are therefore needed. These difficulties were overcome in developing the TO-Reactor system of Lion Fat & Oil Co. Ltd. [3, 4]. This system is based on a reactor with a free-flowing liquid layer intended to ensure an ideal model of “isothermal sulfonation.” A flow of SO3 diluted with an inert gas is fed to the top of the reactor and comes into contact with a thin cooled layer of an organic compound flowing down the reactor walls [5].
Figure 2 shows a schematic of the continuous production of sodium alpha olefin sulfonate in a reactor with a free-flowing liquid film.
Production of alpha olefin sulfonates in a reactor with a free-flowing liquid film [5].
The reaction product enters a separator and is separated into a liquid and a gas. The exhaust gas containing sulfur trioxide and a small amount of sulfur dioxide is washed with an aqueous caustic soda solution and then vented into the atmosphere. The liquid fraction is sent to a circulating flow with caustic soda; some of the flow passes through a heater and is returned to the reactor for subsequent hydrolysis with sodium hydroxide. Hydrolysis is done at temperatures of 150 to 250°C with a residence time in the reactor of less than 1 h. If the mixture contains a large amount of the unreacted residue of AOs, it can easily be removed because less dense AOs are in the upper layer of the reaction space. The obtained mixture is next purified with hydrogen peroxide, and alpha olefin sulfonates at the outlet contain impurities that are 10–15% disulfonates, sultones, sodium sulfate, and a small amount of the unreacted olefins [6].
Alpha olefins can be sulfonated with chemicals other than diluted gaseous SO3. Rigdon et al. described the liquid-phase interaction between C5–C30 alpha olefins and sulfuric acid supported on a granular sorbent at temperatures of −18 to 107°C [7]. This technique has several advantages over conventional ones in which olefins come into direct contact liquid concentrated acid. One is the use of a solid sorbent catalyst with a high specific surface area (e.g., silica gel, alumina, silica, and activated clays). Such sorbents adsorb liquid sulfuric acid, and the resulting composite material acts as a heterogeneous catalyst. Another is that the sorbing support reduces content of sulfuric acid in the olefin-containing phase. Finally, the sorption of sulfuric acid on a highly porous support produces a larger interface between olefin and acid than in homogeneous sulfuric acid.
With alkyl ethoxy sulfates (AESes), the main trends in developing technologies for producing AOSes are improving the equipment used in the sulfonation of alpha olefins and enhancing the technical performance of the resulting products by, e.g., by reducing the content of impurities (including traces of the sulfonating agent). Different ways of modifying sulfonating agents with the deep purification of products are thus under development.
1.2 Producing Linear Alkylbenzenes and Linear Alkylbenzene Sulfonates from LAOs
Linear alkylbenzene sulfonates (LABSes) are widely used synthetic surfactants that include different salts of sulfonated alkyl benzenes. C10–C14 LABS are the surfactants most frequently used in household and industrial detergents (both liquid and powder). C15+ LABSes are poorly soluble in water, so they are often mixed with mineral oils and used as lubricating coolants in the metalworking and drilling industries. LABSes have important advantages over branched alkylbenzene sulfonates (BABSes) that include higher degradability in the environment and better detergency. LABSes have therefore almost completely replaced RABSes in household detergents worldwide.
LABSes are synthesized via sequential alkylation of benzene, sulfonation of the obtained linear alkylbenzene (LAB), and neutralization of the product of sulfonation. Only olefins and haloalkanes are of industrial importance as benzene-alkylating substrates. Alkylation with alpha olefins is performed using homogeneous or heterogenous acid catalysts. Catalysts of alkylation in the current commercial production of LABs are typically homogeneous AlCl3 and HF [8]. UOP uses a proprietary solid catalyst [9].
One of the most important parameters of alkylation is the selectivity of the formation of the desired products. These are monosubstituted alkyl benzenes, which are classified according to the position of the phenyl substituent in the alkane: 2-phenylalkanes (2-LAB), 3-phenylalkanes (3-LAB), and so on. The diversity of alkylation products is one of the problems in the production of LABs. Along with variation in additions of the side chain, LABs can be alkylated at the benzene ring to form undesirable dialkyl- and trialkyl-substituted benzenes. Under certain conditions, migration of the double bond in the olefin is also possible. The position of the alkyl chain added to the benzene ring determines the detergency of the product. Maximum detergency is most commonly reached by adding the benzene ring in the first or second position.
There are different approaches to increasing the yield of the desired products. The selectivity of the production of dialkylate in the final mixture can be by adding an excess of benzene to the reaction mixture, controlling the reaction temperature and the catalyst load, or dealkylation in an external reactor [5].
The distribution of the reaction products depends on the choice of the catalyst. When using aluminum chloride, the contents of isomers are almost equal, with 2-phenylalkanes dominating. If the process is catalyzed by HF, the content of 2-phenylalkanes on the products of alkylation is very low. The dominant isomers are phenylalkanes in which the phenyl radical is in the central part of the chain. The closer the substituent is to the center, the higher the content of the corresponding isomer. Table 1 presents typical ratios between the products of the industrial alkylation of benzene when using different catalysts [2].
The bromine index (BI) characterizes the degree of unsaturation of the alkyl chain of a LAB molecule. A low bromine index for LAB is one sign of high quality. Allowable BI values are BI < 30; the preferred BI values are BI < 10 [2].
Products of alkylation are separated via fractional distillation. Benzene is the first to distill off. Unreacted olefins are next, followed LABs, which are used to obtain LABSes. The remaining dialkylbenzenes are removed and used as lubricants [10].
LABSes result from the interaction between LABs and different sulfonating agents in an equimolar ratio. Part 1 of this review already considered a set of the main sulfonating agents, which include sulfuric acid, oleum, pure SO3 or complexes of it, chlorosulfonic acid, and aminosulfonic acid [2].
Like benzene, phenol and naphthalene react with alpha olefins to form compounds used as detergents and lubricants [11].
The process most widely used to obtain LABs is UOP’s (Fig. 3) [12].
UOP process for producing LAB from n-paraffins [12].
The UOP process for producing LABs has four main steps. At the first one, n-paraffins are dehydrogenated into olefins at temperatures of around 500°C and a low (3 bar) excess hydrogen pressure using a modified platinum catalyst supported on alumina. The conversion of alkanes at this step is kept at 10–15% to minimize the subsequent deeper dehydrogenation of olefins to dienes and aromatic compounds. At the second, Ni/Al2O3 or Pd/C is used for selectively hydrogenating the dienes obtained at the first step into olefins, in order to increase the yield of LABs and improve the general quality of the product. At the third, aromatic compounds are removed from the mixture to extend the service life of the coking-prone DETAL catalyst used at the next step. At the fourth, benzene is alkylated with a mixture of alpha olefins using a solid heterogeneous DETAL catalyst, which is superior to conventional catalysts HF and AlCl3 in the quality of the product. The resulting products are separated into fractions via distillation, which is greatly simplified if olefins without impurities of paraffins are used in alkylation. Because the DETAL process uses substances that do not corrode metal (olefins, benzene, alkylbenzenes), reactors and other equipment and elements can be made of carbon steel. There is no need to use metal parts made of expensive alloys or canned pumps (as with HF). Using HF and AlCl3 also requires neutralization of the final reaction mixture, which raises the final costs [13].
The patent of Knifton et al. describes the production of LABs in a distillation column. An initial mixtuire of benzene and olefin in respective molar ratios of 1 : 1 to 100 : 1 is fed into the reactor [14]. The mixture enters a compacted bed of an acid heterogeneous catalyst based on fluorinated mordenite, in which alkylation occurs. Such process parameters as the temperature in the catalyst bed, the rates of reagent feed, and the size of the catalyst bed can be varied, depending on the reagents that are used. The temperature in the reactor is usually based on the boiling point of benzene at a chosen pressure. The optimum range of temperatures that ensures a high rate of the process without excessive coking of the catalyst is 80 to 140°C. The process can be conducted at different pressures, normally near-atmospheric. The temperature of the vaporizer for the most widely used C10–C14 mixture lies in the range of 200 to 250°C.
The possibility of using fluorinated mordenite catalysts in the considered process was discussed in Anantaneni’s patent [15]. A catalyst was obtained by mixing acidified mordenite LZM-8 and 0.4% hydrofluoric acid. Its selectivity toward 2-phenyl-substituted alkanes (2-LAB) was 70%, a very high value for the alkylation of benzene with alpha olefins.
The use of hydrogen fluoride has a number of obvious drawbacks, the most important of which is its high toxicity. Industrial equipment must meet strict environmental requirements, so one trend in developing of catalysts of alkylation is replacing HF with environmentally friendly (mainly heterogeneous) analogs.
Bordoloi et al. showed that composite materials can be used as catalysts of alkylation [16]. An example of this is the liquid-phase alkylation of benzene with 1-dodecene on regioselective composite catalyst AlMCM-41/beta zeolite. Conversion under certain conditions (120°C, a benzene : 1-dodecene molar ratio of 10 : 1, and a reaction period of 2 h) was 48% with selectivity of up to 2-dodecylbenzene of 76%. This catalyst also exhibited similar activity when using substrates with longer carbon chains.
Saxena et al. studied alkylation using zeolite catalysts of types ZSM-5, MOR, BEA, and HY [17]. The substrate was 1-hexene, and the reaction was conducted at atmospheric pressure with no solvent. The best result was obtained on BEA zeolite. The conversion of benzene was 86.6% (70°C, 3 h, benzene : 1-hexene molar ratio ≈ 1 : 2), and the selectivity toward the 2-LAB product was 38.7% (toward 3-LAB, 47.9%).
Halligudi et al. [18] investigated the alkylation of benzene using heterogenized heteropolyacid. The catalyst was zirconia-supported phosphotungstic acid (PTA). This is an effective stable solid acid catalyst whose activity depends on the content of PTA, the temperature of roasting, and the solvent used to produce the catalyst. The most active catalysts are those produced in methanol by applying a PTA monolayer onto zirconia. The resulting data showed that the effectiveness of such a catalyst is primarily determined by the number of Brønsted acid sites, along with the catalyst’s surface area and the morphology of pores. It was shown that the conversion of benzene grows along with the surface area, and the morphology of pores affects the catalyst’s selectivity toward the 2-LAB product. Under certain conditions (84°C, a benzene : alpha olefin molar ratio of 10 : 1, and a reaction period of 1 h), the most active catalyst displayed relatively high selectivities of 53.5% toward 2-phenyloctane and 47% toward 2-phenyldodecane with a 98% conversion of benzene.
Ahmadpour et al. [19] studied alkylation between benzene and 1-decene with Dawson heteropolyacid supported on titania nanoparticles. Theoretical calculations were made using the CCD (Central Composite Design) and FFD (Fractional Factorial Design) procedures, the results from which converged with experimental data. Variables were such characteristics as the benzene : 1-decene molar ratio, the catalyst load, and the reaction period and temperature. It was found that the contributions from different parameters to the conversion of 1-dodecene decline in the order catalyst load > weight content of the active component in catalyst > reaction period > 1-decene : benzene molar ratio. Nelder–Mead optimization showed that the optimum process parameters were a benzene : 1‑decene molar ratio of 17 : 1, a catalyst load of 35 wt %, and a reaction period of 1 h. The estimated conversion under these conditions was 100%. The yield of LABs was 99.92%, which differs only slightly from the obtained experimental data.
Stefan et al. considered another composite material that was based on AlCl3 immobilized on porous aluminosilicates [20]. Such catalysts have relatively high selectivity, compared to homogeneous analogs. Investigations of AlCl3 immobilized on MCM-41 with regular pore size have demonstrated the string effect of the pore size of MCM-41 on selectivity to LABs. Table 2 presents selectivities of the formation of monoalkyl-substituted benzene at 100% conversion of 1-octene on catalysts with different pore sizes.
The highest selectivity toward the formation of monosubstituted LABs using the most popular substrate (1-dodecene) was 96.2% against 72.5%, achieved using homogeneous AlCl3.
Similar results were obtained on a heterogeneous catalyst, AlCl3 immobilized on γ-Al2O3. At a 95% conversion of 1-dodecene, the total selectivity toward monosubstituted LAB was 92% against a 42% selectivity toward the formation of 2-phenyl-substituted alkanes. Tests of the catalyst’s stability showed it displayed no appreciable changes after 1000 h of operating under the conditions of the reaction [21].
Good results were obtained for the alkylation of benzene with commercially available mixtures of C10–C13 olefins and paraffins using a Zr-containing montmorillonite as a catalyst. The selectivity toward monosubstituted LABs was 72.5% at a more than 98% conversion of benzene. The experiments were performed under different conditions, but the optimum process parameters were reached at a temperature of 150°C, a pressure of 10 bar, and a benzene : alpha olefin molar ratio of 15 : 1 [22].
Preliminary acidic activation of the catalyst increased its activity during alkylation [23]. At a 98% conversion of 1-decene, 100% selectivity toward monosubstituted LABs was reached under optimum conditions (a temperature of 145°C and a benzene : 1-decene molar ratio of 12 : 1).
Numerous Zr-containing catalysts that ensure high selectivity to 2-LAB isomers have been described in the literature. One worthy of note is sulfated zirconia, which exhibits high Lewis acid activity. White et al. [24] determined that high activity in the alkylation of benzene with alpha olefins can be obtained on zirconia roasted at 500–550°C. They produced two types of such catalysts: mesoporous and microporous. The microporous catalyst ensured complete conversion of 1-dodecene and 93% selectivity to LABs, with 43% selectivity toward 2-LAB isomers. However, a comparison of the catalysts after repeated catalytic cycles showed that the microporous sulfated zirconia was deactivated while the mesoporous sample was not. This makes the latter a promising catalyst for the synthesis of LABs.
Another Zr-containing catalyst—a ZrO2/Al2O3 system with a high content sulfate ions—displayed high conversion of 1-dodecene (>90%) and high selectivity toward 2-LAB (>35%) at 150°C and a benzene : 1-dodecene molar ratio of 10 : 1 [25].
Sulfation can be used to impart acidity not only to zirconia. Kang et al. tested sulfated mesoporous tantalum oxides at a temperature of 80°C, a pressure of 1 bar, and a benzene : alpha olefin molar ratio of 10 : 1 [26]. The reaction was conducted for 0.5 h in a round-bottomed flask equipped with a reflux condenser. Full conversion was reached at a catalyst load of 4.0 wt % and a selectivity toward 2-LAB of 41.6%. It was determined that the selectivity and rate of the reaction on this catalyst depend on the pore size.
Complexes of group 3 metal halides and ionic liquids are another type of effective acid catalysts of alkylation. Among different catalytic systems based on ionic liquids with the composition 1-alkyl-3-methylimidazolium halide/aluminum chloride containing different alkyl group cations (butyl-, octyl- and dodecyl-) and different halogen anions (chlorine, bromine and iodine), catalyst [1-butyl-3-methylimidazolium]/Al2Cl6Br ensures 91.8% conversion of 1-dodecene with selectivity of 38% toward 2-LAB products [27].
Kumar et al. studied homogeneous and heterogeneous catalytic systems based on ionic liquids and AlCl3 [28]. It was found that the selectivity provided by homogeneous catalyst [BMIm]+Al2\({\text{Cl}}_{7}^{ - }\) toward 2-LAB products is somewhat lower (40.2%) than that of (45% at a 100% conversion of 1-dodecene) using heterogeneous catalyst SG–N+(C2H5)3–Al2\({\text{Cl}}_{7}^{ - }\) immobilized on silica gel. The reaction was conducted at 27°C on the former and 80°C on the latter at a benzene : olefin molar ratio of 10 : 1 and reaction periods of 15 and 150 min, respectively. It is noteworthy that despite the elevated temperature and the longer reaction period when using the heterogenous catalyst, its greatest advantage is high stability. Tests showed that the resulting homogeneous catalysts lost their activity as early as the fourth catalytic cycle, which was not observed for the heterogeneous catalyst.
The result of DeCastro et al. in [29] is of interest. Benzene was alkylated with 1-dodecene using ionic liquid [1-ethyl-3-methylimidazolium]/MnXn+1 immobilized on the porous support SiO2. The reaction was conducted for 1 h at 80°C and a benzene : olefin molar ratio of 10 : 1. A 99.4% conversion of 1-dodecene with a selectivity of 99.7% toward monoalkylated products was reached under these conditions.
The above data suggest that most of the above catalysts allow fairly high (above 80%) conversion of alpha olefins toward LAB products to be achieved. On the other hand, these catalysts have relatively low (no more than 42%) selectivity toward 2-LAB products (the preferred intermediates for susbequentt processing into derivatives) and the highest biodegradability among possible isomers that retain the required physicochemical properties. Products with high (76%) 2-LAB contents were obtained on AlMCM-41/beta zeolite catalyst, but it did not provide high conversion of alpha olefin into LABs (no more than 48%). The search for effective catalysts of alkylation that can ensure high conversion of alpha olefins with high selectivity toward 2-LAB products thus remains a challenge.
1.3 Producing Industrially Important Derivatives of Amines from LAOs
Linear alpha olefins can be used to produce alkylamines (AAs), which are in high demand in the cosmetics industry because they do not irritate the skin. Of greatest importance to the cosmetics industry are alkyldimethylamines (ADMA) and dialkylmethylamines (DAMA) [30].
Derivatives of amines also have a wide variety of other uses that include the production of amphoteric surfactants: fatty amine oxides (FAOs) synthesized via the reaction between AAs with hydrogen peroxide. C12–C14 alkylkamines are the ones used most often. FAOs exhibit properties of nonionic surfactants in the neutral and alkaline ranges of pH. Amine oxides are good hypoallergenic foam stabilizers, thickeners, water softeners, emulsifiers, and conditioners.
Combining AAs and AESes provides excellent foaming for dishwashing liquids.
Quaternary ammonium halides synthesized via the interaction between AAs and benzyl halides are highly effective biocides and antiseptics.
Betaines, which are easily obtained from AAs via their reacting with sodium chloroacetate, are in turn weak amphoteric surfactants with good foaming and stabilizing properties [10].
Albemarle is one of the largest manufacturers of alkylamines. This company produces a wide range of tertiary amines that are extensively used as active substances for hair care. Alkyldimethylamines are marketed as ADMA Tertiary Amines; dialkylmethylamines, as DAMA Tertiary Amines.
Albemarle’s patents describe ways of processing alpha olefins into valuable derivatives of amines. The patent of McKinnie and Harrod offers a procedure for preparing alkylamine from olefins via hydrohalogenation [31]. Alpha olefins (or mixtures of them and internal olefins) are first hydrohalogenated to form a mixture of haloalkanes. Selective dehydrohalogenation of this mixture is then performed, and 1-haloalkanes are separated from the rest of the mixture. Finally, 1-haloalkanes are aminated to produce amine hydrohalides that are neutralized to yield desired alkylamines. An improvement described in the patent is neutralization of the resulting alkylamine with an aqueous solution of an alkali or alkaline-earth metal hydroxide at the required temperature. One advantage of this is a reduced amount of the flocculent precipitate that forms when alkylamines are stored. Figure 4 shows the structure of alkylamines resulting from this process.
Structure of alkylamines produced using the Albermarle process [30] (R1, R2, R3, and R4 are hydrogens or alkyl groups).
1-Haloalkanes are aminated with amines that contain at least one substitutable hydrogen atom. This yields amine hydrohalides that are then converted to alkylamines via neutralization with a suitable base (e.g., NaOH, KOH, and Ca(OH)2) to form the salt from which the end product is obtained. The aminating agent that reacts with 1-haloalkanes can be ammonia or lower mono-, dialkyl-, or cycloalkylamines with one to six carbon atoms in their alkyl groups. The amine that reacts with 1-haloalkane should be a secondary amine with a short chain (e.g., methylamine, dimethylamine, isopropylamine, or diisopropylamine). Hydrohalogenation is usually conducted at temperatures of 0 to 75°C. At the same time, the temperature of selective dehydrohalogenation is 150–300°C at pressures of 0.5–10 bar. The latter reaction can be accelerated uaing a base metal oxide catalyst (e.g., magnesium oxide).
Amination is typically performed at temperatures of 50 to 200°C and pressures of 10–100 bar with a molar excess of dialkylamine. As already noted, the reaction mixture after the amination is neutralized at a temperature of 120–160°C. Neutralization is done with a fair amount of metal hydroxide in a small molar excess of hydroxide per number of moles of alkylamine hydrohalide, which must be converted to the product. A 6% molar excess of hydroxide is normally sufficient for neutralization [31].
Peterson and Fales [32] proposed aminating C2–C8 olefins with ammonia or derivatives of it on aluminosilicate catalysts (e.g., different zeolites). Researchers succeeded in achieving high (≈80%) selectivity of the formation of amines with respect to polyamines, avoiding the formation of large amounts of such byproducts as polyolefins or nitriles. It was assumed that an important role in catalytic activity and selectivity is played by the acidity of aluminosilicates (which is ensured by metal or hydrogen ions) or the resistance of metal ions to reduction to the metallic state. Transition metal cations are easily reduced to the metallic state, thereby impairing the acidic properties of aluminosilicates by lowering the total conversion of the substrate in the process. Transition metals in the neutral state also catalyze the dehydrogenation of amine to form undesired products (nitriles). The active component can be either triply charged cations of rare-earth elements, irons, aluminum, and chromium, or also doubly charged cations of calcium, magnesium, and copper. It was noted that zinc typically causes the dehydrogenation of amine, so its use is undesirable. Judging from the yields of amines, it is best to use lanthanum and hydrogen cations.
There are other ways in which zeolites are used as acid catalysts. BASF has commercialized processes for producing isopropylamine and tert-butylamine from propene and isobutene via their interaction with ammonia in the presence of beta zeolites (Fig. 5) [33].
Analysis of the literature shows that one trend in developing processes of the catalytic amination of LAOs is searching for the best acid catalysts. One difficulty in using them is selecting a reduction-resistant active component that would not reduce the acidic properties of the catalyst. Literature data indicate that lanthanum and hydrogen cations are best for this. Ways of processing the resulting products are also being developed, including improved neutralization to reduce the formation of flocculent precipitates when storing AAs and improve the overall quality of the products.
1.4 Producing Poly Alpha Olefins from LAOs
Poly alpha olefins (PAOs) are base materials for synthetic lubricants of the the automotive and aviation industries. Derivatives of C20+ alpha olefins are used in lubricating oils and transmission fluids. These wax fractions can also be chemically modified to replace more expensive carnauba wax or ozokerite, which are used to manufacture candles and polishes [1]. Many of these compounds have found other applications as well. PAOs are important components of dielectric liquids and drilling muds [1].
PAOs are obtained via the oligomerization of linear alpha olefins (mainly C10, but also C8, C12, and C14, or mixtures of these fractions) using promoted boron trifluoride as a catalyst. Depending on the conditions, a typical mixture of oligomers is 50–65% trimer and 10–15% dimer. Only low concentrations of the tetramer and higher oligomers form. Oligomers can be converted to stable isoparaffins of high purity by hydrogenation. The high efficiency of PAOs relative to group III base oils allows them to keep a considerable market share in sectors where improved performance of oils is required [34].
PAOs are usually considered the most universal of all lubricants for the automotive industry. Compared to conventional oils, PAOs have good lubricating properties over a wide range of temperatures, high viscosity indices, high thermal and chemical stability, and high resistance to ignition. PAOs have found a special niche in automotive transmission oils. Special PAO-containing oils have been developed for heavy-duty and off-road vehicles to save fuel and ensure the steady operation of engines at high temperatures and speeds [35].
High–molecular weight PAOs synthesized via the polymerization of medium–chain length alpha olefins (e.g., 1-hexene and 1-octene) have found important practical applications as drag-reducing agents. Such agents greatly reduce turbulence at the walls of oil pipelines, thereby lowering drag and friction losses. This reduces the consumption of energy for shipping oil and petroleum products over main pipelines, increases pipeline throughput capacity by at least 20%, and extends the service life of the equipment. Current drag-reducing agents are mainly solutions or suspensions of a high–molecular weight hydrocarbon polymer in a solvent.
Different ways of producing poly alpha olefins are described in the patent literature. ExxonMobil Chemical’s patent offers a process for obtaining poly alpha olefins in which individual AO monomers containing 3 to 24 carbon atoms or mixtures of them come into contact with transition metal bis(cyclopentadienyl) [36]. An anionic activator and optional trialkyl aluminum are also added to the reaction medium. An advantage of this process is that the rise in the temperature inside the reaction zone during oligomerization does not exceed 10°C, and the obtained poly alpha olefins have a kinematic viscosity of less than 20 mm2/s at 100°C.
The ExxonMobil Oil’s patent [37, 38] describes a way of producing synthetic oils from mixtures of C6–C12 alpha olefins. The procedure for olefin processing described by this patent is not restricted to this range. It can be used to polymerize virtually any alpha olefin or mixtures of them. Liquid alpha olefins or mixtures of them are saturated with BF3 at room temperature by, e.g., bubbling BF3 through a mixture of olefins immediately before loading into the reaction zone. To improve the catalytic properties of BF3 in the polymerization of olefins, substances capable of forming complexes (adducts) with BF3 are introduced to the BF3 flow. The flow fed into the reactor thuscontains a 1 : 1 molar complex of BF3 with a promoter compound. When it comes into contact with alpha olefins, this flow catalyzes the polymerization process. A wide range of promoters is used, the most popular of which are water, acetic acid or its anhydride, diethyl ether, ethyl acetate, acetone, and benzaldehyde. The rate at which flows are fed into the reactor is limited only by our ability to remove the heat released during polymerization from the reactor and maintain a given temperature, which is typically in the range of 0–35°C. The reaction can be conducted at atmospheric pressure, but the pressure is usually raised to 34 bar to increase the conversion of olefins.
The patent literature also contains information on ways of obtaining high–molecular weight copolymers of alpha olefins.
The Universal Oil Products (UOP) patent [39] describes procedure for polymerizing olefins to form high–molecular weight polymers using a catalytic system that contains titanium tetrahalide and metallic aluminum. An important role in this process is played by the solvent, a halogenated aromatic hydrocarbon (e.g., chlorobenzene or 2,3-dichlorotoluene). A specific feature of such solvents is their ability to dissolve the polymers that form, thereby cleaning the surface of the catalyst. This allows us to obtain high–molecular weight polymers and prolongs the period of catalyst deactivation. Another important factor is that halogenated aromatic hydrocarbons do not favor the undesirable side process of alkylation, which occurs when using nonhalogenated aromatic solvents in combination with titanium tetrahalides.
The patent [40] granted to Zharov et al. (Nika-Petrotek, Russia) describes the production of copolymers of alpha olefins with ultrahigh molecular weights of more than 5 MDa on a modified titanium–magnesium nanocatalyst. The patent describes a procedure for obtaining an effective catalyst of polymerization by selecting the molar ratio between titanium, magnesium, and n-butyl chloride. An excess of TiCl4 in synthesizing the catalyst results in a very high activity of copolymerization, making the reaction uncontrollable and increasing the rate of heat release. In contrast, an insufficient amount of TiCl4 results in low activity, greatly prolonging the time required to reach the required conversion of alpha olefins.
Ways of producing drag-reducing additives based on high–molecular weight polyhexene have also been patented [41, 42]. Polymerization is done mainly in the liquid phase using an organic solvent (e.g., benzene). Polymerization occurs at temperatures of 20–30°C when using conventional Ziegler–Natta catalysts. Introducing such electron donors as alkoxy/alkyl-containing silicon compounds increases the catalyst’s stereo-regulating ability. High–molecular weight polymers are obtained if the required conditions are maintained.
Numerous transition metal–based catalytic systems for the polymerization of olefins are described in the scientific literature. Guo et al. [43] discussed the polymerization and copolymerization of alpha olefins with polar comonomers (e.g., methyl acrylate) catalyzed by transition metals. The change in the nature of the catalysts allows us to obtain polymers with radically different characteristics. The main problem in using group 8–11 transition metal compounds as catalysts of polymerization is the formation of amorphous and atactic polymers, due to their low regio- and stereo-selectivity and the rapid migration of chains.
Caruthers et al. [44] studied the polymerization of 1‑hexene on zirconocene rac-C2H4(1-Ind)2ZrMe2 activated with B(C6F5)3, especially the mechanism of the reaction. They developed kinetic models that allowed them to predict a wide range of properties of the resulting product, including the molecular weight distribution of the polymer.
Zakharov et al. [45] investigated the production of polyhexene on titanium–magnesium catalysts. They found that both hydrogen and the cocatalyst (triethylaluminum, triisobutylaluminum) strongly affect the activity of the system, along with the molecular weight distribution and isotacticity of the polymer.
Tavtorkin et al. considered the possibility of synthesizing poly alpha olefins on titanium–magnesium, metallocene, postmetallocene, and other catalysts. They concluded that polymers obtained with mixtures of alpha olefins can have properties superior to those of homopolymers, and catalysts sufficiently active for the polymerization of alpha olefins are Ziegler–Natta systems based on titanium(III) chloride. Tavtorkin et al. believed a promising approach was to use a sequence of reactions for oligomerizing ethylene into 1-hexene and 1-octene with subsequent polymerization is [46].
Kaiser and Long [47] described catalysts based on complexes of Pd and Ni, which take full advantage of such popular ligands as phosphine sulfonates, alpha diimines, and N-heterocyclic carbenes. These systems are highly selective toward different substrates (e.g., ethylene, higher alpha olefins, alkynes, and many polar co-monomers). Nickel-based catalysts for the redox polymerization of olefins find special applications because they can control the microstructure of polyolefins, depending on the electronic state of the ligand.
Wang et al. [48] tested naphthyl-alpha-diimine nickel complexes activated by modified methylaluminoxane for use as catalysts in the polymerization of higher alpha olefins (1-hexene, 1-decene, and 1-hexadecene). The results from polymerization suggest the possibility of controlling microstructure by varying the nature of the ligands, the temperature of polymerization, and the nature and concentration of the monomer. The investigated complex showed good catalytic activity and produced branched polymers (42–88 branches per 1000 carbon atoms) with high molecular weights (Mn = (4.3–15.2) × 104 g/mol) and a fairly narrow molecular weight distribution of the resulting polymer.
Our analysis of the literature thus shows that synthetic high–molecular weight polymers (PAOs obtained from LAOs) are products in high demand. PAOs are superior in their properties to ordinary polymers obtained from conventional monomers. A promising trend is investigating processes of the polymerization of higher alpha olefines to produce such polymers as polyhexene and polyoctene. Systems used in the polymerization of ethylene, which include many thoroughly-studied Ziegler–Natta systems, different organometal complexes, and strong Lewis acids (e.g., BF3) can also be catalysts of polymerization.
1.5 Producing Industrially Less Important Derivatives of LAOs
Alpha olefins are used in fields other than their more familiar applications. This section briefly presents data on industrially less important or small-scale products of LAO processing.
A wide range of LAOs are used to produce ketones, esters, pyrazines, and alkylsilanes. Compounds containing S–H bonds can react with alpha olefins under suitable conditions to form sulfur-containing hydrocarbons. Thiols obtained via interaction between alpha olefins and hydrogen sulfide are used in ore flotation, as additives in manufacturing rubber goods, and in other chemical industries. LAOs are also converted into trialkylphosphines, alkylsilanes, and organometal compounds that are then used in different industries [10].
The oxidation of alpha olefins yields a wide range of fatty acids that are used to produce polyester alkyd resins, dies, and stabilizers. Substituted acids are used as biostimulants; esters, as fragrances. A major manufacturer of synthetic fatty acids is OXEA. This company produces heptanoic (C7) and nonanoic (C9) acids from 1-hexene and 1-octene, respectively. Alpha olefins are used to synthesize aldehydes, from which acids are then obtained. Muller et al. described a way of preparing aldehydes by hydroformylating LAOs with hydrogen and carbon monoxide using a homogeneous catalytic system that contains complex compounds HRhCO[P(C6H5)3]3 and RhCl[P(C6H5)3]3. The catalytic system is subsequently separated from the reaction mixture by filtering it through a semipermeable aromatic polyamide membrane under pressure [49]. Hydroformylation is performed at pH ranged within 2.5–4.3, phosphine : Rh molar ratios of 60 and more, and concentrations of rhodium of at least 10−6 by weight on an olefins basis. Instead of aromatic phosphines, we can use special alkylammonium or arylammonium salts, or sulfonated or carboxylated triarylphosphines, which produce high activity and high selectivity in hydroformylation, along with high retention of the catalyst in membrane filtration.
LAOs are hydroformylated at temperatures of 100–140°C and pressures of 0.5–27 MPa. The carbon monoxide : hydrogen ratio in syngas can be varied over a wide range. A ratio of 1 : 1 is typically used. It is also important to maintain the pH of the reaction mixture in the range of 2.5–4.3 during hydroformylation. This is because the pH can fall continuously during hydroformylation, due to the dissociation of sulfonated or carboxylated triphenylphosphine ammonium salts into free amine and the respective triphenylphosphine sulfonate or carboxylate. An appropriate amount of free amine NR1R2R3 or metal hydroxide is added to the system to maintain the required pH. Phosphorus-containing compounds cannot be removed completely from the product, so as much as 5.6% of phosphorus(III) compounds can remain in the final mixture. These can be removed from it via complex distillation with a fairly high loss of the desired product.
The final step in the production of fatty acids is catalytic oxidation of the obtained aldehydes. The Springer and Lappe’s patent describes this process, which is catalyzed by metal compounds of groups 5 and 11. It is important to maintain the optimum weight ratio between the catalyst and the aldehyde being oxidized [50]. According to the patent, the best ratio is 0.5–2 weight parts of the metal to 106 weight parts of the aldehyde. This ratio is considered optimal when using metal compounds or mixtures of them, so the required amount of the catalyst is determined by the content of metal in it.
Oxidation is done at atmospheric pressure in the 20–100°C range of temperatures. The length of the process needed to convert aldehydes to carboxylic acids is typically 3 to 8 h. It is determined by the temperature of the reaction, the natures of the reactants and the catalyst, and their ratio.
C16–C24 LAOs are used to alkylate naphthalene. The obtained product is used as pour-point depressants that lower the pour point of oil. In this application, it competes with polymetacrylate pour-point depressants [51].
The patent of Helton et al. [52] describes a way of obtaining alkyl-substituted naphthalenes with long chains via interaction between naphthalene and C8+ olefin, which is used as an alkylating agent. The process is conducted under conditions that are standard for alkylation using a catalyst that contains highly stable zeolite Y with cation-substituted active acid sites. The initial reactant can be either naphthalene itself or different alkylnaphthalenes with short-chain substituents (e.g., methyl, ethyl, or propyl). The most widely used alkylating agents are C8–C12 AOs. The large size of the molecules of alkylation products greatly limits the size of pores in zeolite, since the products must pass freely through them, removing constraints on diffusion from long-chain alkylating agents. Suitable zeolites with large pores include faujasite, synthetic faujasites (zeolites X and Y), zeolite L, ZSM-4, ZSM-18, ZSM-20, mordenite, and offretite.
LAOs can be converted into alkenyl succinic anhydrides (ASAs) formed by heating linear alpha olefins and maleic anhydride at temperatures to 200°C. The resulting anhydrides are used as dispersing agents in lubricant oils and transmission fluids, and as pour-point depressants. One of the most popular uses for ASAs is paper sizing. C16 or C18 linear alpha olefins are isomerized to form mixtures of olefins with internal double bonds. These olefins then react with maleic anhydride to yield the desired ASAs [1].
According to the Albemarle’s patent [53], maleic anhydride (MA) interacts with aliphatic olefine at temperatures of 190–250°C when using the stabilizing agent triarylphosphite (Fig. 6):
where R1 is the C4–C12 tertiary alkyl group, and R2 is the C1–C12 alkyl group.
The molecular weight of the used olefin can be varied widely, depending on the purpose of the product. Alkenyl groups containing 12–22 carbon atoms are used in synthesizing ASAs for treating paper. Corrosion inhibitors and detergents typically contain C16–C35 alkenyl groups. ASAs used to produce imides, amides, and esters for use as dispersing agents in lubricant oils have alkenyl groups with 40–250 carbon atoms.
Olefins are added to MA inside a sealed (to avoid loss of the reactants) reaction vessel at temperatures of 190–250°C under the intrinsic pressure. The MA : olefin molar ratio can be varied over a wide range, and the optimum ratios range from 3 : 1 to 1 : 3. When using such high–molecular weight olefins as polyisobutylene with average molecular weights of 900–5000 or more, MA is taken in a stoichiometric excess (e.g., 1.1 : 5 mol MA per mole of olefin). The unreacted MA is removed by evaporating it from the reaction mixture. If olefins with lower molecular weights of 200–350 are used, they are typically taken in excess (e.g., in an olefin : MA molar ratio of 1.1 : 3). The optimum amount of tri(ortho-tert-alkylphenyl)phosphite in the reaction mixture is easy to determine experimentally. It is approximately 0.1–1 wt % of the weight of the MA.
Chevron has patented a process for producing alkenyl succinic anhydride from a mixture of alpha olefins and maleic anhydride [54]. Linear alpha olefines are mostly used in this process. The initial mixture of olefines is preliminarily separated into low- and high-boiling fractions, and the higher-boiling LAOs are isomerized using acid catalysts. The resulting mixture is heated in combination with maleic anhydride for ≈3 h inside an autoclave at 230°C. This process allows up to 95% conversion of olefines. Unreacted MA and AOs are removed from the mixture of products by heating to 260°C at ≈0.03 bar while bubbling nitrogen through the melt. The purified ASA is a liquid with a pour point of around 5°C.
Interaction between alpha olefines and peroxides yields epoxides that are used as modifiers of epoxy resins and the polyether components of polyurethanes. Almost all fractions of alpha olefines with different numbers of carbon atoms find use in the production of epoxides [35]. Patents describe different ways of synthesizing epoxides from alpha olefines.
The Chevron’s patent [55] describes a procedure for the epoxidation of alpha olefines, in which hydroperoxide stabilized by barium oxide is added to a mixture of alpha olefines along with a catalyst of epoxidation. It is believed that the catalyst promotes the transfer of oxygen from hydroperoxide to the double bonds of the olefine. A considerable excess of olefine is created in the reaction zone to accelerate epoxidation and simultaneously prevent excessive decomposition of peroxide.
The Vangermain and Wolpers’s patent [56] describes the beneficial effect of slowly adding organic hydroperoxide to an excess of olefine in order to reach the optimum conditions of epoxidation. It is assumed that if there is a shortage of olefine in the reaction zone, the catalyst of oxygen transfer accelerates the undesirable decomposition of hydroperoxide, and any nonselective decomposition of hydroperoxide lowers the yield of epoxide. The reaction thus proceeds quickly in a considerable excess of olefine by gradually addi small amounts of hydroperoxide.
The above patents [55, 56] indicate that having barium oxide in the reaction mixture allows the catalytic epoxidation of olefines with organic hydroperoxides of fairly high selectivity toward epoxide and at relatively low molar ratios between olefine and hydroperoxide in the reaction zone. This is because barium oxide stabilizes hydroperoxide, preventing undesirable decomposition. These patents emphasize the uniqueness of barium oxide because it was determined that none of the other base metals can effectively inhibit the decomposition of hydroperoxide.
Because epoxidation is highly exothermic, gradually adding hydroperoxide reduces the self-heating of the reaction mixture. An advantage of gradually adding hydroperoxide is the possibility of using high concentrations of hydroperoxide while maintaining high yields of the epoxy resin and thus reducing consumption of the reactant.
Any homogeneous catalyst of oxygen transfer (e.g., such soluble molybdenum compounds as molybdenum naphthenate, molybdenum acetylacetonate, and molybdenum carbonyl) can be used for epoxidation [57]. The optimum content of catalyst ranges from 0.05 to 2.5 wt % of the total amount of olefine and organic hydroperoxide.
Chlorination of normal C20–C24 alpha olefines yields products used as secondary plasticizers in PVC compositions [35]. Plasticizers based on chlorinated alpha olefine (40 wt % Cl) can compete with analogs based on chlorinated paraffins (42 wt % Cl) and alkyl aryl hydrocarbons. Estimates of the mechanical properties of these plasticizers showed that chlorinated alpha olefines are equivalent to two other secondary plasticizers in efficiency. At the same time, however, they have relatively high heat resistance and low volatility. Chlorinated poly(1-dodecene), poly(1-tetradecene), and poly(1-hexadecene) can be stable high-temperature additives to emulsions used in metalworking.
CONCLUSIONS
Our analysis of scientific publications and patent literature has identified current trends in approaches to processing LAOs.
A rapidly developing trend in catalytic alkylation with the participation of LAOs is the development of solid acid catalysts, driven by the many obvious advantages of heterogeneous over homogeneous catalysts. Heterogeneous catalysts are easier to separate from the reaction space and thus reduce losses of them and ensure the purity of the resulting products. A fairly new trend is using insoluble salts of heteropoly acids of different compositions, which continue to compete with zeolites, different metal oxides, Zr-containing catalysts, and conventional aluminum chloride, the last being immobilized on different supports for use as a heterogeneous catalyst. Another promising trend is the development of catalytic systems that use different ionic liquids that favor high selectivity toward 2-LAB products. Advantages of these systems are the simplicity of homogenizing the reaction space, preserving the uniformity of the spatial temperature distribution in the reactor during exothermic processes, and preventing the formation of local hotspots that lower the yield of the desired product and the lifetime of the catalyst. Nevertheless, large-scale commercial application of these systems in industrial processes of alkylation remains an open question. The main disadvantages of catalytic systems based on ionic liquids (relative to, e.g., solid acid catalysts) are the extremely high hydrophilicity of ionic liquids and thus the need to comply with special conditions of storing and handling the reagents, along with the high cost of separating and regenerating the catalyst.
In terms of chemistry, the approaches to the synthesis of alpha olefin sulfonates and other surfactants obtained via sulfonation remain mostly conventional. Nevertheless, there are many patents for improving the design of reactors to optimize heat removal and other factors. However, the main sulfonating agent remains SO3 in a flow of dry air. This is how all major companies obtain AOSes.
Our analysis of patent literature on the production of poly alpha olefins showed that BF3 is a promising catalysts. Promoters with which BF3 forms adducts vary in numerous works and patents. Some works propose using different sandwich catalysts. These are widely known, due to their high activity in the polymerization of ethylene.
Our analysis of recent literature data and patent information confirmed the high demand for derivatives of linear C4+ alpha olefines, in both many industries and household use. The most important trends in the processing of alpha olefines are the synthesis of LABs and their subsequent processing into LASes, along with the production of AOSes and fatty alcohols that are converted to alkyl ethoxy sulfates, alkyl ethoxylates, and alkyl sulfates in high demand (see Part 1 of this review). Also of commercial interest are the high-boiling linear alpha olefines (C17+) used in, e.g., synthesizing PAOs and ASAs, which are then used to produce synthetic lubricants.
The main trend in the technological development of the large-scale production of the products obtained from LAOs is improving the economic efficiency of the relevant processes by creating and using new catalysts while modernizing existing reactors and separation systems to increase the yield of end products. The many scientific works and patents published in the last 10–15 years testifies to the attention given this subject by specialists of scientific institutions and universities, and the research departments of commercial companies.
It should be noted that that the economic efficiency of processing LAOs can also be improved by reducing the cost of producing the initial reactant. The main ways of obtaining LAOs are currently the oligomerization of ethylene via SHOP (the Shell Higher Olefin Process™, Shell) and the dehydrogenation of n-alkanes (Pacol™, UOP; see Fig. 3) on modified platinum catalyst supported on alumina. Both ways of producing LAOs are quite costly, due to using high-purity ethylene as a feedstock in the former case, and the high cost of regenerating expensive Pt-containing catalyst in the latter. An alternative way of producing LAOs could be the radiation- or microwave-stimulated cracking of nonstandard feedstock containing high–molecular weight n-alkanes (products from the dewaxing of diesel and oil fractions, oil residues from Fischer–Tropsch synthesis, and solid household wastes with high contents of polymer), a process being developed at the Russian Academy of Sciences’ Boreskov Institute of Catalysis.
REFERENCES
Neodene Linear Alpha and Internal Olefins. Shell Official Website. https://www.shell.com/business-customers/ chemicals/our-products/higher-olefins-and-derivatives/ neodene-linear-alpha-and-internal-olefins.html. Cited July 12, 2021.
Handbook of Detergents, part F: Production, Zoller, U. and Sosis, P., Eds., Boca Raton, FL: Taylor & Francis, 2008.
US Patent 3839391A, 1974.
US Patent 3925441A, 1975.
Porter, M.R., Handbook of Surfactants, New York: Springer, 1991.
Yamane, I., J. Am. Oil Chem. Soc., 1978, vol. 55, no. 1, pp. 81–86.
US Patent 3758608A, 1973.
Alkylation Unit. McKinsey & Company Official Website. www.mckinseyenergyinsights.com/resources/refinery-reference-desk/alkylation-unit/. Cited July 12, 2021.
De Almeida, J., Dufaux, M., Taarit, Y.B., and Naccache, C., J. Am. Oil Chem. Soc., 1994, vol. 71, no. 7, pp. 675–694.
Alpha Olefins Applications Handbook, Lappin, G. and Sauer, J., Eds., New York: Marcel Dekker, 1989.
De Klerk, A. and Nel, R.J., Ind. Eng. Chem. Res., 2007, vol. 46, no. 22, pp. 7066–7072.
US Patent 4523048A, 1985.
Surfactants. A Practical Handbook, Lange, R. K., Ed., Synthesis, Properties, Analysis, Application), Munich: Hanser Publishers, 1999.
WO Patent 1997029063A1, 1997.
US Patent 6166281A, 2000.
Bordoloi, A., Devassy, B.M., Niphadkar, P., Joshi, P., and Halligudi, S., J. Mol. Catal. A: Chem., 2006, vol. 253, nos. 1–2, pp. 239–244.
Saxena, S.K., Viswanadham, N., and Ala’a, H., Mater. Today Chem., 2017, vol. 4, pp. 45–52.
Devassy, B.M., Lefebvre, F., and Halligudi, S., J. Catal., 2005, vol. 231, no. 1, pp. 1–10.
Toutounchian, N., Ahmadpour, A., Heravi, M.M., Bamoharram, F.F., Ayati, A., and Deymeh, F., Res. Chem. Intermed., 2016, vol. 42, no. 4, pp. 3283–3301.
Hu, X., Foo, M.L., Chuah, G.K., and Jaenicke, S., J. Catal., 2000, vol. 195, no. 2, pp. 412–415.
Shang, L., Ji, M., Cai, T., Shan, W., He, M., and Jiang, S., React. Kinet. Catal. Lett., 2005, vol. 87, no. 1, pp. 101–106.
Awate, S., Waghmode, S., and Agashe, M., Catal. Commun., 2004, vol. 5, no. 8, pp. 407–411.
Faghihian, H. and Mohammadi, M.H., Appl. Surf. Sci., 2013, vol. 264, pp. 492–499.
Clark, J.H., Monks, G.L., Nightingale, D.J., Price, P.M., and White, J.F., J. Catal., 2000, vol. 193, no. 2, pp. 348–350.
Borutskii, P., Kozlova, E., Podkletnova, N., Gil’chenok, N., Sokolov, B., Zuev, V., and Shatovkin, A., Pet. Chem., 2007, vol. 47, no. 4, pp. 250–261.
Kang, J., Rao, Y., Trudeau, M., and Antonelli, D., Angew. Chem., 2008, vol. 120, no. 26, pp. 4974–4977.
Xin, H., Wu, Q., Han, M., Wang, D., and Jin, Y., Appl. Catal., A, 2005, vol. 292, pp. 354–361.
Kumar, R., Kumar, A., and Khanna, A., Catal. Ind., 2015, vol. 7, no. 3, pp. 188–197.
DeCastro, C., Sauvage, E., Valkenberg, M., and Hoel-derich, W.F., J. Catal., 2000, vol. 196, no. 1, pp. 86–94.
Surfactant Science Series, vol. 68: Surfactants in Cosmetics, Rieger, M.M. and Rhein, L.D., Eds., New York: Marcel Dekker, 1985.
US Patent 5347053A, 1994.
US Patent 4375002A, 1983.
Beller, M., Seayad, J., Tillack, A., and Jiao, H., Angew. Chem., Int. Ed., 2004, vol. 43, no. 26, pp. 3368–3398.
Oil Categories. American Petroleum Institute Official Website. www.api.org/products-and-services/engine-oil/eolcs-categories-and-classifications/oil-categories. Cited July 13, 2021.
Greiner, E.C. and Inoguchi, Y., Chemical Economics Handbook: Linear Alpha-Olefins, Menlo Park, CA: SRI Consulting, 2010. https://cdn.ihs.com/www/pdf/CEH-Linear-Alpha-Olefins-sample-report-2010.pdf. Cited July 13, 2021.
US Patent 8207390B2, 2012.
US Patent 3149178A, 1964.
US Patent 3382291A, 1968.
US Patent 3019216A, 1962.
EA Patent 026852B1, 2017.
US Patent 9074024B2, 2015.
RF Patent 2230074C1, 2004.
Guo, L., Liu, W., and Chen, C., Mater. Chem. Front., 2017, vol. 1, no. 12, pp. 2487–2494.
Novstrup, K.A., Travia, N.E., Medvedev, G.A., Stanciu, C., Switzer, J.M., Thomson, K.T., Delgass, W.N., Abu-Omar, M.M., and Caruthers, J.M., J. Am. Chem. Soc., 2010, vol. 132, no. 2, pp. 558–566.
Matsko, M., Echevskaya, L., and Zakharov, V., Kinet. Catal., 2020, vol. 61, no. 1, pp. 40–57.
Ivchenko, P., Nifant’ev, I., and Tavtorkin, A., Pet. Chem., 2016, vol. 56, no. 9, pp. 775–787.
Kaiser, J.M. and Long, B.K., Coord. Chem. Rev., 2018, vol. 372, pp. 141–152.
Wang, F.-Z., Tian, S.-S., Li, R.-P., Li, W.-M., and Chen, C.-L., Chin. J. Polym. Sci., 2018, vol. 36, no. 2, pp. 157–162.
US Patent 5773667A, 1998.
US Patent 6800783B2, 2004.
Guo, H., Liang, Y., Qiao, W., Wang, G., and Li, Z., Stud. Surf. Sci. Catal., 2002, vol. 142, pp. 999–1006.
US Patent 5457254A, 1995.
US Patent 5021169A, 1991.
US Patent 4431826A, 1984.
US Patent 4217287A, 1980.
US Patent 3526645A, 1970.
Barlan, A.U., Basak, A., and Yamamoto, H., Angew. Chem., Int. Ed., 2006, vol. 45, no. 35, pp. 5849–5852.
Funding
This work was supported by the Russian Scientific Foundation, project no. 17-73-30032.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Golub’, F.S., Bolotov, V.A. & Parmon, V.N. Current Trends in the Processing of Linear Alpha Olefins into Technologically Important Products: Part 2. Catal. Ind. 13, 203–215 (2021). https://doi.org/10.1134/S2070050421030053
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070050421030053