Abstract
Comparative estimation is performed for the repeatability of results from measuring the two main parameters (peak areas and retention times) of components (carbon dioxide, sulfur compounds) of a model mixture similar in composition to natural gas. The measurements are made on a capillary column prepared for the first time with nonpolar phase poly(1-trimethylsilyl-1-propyne) (PTMSP) with dimensions of 30 m × 0.53 mm and a sorbent film thickness of 2.82 μm, and a commercial Rt–Q–BOND column 30 m long and 0.32 mm in diameter, with a nonpolar polydivinylbenzene sorbent layer 10 μm thick. The use of these columns ensures satisfactory repeatability of the chromatographic characteristics of the analyzed compounds. The relative mean standard deviation (RMSD) for retention times is no greater than 0.10%. The RMSD for the measured peak areas is less than 1.92%. A slight difference between the peak areas for sulfur dioxide and carbon disulfide (five consecutive injections) is observed on an Rt-Q-BOND capillary column. The corresponding values of the RMSD are 4.22 and 2.52%. The magnitude of the signal response of the analyzed compounds is recorded with a microcatarometer. The limits for detecting sulfur-containing compounds on the PTMSP column change from 0.40 × 10−3 to 0.82 × 10−3 mg/mL. The possibility of selectively separating the macrozone of methane and the propane–butane fraction from trace hydrocarbons, sulfur compounds that contain carbon dioxide, hydrogen sulfide, and mercaptans on a capillary column with a film thickness of 2.82 μm PTMSP is also demonstrated for the first time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Many sources of natural gas and other hydrocarbon fuels contain sulfur compounds, which not only have an unpleasant odor but damage human health as well. In a wide fraction of light hydrocarbons (C2–C5) impurities contain carbonyl sulfide and carbon disulfide in addition to hydrogen sulfide and mercaptans. Such sulfides corrode equipment and poison catalysts in the processes of petrochemical synthesis. In addition, substances are added to natural gas to give it a distinctive odor, in order to identify possible leaks. Sulfur-containing compounds (mercaptans) are usually used as odorants [1].
Gas chromatography is the main way of determining the component composition of natural gas, products of gas and oil refining, anthropogenic contamination with sulfur compounds in the environment (the atmosphere, the air of a workspace, soil, water). In both domestic (GOST) and foreign (ASTM) procedures, sulfur compounds are nowadays analyzed on columns with polysiloxane stationary liquid phases, silica sorbents, and porous polymers [2–5]. If all conditions are met and determination of sulfur-containing compounds is done in the 0.00010 to 0.50% range of measuring the mass fraction in natural gas on columns with silica sorbents, the repeatability of the measuring results should not exceed 12% [5].
Over the last decade, the use of different detectors (flame photometric (FPD), pulsed flame photometric (FPPD), microcatarometer (μ-TCD), and differential ion mobility (DPD)) and combinations of them has allowed detection of sulfur compounds in natural gas, in the range of 10 ppbv to 5 ppmv. When these compounds are chromatographed on capillary columns with dimethylpolysiloxane phases CP-Sil 5, DB-1. and Optima 5, or on columns with such porous polymers such as PoraPLOT U and PoraBOND Q, the repeatability index for, e.g., hydrogen sulfide with a concentration of 1–3 ppm is 2–8% [6].
The PAC Corporation offers the AC ASTM 5623 analytical system with a new SeNse chemiluminescence detector from Analytical Controls for analyzing ultra-low concentrations of sulfur compounds in natural gas and gaseous fuels. When checking this system, the methodology for analyzing sulfur-containing compounds using a metering valve and a capillary column with a thick film of a polysiloxane stationary liquid phase, the maximum RMSD value for the retention time was 0.04% for hydrogen sulfide and carbonyl sulfide. The maximum RMSD of the repeatability of the results of the peak areas when a mixture of sulfur-containing gases was introduced with a metering valve was 0.10% for hydrogen sulfide and methyl mercaptan [7].
The authors of [1, 8–11] described approaches to analyzing complex matrices that contain hydrocarbon and sulfur compounds in studying the reduction of sulfur dioxide with methane or synthesis gas, the catalytic dehydrogenation of isobutane, and the catalytic pyrolysis of ethylbenzene on packed and capillary columns prepared on the basis of glassy nonpolar porous polymer poly(1-trimethylsilyl-1-propyne). The main chromatographic parameters (retention characteristics, selectivity of separation of pairs of components, column efficiency) were determined. A capillary column 30 m long and 0.32 mm in diameter with a film thickness of 0.8 µm PTMSP was used in studying the composition of products of the catalytic pyrolysis of ethylbenzene in [12]. The analysis proceeded one and a half times faster using this column than on a commercial Rt–Q–BOND (polydivinylbenzene non-polar porous polymer) column of the same size with a sorbent layer of 10 μm. However, the PTMSP column is inferior to the commercial one in terms of separation capacity for some aromatic hydrocarbon vapors. For example, the resolution (Rs) of a styrene/ethylbenzene pair was 0.9, while Rs = 1.25 for the same pair on the Rt–Q–BOND column. Incomplete separation of the butene-1/isobutane pair was also observed on a column with PTMSP with a layer thickness of 0.8 μm (for which Rs = 0.45) when analyzing a mixture of hydrocarbons with accompanying impurities of sulfur compounds [1].
To improve the properties of separation, a capillary column with dimensions of 30 m × 0.53 mm and a layer of nonpolar poly(1-trimethylsilyl-1-propyne) 2.82 μm thick was prepared for the first time and used in this work to analyze a mixture close in composition to natural gas.
It is known from the literature that a sorbent (e.g., Porapak Q) obtained on the basis of a copolymer of ethylstyrene and divinylbenzene contains active sites in the amount of 1% of its surface. The authors associate the presence of such sites with traces of the catalyst that remain during synthesis (remnants of acid anhydride) [13].
Since commercial Rt–Q–BOND with polydivinylbenzene sorbent was selected as the comparison column, it was important to study the effect of possible acidic sites on chemical behavior, relative to the areas of the chromatographic peaks of sulfur compounds.
Monitoring the accuracy of measuring the chromatographic parameters of the analyzed components on a capillary column with PTMSP was also not studied earlier. The index of the repeatability of the results from measuring the two main chromatographic parameters (peak area and retention time) for carbon dioxide and light sulfur compounds was evaluated via gas chromatography using a microcatarometer (μ-TCD). The limits of detection for sulfur compounds analyzed on the column with PTMSP were calculated. We also compared the selectivity of the separation of these compounds on a capillary column prepared on the basis of poly(1-trimethylsilyl-1-propyne) with a film thickness of 2.8 μm, and on a commercial Rt–Q–BOND column with a polydivinylbenzene sorbent layer 10 μm thick.
EXPERIMENTAL
Reagents
In this work, we used nonpolar poly(1-trimethylsilyl-1-propyne) synthesized at the Boreskov Institute of Catalysis [8, 9].
Column Preparation
The sorbent was applied to the inner surface of the capillaries using the high pressure static procedure. A solution of PTMSP in toluene was prepared with 0.2 g of polymer dissolved in 9.4 mL of toluene. The procedure for preparing the column was described in [9].
Column I with dimensions of 30 m × 0.53 mm and a polymer film thickness of 2.82 μm was prepared on the basis of PTMSP for the first time.
A commercial Rt–Q–BOND capillary (Restek, United States) (column II) with dimensions of 30 m × 0.32 mm × 10 μm and a polyvinylbenzene sorbent was used as our comparison column. The choice of the comparison column was due to polydivinylbenzene sorbent, like PTMSP, being a nonpolar stationary phase and displaying similar chromatographic properties [14–16]. It would probably be more correct to perform a comparative analysis with an Rt–Q–BOND column having dimensions of 30 m × 0.53 mm. We may assume, however, that (a) the period of elution of the components would grow considerably and (b) the efficiency of the column would fall, since broad peaks of butane isomers were already observed on a 30 m × 0.32 mm × 10 μm polydivinylbenzene column.
Preparing Model Mixtures
Mixture I for testing was prepared by mixing carbon dioxide, carbonyl sulfide, sulfur dioxide, and carbon disulfide. Mixture II was a calibration gas mixture (Table 1) prepared at OOO PGS-service (Zarechny, Sverdlovsk region). The butene–butane fraction (BBF) was 1.3-butadiene, iso-butene, 1-butene, iso-butane, trance-2-butene, cis-2-butene, and n-butane. Mixture III was a mixture of light hydrocarbons C1–C4, carbon dioxide, carbonyl sulfide, sulfur dioxide, and carbon disulfide; a test gas mixture; and a butene-butane fraction. The methane content in this mixture was >90%. The composition of model mixture III was close to the qualitative composition of natural gas, with the exception of sulfur dioxide. Natural gas does not contain sulfur dioxide.
Chromatographic characteristics (peak area S, μV × min, and retention time t, min) were studied in the separation of carbon dioxide, sulfur-containing gases, and mercaptans.
Calculations of the convergence index under conditions of repeatability relative to the measured peak areas of the analyzed mixture components were performed according to RMG 61–2010 [17].
Obtaining Chromatograms
To separate test mixtures I and II on columns I and II, we used an Agilent 7890 chromatograph equipped with a thermal conductivity detector (μ-TCD). The temperature of the injector was 200°С. That of the detector was 180°C, and the carrier gas was helium. To determine the values of the peak areas of mercaptans, we used a Kristall 2000 chromatograph equipped with a flame ionization detector (FID) (Kupol, Izhevsk). The injector temperature was 250°С. The detector temperature was 230°C, and the carrier gas is nitrogen. Chromatography of the mixtures was done in the isothermal mode and under conditions of temperature programming. The temperature of the column oven was maintained with an accuracy of ±0.5°C. The ChemStation (Agilent) and NetChrom (Metachromium) software were used to process the chromatographic data.
RESULTS AND DISCUSSION
A metrological assessment of the repeatability index for retention times and peak areas of the carbon dioxide and sulfur compounds was performed using model mixtures I, II, and III on column I with PTMSP and on column II with polydivinylbenzene sorbent. Model mixtures were taken with gas syringes having capacities of 100 and 1000 μL and injected into the injector of the chromatograph.
A chromatographic analysis of model mixture I was performed on columns I and II under isothermal conditions using an Agilent 7890 chromatograph (μ-TCD). A chromatographic analysis of calibration mixture II was performed on columns I and II under isothermal conditions using a Crystal 2000 chromatograph (FID).
The repeatability of the retention times was measured over five successive analyzes of mixtures I and II. The repeatability of the retention times of carbon dioxide and sulfur compounds are given in Tables 2 and 3 along with their relative standard deviations (RMSD).
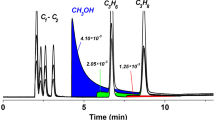
Figure 1 shows the repeatability of retention times for mixture I (the overlapping of 5 chromatograms) on a PTMSP column.
ASTM D5623 determine that chromatographic parameters should ensure repeatability (convergence) of retention times of 0.05 min (3 s) within the period of analysis [7].
Analysis of the data presented in Tables 1 and 3 and in Fig. 1 confirms the accuracy of our experiments and the stable operation of the capillary column with PTMSP, since the difference between the convergence of the retention times is 2–3 s, and this meets the requirements of ASTM D5623 (a capillary column with polysiloxane stationary liquid phase).
The repeatability of the concentration measurements was estimated from the results of the areas of chromatographic peaks (S, μV min) of the components of mixtures I and II on columns I and II. Satisfactory repeatability values were obtained (Tables 4 and 5).
We also calculated the limits of detection for sulfur compounds analyzed on a column with poly(1-trimethylsilyl-1-propyne) using μ-TCD (see Table 1).
The limits of detection (Cmin) for sulfur compounds were calculated with the formula
where
—σ is the background signal of the detector, μV;
—G is the content of the component, mg/mL;
—S is the area of the component’s peak, μV × min;
—V is the volume of injected sample, mL;
—Vgn is the velocity of the carrier gas, mL/min.
These results are in good agreement with the data published in [6]. For example, when analyzed on a Pora PLOT U capillary column in combination with a micro-TCD, the limit of detection for hydrogen sulfide in natural gas was 0.50 × 10−3 mg/mL.
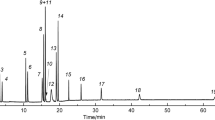
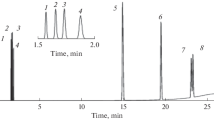
Figures 2 and 3 show chromatograms of a model mixture of carbon dioxide, light hydrocarbons, sulfur-containing gases, the butene–butane fraction, and mercaptans, analyzed on two types of columns. Chromatography of the components of the model mixture was done under conditions of temperature programming with an initial temperature of 35°C, holding for 3 min, and then heating to 180°C at a rate of 7°C/min. At a temperature of 180°C, they were held until there was complete elution of all components. The carrier gas was helium; P = 0.6 bar.
Chromatogram of a model mixture of carbon dioxide, light hydrocarbons, sulfur-containing gases, the butene–butane fraction, and mercaptans on a column with PTMSP: (1) methane, (2) carbon dioxide, (3) acetylene, (4) ethylene, (5) hydrogen sulfide, (6) ethane, (7) sulfur dioxide, (8) COS, (9) propylene, (10) n-propane, (11) methyl mercaptan, (12) 1.3-butadiene, (13) isobutene + 1-butene, (14) isobutane, (15) trans-2-butene, (16) cis-2-butene, (17) n-butane, (18) ethyl mercaptan, (19) CS2, (20) isopropyl mercaptan, (21) propyl mercaptan.
Chromatogram of a mixture of carbon dioxide, light hydrocarbons, sulfur-containing gases, the butene–butane fraction, and mercaptans on a commercial Rt–Q–BOND column: (1) methane, (2) carbon dioxide, (3) acetylene, (4) ethylene, (5) ethane, (6) hydrogen sulfide, (7) water, (8) carbonyl sulfide, (9) propylene, (10) n-propane, (11) sulfur dioxide, (12) methyl mercaptan, (13) isobutane, (14) 1,3-butadiene, (15) isobutene, (16) 1-butene, (17) n-butane + cis-2-butene, (18) trans-2-butene, (19) ethyl mercaptan, (20) CS2, (21) isopropyl mercaptan, (22) propyl mercaptan.
Carbonaceous and sulfur compounds were separated satisfactorily in both columns. The components of the butene–butane fraction eluted from the surface of the polydivinylbenzene sorbent in broad peaks with extended tails; n-butane and cis-2-butene were not separated, and the resolution (Rs) for the two peaks of 1.3-butadiene/isobutene was considerably less than 1. On the column with PTMSP, isobutene and 1-butene appear as one peak, while the resolution for the other components of the butene–butane fraction was close to or higher than unity. Symmetric and narrow peaks of the separated components were in this case observed.
CONCLUSIONS
We compared the indices of repeatability for the main chromatographic characteristics measured in analyzing a model mixture of carbon- and sulfur-containing compounds on capillary columns with nonpolar stationary phases poly(1-trimethylsilyl-1-propyne) (PTMSP) and commercial ones with polydivinylbenzene sorbent. It was found that the standard deviation did not exceed 0.10% for retention times, and was less than 1.92% for the measured peak areas. The minimum signals of the response of sulfur compounds, determined on the column with PTMSP, were 0.40 × 10−3 mg/mL for methyl mercaptan and 0.82 × 10−3 mg/mL for butyl mercaptan. A differency between the values of the peak areas of sulfur dioxide and carbon disulfide was observed on an Rt–Q–BOND capillary column (OCCO was 4.22 and 2.52%, respectively), due possibly to the sorption of these components on a divinylbenzene sorbent.
The prospects of using a capillary column 30 m long, 0.53 mm in diameter, and a sorbent film thickness of 2.82 μm PTMSP for the analysis of natural gas, refined products, and products of the catalytic dehydrogenation of alkanes and alkenes due to the high selectivity of separation of light hydrocarbons (including the butene–butane fraction) and sulfur compounds were demonstrated for the first time.
The time needed for analyzing a mixture of carbon- and sulfur-containing compounds on a column with a 2.82 µm PTMSP layer was approximately 1.4 times shorter than on a commercial column with a film thickness of 10.0 µm divinylbenzene sorbent.
REFERENCES
Yakovleva, E.Yu., Patrushev, Yu.V., and Pai, Z.P., Russ. J. Phys. Chem. A, 2018, vol. 92, no. 5, pp. 1018–1024.
Arystanbekova, S.A., Lapina, M.S., Volynskii, A.B., Ustyugov, V.S., and Almametov, A.I., J. Anal. Chem., 2012, vol. 67, no. 7, pp. 669–676.
GOST R (Russian State Standard) 53367-2009: Combustible Natural Gas. Determination of Sulfur-Containing Components Using Chromatographic Method, 2010.
GOST R (Russian State Standard) 56718-2015: Distillates and Stable Gas Condensate. Determination of Sulfur Compounds by Gas Chromatography Method, 2016.
ISO (ISO Standard) 19729:2004: Natural Gas—Determination of Sulfur Compounds Using Gas Chromatography, 2004.
Frito, R. and Quimby, B., A Comparison of Sulfur Selective Detectors for Low Level Analysis in Gaseous Streams-Application, Agilent Technologies 5988-2426EN, 2001.
PAC methodical notes. http://paclp.tech/lab_instruments/sense/AN_-_SeNse_ASTM_D5623_RU.pdf. Cited June 17, 2019.
Belotserkovskaya, V.Y. and Yakovleva, E.Y., Russ. J. Phys. Chem. A, 2011, vol. 85, no. 5, p. 851–856.
Patrushev, Y.V., Yakovleva, E.Y., Shundrina, I.K., Ivanov, D.P., and Glazneva, T.S., J. Chromatogr. A, 2015, vol. 1406, pp. 291–298.
Yakovleva, E.Yu., Patrushev, Yu.V., and Pai, Z.P., Katal. Prom-sti, 2017, no. 6, pp. 460–464.
Yakovleva, E.Yu., Yakushina, I.A., and Skrypnik, O.V., Katal. Prom-sti, 2006, no. 6, pp. 28–32.
Yakovleva, E.Yu., ShanShan, Y., and, Pai, Z.P, Catal. Ind., 2019, vol. 11, no. 4, pp. 295–300.
Sakodynskii, K.I. and Panina, L.I., Polimernye sorbenty dlya molekulyarnoi khromatografii (Polymeric Sorbents for Molecular Chromatography), Moscow: Nauka, 1977.
Berezkin, V.G., Popova TP., Shiryaeva V.E., Kozlov S.P., Vlasenko E.V., Zavod. Lab., Diagn. Mater., 2003, vol. 69, no. 10, pp. 3–7.
Berezkin, V.G., Popova, T.P., and Shiryaeva, V.E., Russ. Chem. Bull., 2001, vol. 50, no. 2, pp. 233–236.
Belotserkovskaya, V.Y. and Yakovleva, E.Y., J. Chromatogr. A, 2013, vol. 1298, pp. 109–117.
RMG (Interstate Standardization Recommendations) 61-2010: State System for Ensuring the Uniformity of Measurements. Accuracy, Trueness, and Precision Measures of the Procedures for Quantitative Chemical Analysis. Methods of Evaluation, 2012.
Funding
The work was performed as part of a State Task for the Boreskov Institute of Catalysis, project no. АААА-А17-117041710081-1.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yakovleva, E.Y., Patrushev, Y.V. Analysis of Light Hydrocarbons and Sulfur Compounds on Porous Layer Capillary Columns with a Nonpolar Phase. Catal. Ind. 12, 280–286 (2020). https://doi.org/10.1134/S2070050420040108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070050420040108