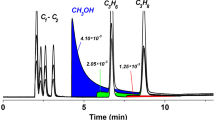
Abstract
A comparative assessment is performed of the separation capability of three types of PLOT capillary columns: PTMSP032 (poly(1-trimethylsilyl-1-propyne)), Rt-Q-BOND (polydivinylbenzene), GS-GasPro (silica), and it is studied of the possibility of their use for the determination of impurities in commercial (target, technical) n-butane. Satisfactory separation of alkanes, alkenes, arenes and methanol was achieved on PTMSP032 and Rt-Q-BOND columns. The effect of the main component amount of the mixture on the separation of trace amounts of hydrocarbons and methanol is studied. It is shown that the macrozone (> 99%) of n-butane is selectively separated from the accompanying impurities. With an increase in the injected sample volume, the resolution for the peaks of C1–C2 hydrocarbons and the structural isomers of o-, m-, and p-xylenes on the PTMSP032 column is significantly higher than on the Rt-Q-BOND column. A comparative assessment of the precision of the measurements of the hydrocarbons and methanol retention times on PTMSP032 and Rt-Q-BOND is characterized by a relative standard deviation (RSD) of not more than 0.3%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural gases and associated petroleum gases are valuable minerals and mainly consist of alkanes, a small amount of cycloalkanes and aromatic hydrocarbons, nitrogen and argon, as well as traces of helium and hydrogen. In addition, the gases sometimes contain hydrogen sulfide, mercaptans and carbon dioxide. The composition of gases released from oil during its production differs significantly from the composition of free gases extracted from gas-bearing reservoirs. Gases are classified as natural gases consisting mainly of 90–98% methane (dry gases), and associated and gas condensate, which usually contain 50–100 g·m−3 of hydrocarbons from C3 and above [1].
n-Butane is found in sufficient quantities in natural gas, shale and associated petroleum gas (APG) and it is not produced artificially. n-Butane is released during the purification and separation of natural gas and APG, during the distillation of oil and during the cracking of petroleum products. n-Butane mixed with propane is widely used as fuel for household stoves, transport means and heating appliances due to its high flammability. n-Butane is today used as a refrigerant in refrigeration systems (as a safer gas for the environment than freons). Gasoline with a high octane number (a measure of detonation resistance that is the ability of a substance to resist self-ignition during compression) also contains n-butane. n-Butane is used as a raw material in the chemical and petrochemical industries to produce butylene which is used as a fuel (mixed with other gases) for the synthesis of butyl alcohols, methyl ethyl ketone, 1,3-butadiene using for the production of synthetic rubber and other chemicals, in metal cutting and gas-oxygen welding. It is also used in the food industry as a food additive E943a—a solvent of edible oils and flavorings [2].
It should be noted that the determination of the quality of the raw material (target, technical or commercial product) is one of the most important conditions for its further effective use. For example, the admixture of acetylene hydrocarbons in n-butane has a negative effect on the production of 1,3-butadiene. Small amounts of i-butane increase the yield of low-value products such as acetone and methyl acetate and an admixture of butenes and propene reduces the yield of acetic acid due to the formation of olefinglicol diacetates [3].
Methanol is added to gas condensates and oil in the fields (for example, the Urengoyskoye field) to inhibit hydrate formation and which is present in the technological flows in the process of gas and oil refining together with hydrocarbons. If the content of methanol in the streams of light hydrocarbons, for example, in the propane fraction, exceeds the permissible concentration of 0.005% [4,5,6], as a result the production efficiency of this product is significantly reduced and the sales market is decreasing [7]. Aromatic hydrocarbons (benzene, toluene, xylenes, and other arenes have pronounced carcinogenic activity) and acyclic hydrocarbons C4 and higher are also part of associated gases, gas condensates and some types of oil, and their trace amounts may be present when the target product is obtained.
The main method for analyzing the raw materials used, including commercial (target and technical) n-butane, trace amounts of saturated, unsaturated, aromatic hydrocarbons and methanol is the gas chromatography method. The methods for determining the hydrocarbon composition of liquefied hydrocarbon gases, impurities in the air of the working area and other objects use packing columns prepared on the basis of porous polymers, silica, aluminum oxide or modified by different stationary phases of the diatomite carrier Chromosorb P (Chromosorb P NAW), as well as capillary PLOT columns Al2O3/KCl, Al2O3/S with aluminum oxide, HP-PLOT Q, PoraPLOT U with porous organic polymers [8, 9], GS-GasPro with a porous silica layer [10, 11].
The hydrocarbons C1-C2 and 1-butene with i-butane are eluted as single peak when analyzing commercial n-butane on packed columns with di-n-butyl maleate and on columns with vaseline oil or with a mixed stationary phase of di-n-butyl maleate + β,β-oxydipropionitrile, ethane and ethylene are not separated [8]. The use of a capillary chromatographic column with a porous layer of Al2O3/KCl makes it possible to selectively determine the impurities of methane, saturated and unsaturated hydrocarbons C1–C6 in technical butane [8] with the exception of methanol which is strongly sorbed with tailing peak on the surface of aluminum oxide.
A method has been developed that requires additional sample preparation of gas samples for the determination of methanol in liquefied petroleum gases (LPG), in gas condensate and in a wide fraction of light hydrocarbons. Sample preparation included the concentration of methanol by extraction with water from hydrocarbon gases. The aqueous extract was injected into the injector of chromatograph and analyzed under temperature programming conditions [12].
Another method of determining the composition of liquefied hydrocarbon gases (LPG) without preliminary sample preparation (concentration) consists in introducing a sample into the column using a 6-port sampling valve from the pipeline, sampler or cylinder. The total hydrocarbon composition of LPG including saturated and unsaturated hydrocarbons with one or two double bonds is analyzed on the capillary column PLOT Al2O3/S. The content of methanol and the component composition of LPG that does not contain unsaturated hydrocarbons or in cases where the content of unsaturated hydrocarbons in the LPG is not normalized is analyzed on a capillary chromatographic column with an organic porous polymer HP-PLOT Q [9].
In interstate standards the determination of the hydrocarbons composition is carried out on two [13] or three packed columns [14] as well as on two capillary columns: one of which is PoraPLOT U with the divinylbenzene/ethyleneglycol dimethacrylate sorbent and the second—with a non-polar methylpolysiloxane stationary liquid phase [15]. It should be noted that none of the above columns can achieve complete separation of the structural isomers of o-, m-, and p-xylenes.
In 2002, it was first proposed to use a glassy unsaturated polyacetylene poly (1-trimethylsilyl-1-propyne) (PTMSP) as a chromatographic sorbent in capillary gas chromatography [16] and a stationary phase for the preparation of packing (as composition of Chromosorb W + 10 wt. % PTMSP) [17] and multicapillary [18] columns. It turned out that PTMSP is characterized by unusual properties due to the structure of the polymer itself, namely, the bimodal distribution of pores with sizes of 4.5 and 10–13 Å, a large fraction of internal free volume (more than 20%), and relatively high solubility in organic solvents (for example, in toluene, chloroform) [19,20,21,22,23]. Further studies of PTMSP as a chromatographic stationary phase have shown that this material is used to solve such analytical tasks as the determination of hydrides [24], thiophene in the target benzene [25], structural isomers [26], as well as products of catalytic reactions [27,28,29,30]. The prospects of using capillary columns with a diameter of 0.32 and 0.53 mm with different film thicknesses of PTMSP for the analysis of natural gas and petroleum products were also demonstrated. The high separation selectivity of saturated and unsaturated hydrocarbons, sulfur compounds, as well as the determination of their micro-impurities in the methane macrozone was shown [31].
The aim of this work is to determine the residual amounts of aromatic hydrocarbons, saturated and unsaturated aliphatic hydrocarbons C1–C10 and methanol in n-butane on a PLOT column with a stationary phase poly(1-trimethylsilyl-1-propyne).
Experimental
Materials
Poly(1-trimethylsilyl-1-propyne) synthesized at the Institute of Catalysis SB RAS according to the method described in [32, 33] was used in this work.
Column Preparation
A capillary column with a diameter of 0.32 mm was prepared by a static high-pressure method. The fused-silica capillary (Fiberguide Industries Inc., Stirling, NJ, USA) was filled with a 2.1% solution of PTMSP in toluene. After that one end of the capillary was sealed and the open end was introduced at a constant speed into the air oven at a temperature of 200 °C [31, 34]. The prepared capillary column had a length of 30 m, a diameter of 0.32 mm and a polymer film thickness of 1.55 μm (PTMSP032).
Commercial capillary columns were used as comparison columns: Rt-Q-BOND (Restek, USA) with a size of 30 m × 0.32 mm × 10 μm with a polydivinylbenzene sorbent and GS-GasPro (Agilent, USA) with a size of 30 m × 0.32 mm with a silica as stationary phase. The choice of comparison columns is determined by the following: the Rt-Q-BOND column is made on the basis of a polydivinylbenzene sorbent, which is a non-polar stationary phase and exhibits similar chromatographic properties as PTMSP [27,28,29,30,31, 35]; the GS-GasPro column is used to determine the hydrocarbon composition in gas and oil refining products [10, 11].
Model Mixtures
Mixture I—calibration gas mixture of aliphatic hydrocarbons C1–C10 prepared in a cylinder with a volume of 5 L in Limited liability company (LLC) "PGS-service" (Zarechny, Sverdlovsk region, Russia). The composition of the mixture is shown in Table 1;
Mixture II—n-butane (> 99%, LLC “Chistye Gazy”, Novosibirsk, Russia) + mixture I + methanol, benzene, toluene, o-, m-, p-xylenes prepared in a gas syringe with a volume of 500 ml (Hamilton, USA).
Obtaining Chromatograms
The precision of the retention times measurements of the components of mixtures I and II and the study of the separation capability of three types of columns (PTMSP032, Rt-Q-BOND and GS-GasPro) were performed under the conditions of temperature programming on a Crystal 2000 chromatograph with a flame ionization detector (FID) (Kupol production, Izhevsk, Russia). The temperature of the injector is 250 °C, the detector is 230 °C, the carrier gas is nitrogen. The temperature of the column oven was maintained with an accuracy of ± 0.5 °C. The NetChrom (Meta-chrome, Yoshkar-Ola, Russia) software was used to process the chromatographic data.
Calculations of the precision index under repeatability conditions for the retention times of the components of the mixtures were carried out in accordance with the Recommendation on Interstate Standardization (RMG) 61–2010 [36].
Results and Discussion
The Separation of Aliphatic Hydrocarbons C1–C10 on Capillary Columns PTMSP032, Rt-Q-BOND, GS-GasPro
Today the capillary columns with sorbents based on alumina, silica, and polydivinylbenzene sorbents are most often used to analyze the component composition of natural and associated petroleum gas, gas condensate, and oil [9, 12]. The selective separation of components was observed in the analysis of natural gas on capillary columns with PTMSP in previously published works [31]. The chromatographic conditions were previously selected to determine and separate selectively of the accompanying impurities in n-butane which is a part of natural, associated gases and other products of gas and oil refining. The separation capability of the Rt-Q-BOND column, GS-GasPro column and PTMSP032 column was compared using a calibration gas mixture I which was injected in a volume of 1 mL (Figs. 1, 2 and 3).
Chromatogram of the components of Mixture I on the Rt-Q-BOND column. Temperature programming: 35 °C—3 min, then heating at a speed of 7 °C / min to 180 °C. At a temperature of 180 °C, they were kept until all the components were completely eluted. The carrier gas is nitrogen, split ratio is 1:20. 1—methane, 2—acetylene, 3—ethylene, 4—ethane, 5—propylene, 6—propane, 7—i-butane, 8—1-butene, 9—trans-2-butene, 10—cis-2-butene, 11—n-butane, 12—neo-pentane, 13—i-pentane, 14—n—pentane, 15—n-hexane, 16—n-heptane, 17—n-octane, 18– n-nonane, 19—n-decane
It can be seen from the chromatogram shown in Fig. 1 that n-butane and trans-2-butene are eluted as a single peak on the Rt-Q-BOND column. Two pairs of compounds, 1-butene-/neo-pentane and cis-2-butene/i-pentane, are also not separated on the GS-GasPro column under these chromatography conditions (Fig. 2). Complete separation of the components of the mixture I including the structural isomers of n-butane and n-pentane was achieved on the PTMSP032 column (Fig. 3).
Chromatogram of the components of Mixture I on the GS-GasPro column. Temperature programming: 40 °C—11 min, then heating at a speed of 7 °C / min to 220 °C. At a temperature of 220 °C, they were kept until all the components were completely eluted. The carrier gas is nitrogen, split ratio is 1:20. Peak designations-see Fig. 1
Chromatogram of the components of Mixture I on the PTMSP032 column. Temperature programming: 40 °C—11 min, then heating at a speed of 7 °C / min to 85 °C, kept for 0.5 min, then heating at a speed of 10 °C / min to 220 °C. At a temperature of 220 °C, they were kept until all the components were completely eluted. The carrier gas is nitrogen, split ratio is 1:20. Peak designations-see Fig. 1
Aromatic hydrocarbons such as benzene, toluene, o-, m-, p-xylenes and methanol along with aliphatic hydrocarbons may also be present as co-impurities in n-butane. The separation of the structural isomers of xylenes is not achieved on any of the known sorbents [13,14,15]. It is known that a mixture of LPG and methanol is analyzed on two columns: LPG—on the PLOT Al2O3 column, the symmetric methanol peak—on HP-PLOT Q [9]. The peak of methanol is not possible to register on the GS-GasPro column because it is sorbed irreversibly on silica [37]. Further studies of the chromatographic behavior of the components of mixture I and mixture II including aromatic hydrocarbons and methanol will be carried out on the PTMSP032 and Rt-Q-BOND columns.
The precision of measurements is indicated by a relative standard deviation (RSD, %) [36]. Therefore the gas mixture I and mixture II were repeatedly and sequentially introduced into the PTMSP032 and Rt-Q-BOND columns. The standard deviation under repeatability conditions for the retention times of the components of mixture I and mixture II on the PTMSP032 column and on the Rt-Q-BOND column is not more than 0.3%. The satisfactory stability of the retention parameters on the two types of porous-layer columns indicates the accuracy of the experiments and their suitability for quantitative analysis (Table 2).
Determination of Hydrocarbon and Methanol Impurities in Target n-Butane
Mixture II that contains n-butane, mixture I, aromatic hydrocarbons and methanol was introduced into the injector of chromatograph in a volume of 1 ml and analyzed on Rt-Q-BOND and PTMSP032 columns (see Figs. 4, 5 and 6 and Table 2).
Chromatogram of the components of Mixture II on the Rt-Q-BOND column. Temperature programming: 35 °C—3 min, then heating at a speed of 7 °C/min to 180 °C. At a temperature of 180 °C, they were kept until all the components were completely eluted. The carrier gas is nitrogen, split ratio is 1:20. 1—methane, 2—acetylene, 3—ethylene, 4—ethane, 5—propylene, 6—propane, 7—methanol, 8—i-butane, 9—n-butane + 1-butene + trans-2-butene + cis-2-butene, 10—neo-pentane, 11—i-pentane, 12—n-pentane, 13—n-hexane, 14—benzene, 15—n-heptane, 16—toluene, 17– n-octane, 18—p—xylene, 19—m-,o—xylenes, 20—n-nonane, 21—n-decane
The asymmetric peak of methanol elutes to the baseline on the PTMSP032 column. The well-resolved peaks of propylene and n-propane are found on the tail of methanol peak. The shape of these peaks is close to Gaussian. It should be noted that a large amount of methanol in the sample of mixture II does not interfere with the determination of propylene and n-propane (see frame in Fig. 5). 1-Butene, i-butane, trans-2-butene and cis-2-butene as well as neo-pentane, i-pentane and n-pentane are well separated from the n-butane macrozone (mixture II) on the PTMSP032 column. This indicates a high selectivity of this column. In contrast, on the Rt-Q-BOND column the structural isomers (1-butene and trans-2-butene) are eluted simultaneously with n-butane (Fig. 4, Table 2).
Chromatogram of the components of Mixture II on the PTMSP032 column. Programming temperature: 40 °C − 11 min, then heating at a speed of 7 °C / min to 85 °C, kept for 0.5 min, then heating at a speed of 10 °C/ min 220 °C. At a temperature of 220 °C, they were kept until all the components were completely eluted. The carrier gas is nitrogen, split ratio is 1:20. 1—methane, 2—acetylene, 3—ethylene, 4—ethane, 5—methanol, 6—propylene, 7—propane, 8—1-butane, 9—i—butane, 10—trans-2-butene, 11—cis-2-butene, 12—n-butane, 13—neo-pentane, 14—i—pentane, 15—n—pentane, 16—n—hexane, 17- benzene, 18—n-heptane, 19—toluene, 20– p-xylene, 21—m—xylene, 22—o—xylene, 23—n—octane, 24—n—nonane, 25—n—decane
In addition, the peak resolution Rs for p-, m-xylenes and m-, o-xylenes pairs is close to or greater than 1 on the PTMSP032 column (Fig. 5), whereas m- and o-xylenes are eluted as a single peak on a column with a polydivinylbenzene sorbent (Fig. 4).
Column overload
Column overload was studied by measuring the efficiency at different amounts of the introduced sample. The measurements were performed for the PTMSP032 and Rt-Q-BOND columns using ethylbenzene as standard (99%, Sigma-Aldrich) and which was dissolved in n-hexane (99.9%, Sigma-Aldrich).
The flame ionization detector was calibrated prior to the measurements. Dinitrogen was used as the carrier gas. Solutions of ethylbenzene in n-hexane of different concentrations were used. The solvent was selected so that the peaks of the solvent and the standard did not overlap. A more detailed method for determining the loading capacity of columns is described in previously published works [31, 38].
The loading capacity depends on the specific surface area of the sorbent in porous capillary columns. In gas–liquid chromatography the analyte is sorbed by the entire volume of the stationary phase. In gas-adsorption chromatography for typical organic and inorganic adsorbents the sorption–desorption processes occur at the interface of the phases. However, PTMSP is not a classical adsorbent from this point of view and apparently it is able to absorb analyte molecules by the entire volume due to the high fraction of free internal volume. The share of free volume in PTMSP is so large (over 20%) that microvoids form a continuous network of channels. Microvoids are distributed bimodally: submicropores 4–5 Å and micropores 10–15 Å. The reason for the presence of a large free volume is the PTMSP structure; rigid double bonds in the polymer backbone and bulky trimethylsilyl groups reduce the chain mobility and prevent the formation of effective packing [16, 33,34,35].
Table 3 shows that the volume of the sorbent inside the Rt-Q-BOND column is approximately 6.5 times larger than in the PTMSP032 column with a layer thickness of 1.55 μm. Despite this, the loading capacity of the PTMSP032 and Rt-Q-BOND columns is approximately the same (Table 3). This may be due to the fact that the porous structure of PTMSP formed as a result of the three-dimensional conformation of a linear polymer is permeable to sorbate molecules to a great depth. This makes it possible to use this polymer for the manufacture of membranes [21]. Therefore, the load properties of the PTMSP should not be similar to a traditional sorbent but to a stationary liquid phase and absorb analytes in the entire volume of the sorbent layer.
The efficiency of the PTMSP032 and Rt-Q-BOND columns in the separation of aromatic and aliphatic hydrocarbons C3–C10 and methanol did not change with an increase in the volume of the introduced gas mixture II. A distortion of the peak shape was observed on both columns for C1–C2 hydrocarbons.
The Rs value calculated for the component pairs is greater than 1 when the gas mixture II sample was chromatographed in volumes of 1 mL and 2 mL on the PTMSP032 column. The peak resolution decreased slightly only for the methane/acetylene pair and was 0.89 when introducing 4 mL of mixture II (Table 4). However, the shape of the peaks is distorted (Fig. 6a).
The Rs value for the component pairs of acetylene/ethylene decreased significantly from 0.75 to 0.33, when the 1 mL and 2 mL samples respectively, were introduced on the Rt-Q-BOND column. The peaks of acetylene and ethylene are practically not separated and it is not possible to determine the Rs value with an increase in the volume of sample to 4 mL (Fig. 6b).
At the end, an example of separation of impurities in a real sample on PTMSP032 column is presented. Figure 7 shows a separation of industrial liquefied butane fraction produced by «Gazpromneft- Omsk Oil Refinery». The fraction contains 98.82% n-butane, 0.28% i- butane, 0.01% 1-butene, 0.04% trans-2-butene, 0.02% cis-2-butene, 0.83% neo-pentane and 0.02% n-pentane.
Separation of impurities in industrial liquefied butane fraction on the PTMSP032 column. Temperature programming: 40 °C—11 min, then 7 °C / min to 85 °C, kept for 0.5 min, then 10 °C / min to 220 °C. The carrier gas is nitrogen, split ratio is 1:20. 1—1-butene, 2—i-butane, 3—trans-2-butene, 4—cis-2-butene, 5—n-butane, 6—neo-pentane, 7—n-pentane
Conclusion
For the first time the prospects of using a capillary column with a length of 30 m, a diameter of 0.32 mm and a PTMSP film thickness of 1.55 μm for the analysis of accompanying hydrocarbons and methanol in n-butane with a content of more than 99% were demonstrated. The high selectivity of the separation of aromatic and aliphatic hydrocarbons C1–C10 including the structural isomers of the main component as well as p-, m-, o-xylenes and methanol is shown.
It is shown that at the high sample volume, the resolution of pairs of peaks of hydrocarbons C1–C2 on the column PTMSP032 with a poly (1-trimethylsilyl-1-propine) film is higher than on the column Rt-Q-BOND with a porous layer of polydivinylbenzene at close values of the loading capacity of both columns.
The pore-layer column PTMSP032 allows the introduction of a large sample volume to determine the residual amounts of hydrocarbons and methanol in the target (commercial, technical) n-butane without the stage of concentration, stack injection and backflush of the same sample.
References
Drugov YuS, Rodin AA (2013) Gas chromatographic analysis of natural gas. Binom, Moscow
Molchanov SA (2013) Complex preparation and processing of multicomponent natural gases at gas-chemical complexes. Nedra, Moscow
Chemist's Handbook 21. Chemistry and chemical technology. https://www.chem21.info/info/1159614.
Russian national standard GOST 20448–90 Liquefied hydrocarbon fuel gases for domestic use. Specifications.
Russian national standard GOST 21443–75 Liquefied hydrocarbon gases for export. Specifications.
Russian national standard GOST P 21443–75 Exported liquefied hydrocarbon gases from Russian region. Specifications.
Babichevskaya AM (2010) Technology of purififcation of light hydrocarbon raw materials from methanol on the example of the Surgut condensate stabilization plant Petrochemistry, Abstract of the dissertation for the degree of Candidate of Technical Sciences, Kazan
ISO 7941: 1988 Interstate standart propane and butane commodity. Determination of the hydrocarbon composition by gas chromatography
Russian national standard GOST R 54484–2011 Liquefied petroleum gases. Methods for determining the hydrocarbon composition
Krylov VA, Chernova OIu, Sozin AIu, Kotkov AP, Pushkarev GV (2013) Chromato-mass-spectrometric determination of impurities in high-purity phosphine using capillary adsorption chromatographic columns. Analytics and control 17:452–458
Yuzhakova T, Kovács J, Rédey A, Scurtu R, Kovács Z, Somogyi V, Domokos E, Ráduly I, Ráduly L (2012) PtPd-CeO2/γ-Al2O3 Catalysts for VOC treatment of exhaust gases. Environ Eng Manag J 11:1963–1968. https://doi.org/10.30638/eemj.2012.245
Russian national standard GOST R 55997–2014 Stable gas condensate, broad fraction of light hydrocarbons, liquefied petroleum gases. Determination of methanol by gas chromatography
ISO 6974–4:2000 Natural gas—Determination of composition with defined uncertainty by gas chromatography—Part 4: Determination of nitrogen, carbon dioxide and Ci to C5 and C6+ hydrocarbons for a laboratory and on-line measuring system using two columns
ISO 6974–5:2000 Natural gas. Determination of composition with defined uncertainty by gas chromatography method. Part 5. Determination of nitrogen, carbon dioxide and C1 to C5 and C6+ hydrocarbons for a laboratory and on-line process application using three columns
ISO 6974–6:2000 Natural gas - Determination of composition with defined uncertainty by gaschromatography method. Part 6. Determination of hydrogen, helium, oxygen, nitrogen, carbon dioxide and C1–C8 hydrocarbons using three capillary columns
Berezkin VG, Popova TP, Shiryaeva VE, Kozlov SP, Vlasenko EV (2004) On some features of the separation of inorganic and organic gases on a new polymer adsorbent polytrimethylsilylpropine. Diagnostika materialov 69:3–7 (in Russian)
Berezkin VG, Korolev AA, Malyukova IV, Popova TP et al (2002) Poly[1-(trimethylsilyl)-1-propine] as chromatographic adsorbent and prospects of its application in packed and capillary columns. J Chromatogr A 960:151–158. https://doi.org/10.1016/S0021-9673(02)00333-3
Berezkin VG, Khotimskiǐ VS, Sidel'nikov VN, Patrushev YuV (2004)
A gas-adsorption multicapillar column and its application to the separation of light hydrocarbons. Russion Journal of Physical Chemistry A 78:432–435
Vasilyev GB, Mironova MV, Litvinova E, Volkov VV et al (2013) Rheological properties of poly(1-trimethylsilyl-1-propyne) solutions. Polym Sci, Ser A 5:510–517. https://doi.org/10.1134/S0965545X13070067
Khotimsky VS, Tchirkova MV, Litvinova EG, Rebrov AI et al (2003) Poly[1-(trimethylgermyl)-1-propyne] and poly[1-(trimethylsilyl)-1-propyne] with various geometries: Their synthesis and properties. Journal of Polymer Science. Part A Polymer Chemistr 41:2133–2155. https://doi.org/10.1002/pola.10757
Sato S, Suzuki M, Kanehashi S, Nagai N (2010) Permeability, diffusivity, and solubility of benzene vapor and water vapor in high free volume silicon- or fluorine-containing polymer membranes. J Membr Sci 360:352–362. https://doi.org/10.1016/j.memsci.2010.05.029
Starannikova LE, Teplyakov VV (1997) Gas permeability of poly[1-(trimethylsilyl)-1-propyne]: Evaluation of experimental data and calculation methods. Polymer Science Serires A39:1142–2114
Baschetti M, Ghisellini M, Quinzi M, Doghieri F et al (2005) Effects on sorption and diffusion in PTMSP and TMSP/TMSE copolymers of free volume changes due to polymer ageing. J Mol Struct 739:75–86 https://doi.org/10.1016/j.molstruc.2004.08.027
Krylov VA, Berezkin VG, Korolev AA, Chernova OYu, Salganskii YuM (2003) Gas-adsorption chromatography of reactive compounds on open-tubular columns with poly(trimethylsilylpropyne). J Anal Chem 58:372–374
Berezkin VG, Muhina VP, Korolev AA, Faktullina AF, Seroshtan VA (2004) Poly (1-trimethylsilyl-1-propine) - a new porous polymer sorbent for capillary gas chromatography of hydrocarbon raw materials. Zavodskaya laboratoriya Diagnostika materialov 5:9–12 (in Russian)
Belotserkovskaya VYu, Yakovleva EYu (2011) Chromatographic properties of poly-(1-trimethylsilyl-1-propyne). Russ J Phys Chem A 85:939–934
Yakovleva EYu, Belotserkovskaya VYu (2013) Separation of hydrocarbon and sulfur-containing gases on a new poly-(1-trimethylsilyl-1-propyne)/poly-(1-phenyl-1-propyne) mixed stationary phase in the presence of water. J Anal Chem 68:646–651. https://doi.org/10.1134/S1061934813070125
Yakovleva EYu, Patrushev YuV, Belotserkovskaya VYu (2015) Determination of the composition of the reaction products of the catalytic synthesis of pentafluoroethane by hydrofluorination of perchloroethylene in the mixed stationary phase of poly-(1-trimethylsilyl-1-propine/poly - (1-phenyl-1-propine) by gas chromatography. Kataliz v Promyshlennosti 15:15–19
Yakovleva EYu, Patrushev YuV (2020) Analysis of Light Hydrocarbons and Sulfur Compounds on Porous Layer Capillary Columns with a Nonpolar Phase. Catal Ind 20(12):280–286. https://doi.org/10.1134/S2070050420040108
Yakovleva EYu, Patrushev YuV, Pai ZP (2017) Analysis of Isobutane Dehydrogenation Products by Gas Chromatographic Method. Kataliz. v Promyshlennosti 17: 460–464. https://doi.org/10.18412/1816-0387-2017-6-460-464
Yakovleva EYu, Patrushev YuV, Pai ZP (2018) Capillary Columns with a Sorbent Based on Functionalized Poly(1-Trimethylsilyl-1-Propyne) for the Elution Analysis of Natural Gas. Russ J Phys Chem A 92:1018–1024. https://doi.org/10.1134/S0036024418050357
Masuda T, Isobe T, Higashimura T, Takada K (1983) Poly[1-(trimethylsilyl)-1-propyne]: a new high polymer synthesized with transition-metal catalysts and characterized by extremely high gas permeability. Journal of the Americal Chemical Society 105:7473–7474
Masuda T, Isobe E, Hamano T (1986) Synthesis of poly[1-(trimethylsilyl)-1-propyne] with extremely high molecular weight by using tantalum pentachloride-triphenylbismuth (1:1) catalyst. Macromolecules 19:2448–2450. https://doi.org/10.1021/ma00163a020
Patrushev YuV, Yakovleva EYu, Shundrina IK, Ivanov DP et al (2015) The properties of capillary columns with sorbents based on poly-(1-trimethylsilyl-1-propyne) modified with nitrous oxide. J Chromatogr A 1406:291–298. https://doi.org/10.1016/j.chroma.2015.06.013
Yakovleva EYu, Patrushev YuV (2021) Effect of poly(1-trimethylsilyl-1-propine) film thickness on chromatographic characteristics of the LC capillary column. Russian Journal of Physical Chemistry 95: 1124–1130. https://doi.org/10.31857/S0044453721070281
RMG (Interstate Standardization Recommendations) 61–2010 (2012) State System for Ensuring the Uniformity of Measurements. Accuracy, Trueness, and Precision Measures of the Procedures for Quantitative Chemical Analysis. Methods of Evaluation
Grob RL, Barry EF (2004) Modern practice of gas chromatography. Wiley, New Jersey
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation within the governmental order for Boreskov Institute of Catalysis (projects AAAA-A21-121011390007-7, AAAA-A21-121011390053-4).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yakovleva, E.Y., Patrushev, Y.V. Determination of Hydrocarbons in n-Butane on Porous Layer Capillary Columns with Nonpolar Stationary Phase. Chromatographia 84, 1095–1104 (2021). https://doi.org/10.1007/s10337-021-04092-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-021-04092-1