Abstract
This study investigated the separation selectivity for C1–C10 aromatic and aliphatic hydrocarbons (both saturated and unsaturated) and methanol contained as contaminants in n-butane (>99% pure). It was shown that a 30 m × 0.32 mm porous-layer capillary column with a 1.55 μm poly(1-trimethylsilyl-1-propyne) film (PTMSP032) provides a significantly higher peak resolution for C1–C2 hydrocarbons and structural isomers (o-, m-, and p-) of xylene, than a commercial 30 m × 0.32 mm column with a 10 μm polydivinylbenzene layer (Rt-Q-BOND). A PTMSP032-based GC method was developed, which avoids the need for an extra sample-preparation step and assures the rapid and accurate identification of hydrocarbon and methanol impurities in an n-butane gas sample. The FID detection limit was evaluated to range from 3.21 × 10–12 to 6.68 × 10–12 g/s for hydrocarbons and to be 2.78×10–11 g/s for methanol. The repeatability, determined as the root-mean-square deviation (RMSD) of peak area, amounted to 4.20% for hydrocarbons, and varied from 4.96 to 0.29% for methanol in concentrations of 0.06×10–3 to 1.01×10–2 mg/mL, respectively.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The rapid advances in the chemical and petrochemical industries have stimulated a growing demand for crude oil, not only to enhance the output of fuels and oils, but also to obtain valuable feedstocks for the production of synthetic rubbers, fibers, plastics, surfactants, detergents, plasticizers, additives, dyes, etc. Among such feedstocks derived from crude oil, the following hydrocarbons have been used most widely: paraffins (methane, ethane, propane, butanes, pentanes, and hexanes); naphthenes (cyclohexane); aromatics (benzene, toluene, xylenes, and ethylbenzene); mono-olefins and diolefins (ethylene, propylene, and butadiene); and acetylene.
One of the most valuable hydrocarbons is n-butane. It is used for various purposes: (i) in the chemical and petrochemical industries, as a raw material to obtain butylene, as well as to synthesize butyl alcohols, methyl ethyl ketone, and butadiene-1,3 in the production of synthetic rubbers; (ii) as fuels (in a mixture with propane) for household heating stoves and vehicles; (iii) as a refrigerant gas (more environmentally friendly than freons) for refrigerators; and (iv) to prepare gasoline with a high octane number (a measure of detonation resistance, i.e. the fuel’s ability to resist self-ignition under pressure) [1, 2].
n-Butane is generally recovered during the treatment and separation of natural gas and associated petroleum gas (APG), as well as during oil refinery and the cracking of petroleum products. Both commercial and target n-butane products may contain (as impurities) C1–C10 aromatic and aliphatic (both saturated and unsaturated) hydrocarbons, as well as methanol––added as an inhibitor to prevent hydrates from being formed during the production and treatment of natural gas. Therefore, the proper quality control of n-butane is critical for its further efficient use. For example, the presence of isobutane as an impurity in n-butane increases the yields of low-value products such as acetone and methyl acetate, whereas the presence of butenes and propene lowers the yield of acetic acid [3].
The proper control of impurities, in particular aromatics and methanol, in n-butane is also essential to prevent atmospheric chemical pollution and adverse impacts on humans [4, 5].
Currently, various methodological approaches are employed to identify the composition of feedstocks and products in gas and oil refining. When developing relevant analytical methods, gas chromatography is used extensively. To identify the feedstock and product compositions, researchers utilize packed columns based on porous polymers, silica gel, alumina, or on a Chromosorb P/W-NAW diatomite support modified with various stationary phases. Alternatively, capillary porous-layer columns are used with a layer of alumina, porous organic polymers [7], or silica gel [8, 9].
In accordance with applicable regulations (Russian national and Commonwealth of Independent States interstate standards, GOST), two or three chromatography columns (packed or capillary) must be used to identify hydrocarbons and methanol in refinery feedstocks and products, including n-butane [6–13].
To measure methanol in liquefied petroleum gases (LPG), gas condensate, and natural gas liquid (NGL), the procedure may either include the preparation of a gas sample [10] or omit the need for this step [11]. The sample-preparation step, when utilized, involves concentrating methanol by water extraction from petroleum gases, followed by introducing the sample with a syringe into the GC injector [10]. To identify the LPG composition without preliminary sample preparation (i.e., without concentrating), the sample must be introduced into the column from a pipeline, sampling container, or cylinder using a sampling valve. The identification procedure must ensure that the total hydrocarbon composition of the LPG, including saturated and unsaturated hydrocarbons (the latter having one or two double bonds), is measured on PLOT Al2O3/S and HP PLOT Q capillary columns [7] or on a PoraPLOT U porous-layer column with a divinylbenzene/ethylene-glycol-dimethacrylate adsorbent and with a non-polar methyl-silicone stationary liquid phase [13]. It should be noted that none of the columns specified above can achieve the complete separation of xylene structural isomers, namely o-xylene, m-xylene, and p-xylene. The methanol content must be determined on a capillary column with an HP PLOT Q or similar organic adsorbent [7].
In recent years, a porous polymer such as poly(1-trimethylsilyl-1-propyne) (PTMSP), which was originally used as a material for membrane technology, has been increasingly utilized as a chromatographic adsorbent for various separation applications. Due to its high gas permeability, this polymer serves as a material from which capillary columns [14], packed columns (with an adsorbent that combines Chromosorb W and 10 wt % PTMSP) [15], and multicapillary columns [16] are fabricated. PTMSP has proven to be a promising chromatographic adsorbent due to its anomalously large free volume and relatively high solubility in organic solvents (e.g., toluene or chloroform) [17–21]. Research on PTMSP as a chromatographic stationary phase has shown that this material is well suited for analytical purposes, in particular to measure silicon, phosphorus, germanium, and arsenic hydrides [22], thiophene in target benzene products [23], structural isomers of hydrocarbons, and chlorine-based compounds [24], as well as to analyze the products of the catalytic reduction of sulfur dioxide by methane or syngas, the catalytic dehydrogenation of isobutane, and the catalytic pyrolysis of ethylbenzene [25–28]. Further, the promising applicability of 0.32 and 0.53 mm capillary columns with different PTMSP film thicknesses has been demonstrated for the analysis of model mixtures with a composition similar to that of natural gas [29].
The high separation selectivity for various classes of compounds achieved with PTMSP-based capillary columns suggests that the analytical method applied to identify hydrocarbons in propane and butane products [19], which currently involves the simultaneous operation of two or three packed or capillary columns [7–13], can be substantially improved.
Thus, the purpose of the present study was to develop a GC method to identify residual C1–C10 aromatic and aliphatic hydrocarbons and methanol simultaneously present in n-butane on a single capillary column with a porous layer of poly(1-trimethylsilyl-1-propyne).
EXPERIMENTAL
Reagents. We used poly(1-trimethylsilyl-1-propyne) synthesized at the BIC SB RAS facilities according to the method described in references [30, 31].
Column. A capillary column 0.32 mm in diameter was prepared by a static high-pressure method. For this purpose, a 0.32 mm fused silica capillary was filled with a 2.1% PTMSP solution in toluene. One end of the capillary was then sealed, while the open end was introduced into an air thermostat at a constant rate at 200°C [29, 32]. The prepared capillary column was 30 m long and 0.32 mm in diameter, with a 1.55 μm polymer film on the inner capillary surface (PTMSP032).
A commercial Rt-Q-BOND 30 m × 0.32 mm × 10 μm capillary column (Restek, USA) with a polydivinylbenzene adsorbent was used as a reference column. This choice was motivated by the fact that, like PTMSP, polydivinylbenzene is a non-polar stationary phase. Both columns exhibit similar chromatographic propeties [25–29, 33].
Model mixtures. Mixture I: a calibration gas mixture of C1–C10 aliphatics prepared in a 10 L cylinder by OOO PGS-Service in Zarechnyi, Sverdlovsk oblast, Russia. The composition of the mixture is presented in Table 1.
Mixture II: n-butane (>99%, OOO Pure Gases, Novosibirsk, Russia) + mixture I + methanol, benzene, toluene, o-, m-, and p-xylene. The mixture was prepared in a 500 mLgas-tight syringe (Hamilton, USA). The base of mixture II was made up according to GOST 33012-2014 [9], followed by the addition of the aromatics and methanol.
Mixture II was sampled with a 1 mL syringe (SGE, Australia) and introduced into the GC injector. The retention times and peak areas of the mixture components were then measured.
Chromatography. The separation performance of both the PTMSP032 and Rt-Q-BOND capillary columns was examined. To develop the analytical method, evaluate the detection limit, and estimate the precision of the retention times and peak areas for mixture II components, PTMSP032 was used in a FID-equipped Crystal 2000 chromatograph (manufactured by the KUPOL electromechanical plant, Izhevsk, Russia) under temperature-programmed conditions, at injector and detector temperatures of 250 and 230°C, respectively, with nitrogen as a carrier gas. The column oven temperature was maintained with an accuracy of ±0.5°C. NetChrom software (designed by Meta-Chrome) was used to process the chromatography data.
Preparation of methanol/nitrogen gas–vapor mixture. To prepare the gas–vapor mixtures, we used methanol (99.9%, Fluka), commercial nitrogen (99.6%, with 0.4% oxygen and 0.009% moisture, manufactured by SIBTEHGAZ, Novosibirsk, Russia), a 1 µL microsyringe (SGE, Australia), and 50 mL gas syringes (Hamilton, USA).
A calibration gas–vapor mixture was prepared with a methanol concentration of 1.01×10–2 mg/mL under the following procedure. Nitrogen was taken from the cylinder through a short connecting hose by a 50 mLgas syringe, after which the hose was tightly clamped, and 0.64 µL of methanol was added (at 760 mmHg and 22°C). After five minutes (required for the complete vaporization and mixing of methanol and nitrogen), the gas–vapor mixture was taken with a 1 mLgas syringe and introduced into the injector.
To calibrate the detector, calibration gas–vapor mixtures with methanol concentrations of 1.01×10–2, 5.05×10–3, 2.50×10–3, 1.25×10–3, and 0.06×10–3 mg/mL were prepared in a similar manner. Each newly-prepared calibration gas–vapor mixture was subjected to five parallel measurements. The repeatability precision for the retention times and peak areas of the mixture II components was calculated according to the CIS (Commonwealth of Independent States) Standardization Recommendation No. 61-2010 [34].
RESULTS AND DISCUSSION
Separation performance of PTMSP032 and Rt-Q-BOND capillary columns. It was noted above that to measure the qualitative and quantitative compositions of natural gas, APG, and gas condensate, and to identify hydrocarbons and methanol as impurities in a refinery product, the common method of choice is gas chromatography using columns prepared from selective adsorbents such as alumina, silica, or porous polymers [6, 7, 9, 10]. The n-butane product quality is normally tested by one of the two methods defined by the applicable interstate standard (GOST), as appropriate [6]. The first method is designed to analyze propane, butane, and their commercial mixtures that contain C2–C5 saturated/unsaturated hydrocarbons, provided that the concentration of the tested components is at least 0.1 wt %. This method employs packed columns prepared from Chromosorb P and a di-n-butyl maleate stationary phase (represented either by 1,8-dicyanoctane or white paraffin oil). The second method is designed to measure the weight concentrations (at least 0.001 wt %) of methane, C2–C5 saturated/unsaturated hydrocarbons, and C6 hydrocarbons. This method uses a 30 m×0.53 mm porous layer capillary column prepared from Al2O3/KCl. The referenced standard is not applicable to aromatics or methanol. The Russian national standard provided in reference [7] requires two columns to be used to analyze hydrocarbons: a 30 m × 0.53 mm capillary column with an Al2O3/S adsorbent for C1–C5 hydrocarbons, and a 30 m×0.53 mm HP PLOT Q column with a polydivinylbenzene adsorbent for C1–C4 hydrocarbons and methanol. Reference [35] describes a test method that measures C1–C4 hydrocarbons and methanol by multidimensional gas chromatography, also on two capillary columns, specifically HP-INNOwax and Alumina HP-PLOT M.
Multidimensional gas chromatography is designed to measure methanol in crude oil in the concentration range of 15 ppm to 900 ppm [36]. The combined limit of quantification is 15 ppm. An improved analytical method (based on low-temperature sample injection, the operation of a CP-Sil 5 CB pre-column, and backpurging) identifies methanol down to as low as 0.8 ppm in crude oil. In this case, hydrocarbons are analyzed on a 60 m × 0.53 mm × 5 μm capillary column with dimethyl polysiloxane, and methanol on a 10 m × 0.53 mm × 10 μm Varian CP-Lowox column [36, 37]. Satisfactory separation at similar C1–C5 hydrocarbon concentrations was demonstrated on an Rt-Q-BOND column [38].
One of our previous studies was focused on the selective separation of a model hydrocarbon mixture similar to natural gas in composition (particularly, a methane content above 90%), which was tested on a capillary column with a porous PTMSP layer [29]. Based on that work, in order to measure the concomitant impurities in n-butane, we initially selected appropriate test conditions and compared the separation performance of two column types that exhibit similar chromatographic parameters, namely PTMSP032 and Rt-Q-BOND. One milliliter of mixture II was introduced into the injector and analyzed under temperature-programmed conditions (see Figs. 1 and 2).
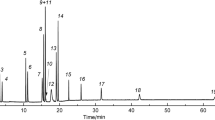
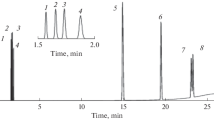
Chromatogram of mixture II components in Rt-Q-BOND column. Temperature programming: initially hold at 35°C for 3 min, heat to 180°C at a rate of 7°C/min, and hold at 180°C until all components are completely eluted. The carrier gas was nitrogen: (1) methane; (2) acetylene; (3) ethylene; (4) ethane; (5) propylene; (6) propane; (7) methanol; (8) isobutane; (9) n-butane + but-1-ene + trans-but-2-ene + cis-but-2-ene; (10) neopentane; (11) isopentane; (12) n-pentane; (13) n-hexane; (14) benzene; (15) n-heptane; (16) toluene; (17) n-octane; (18) p-xylene; (19) m-/o-xylene; (20) n-nonane; and (21) n-decane.
Chromatogram of mixture II components in PTMSP032 column. Temperature programming: initially hold at 40°C for 11 min, heat to 85°C at a rate of 7°C/min, hold for 0.5 min, heat to 220°C at a rate of 10°C/min, and hold at 220°C until all components are completely eluted. The carrier gas was nitrogen: (1) methane; (2) acetylene; (3) ethylene; (4) ethane; (5) methanol; (6) propylene; (7) propane; (8) but-1-ene; (9) isobutane; (10) trans-but-2-ene; (11) cis-but-2-ene; (12) n-butane; (13) neopentane; (14) isopentane; (15) n-pentane; (16) n-hexane; (17) benzene; (18) n-heptane; (19) toluene; (20) p-xylene; (21) m-xylene; (22) o-xylene; (23) n-octane; (24) n-nonane; and (25) n-decane.
The chromatogram for Rt-Q-BOND (Fig. 1) clearly shows that but-1-ene, trans-but-2-ene, and cis-but-2-ene are not separated from the macrozone of n-butane, the purity of which exceeds 99%, whereas m-xylene and o-xylene are eluted as a single peak. The PTMSP032 column (Fig. 2) achieves the complete separation of mixture II, including the structural isomers of n-butane and n-pentane, as well as o-, m-, and p-xylene. The peak resolution (Rs) for pairs such as n-butane/trans-but-2-ene, trans-but-2-ene/cis-but-2-ene, p-/m-xylene, and m-/o-xylene is near to or greater than 1 (Fig. 2).
On the PTMSP032 column, an asymmetric methanol peak is eluted to baseline, and well-resolved nearly-Gaussian peaks of propylene and n-propane are on the tail of the methanol peak. It is worth noting that the large amount of methanol in the tested sample does not impede the measurement of propylene or n-propane. At a concentration of 0.06×10–3 mg/mL, methanol is eluted as a separate peak after propane (Fig. 4). PTMSP032 exhibits high selectivity, as indicated by the good separation of but-1-ene, isobutane, trans-but-2-ene, and cis-but-2-ene, as well as neopentane, isopentane, and n-pentane, from n-butane’s macrozone. Therefore, to develop a method for the identification of residual hydrocarbons and methanol in n-butane (>99% pure), we used a 30 m × 0.32 mm PTMSP032 capillary column with a 1.55 µm poly(1-trimethylsilyl-1-propyne) film on the inner capillary surface.
Identification of hydrocarbon and methanol impurities in n-butane (>99%). Mixture II was introduced repeatedly and sequentially into the injector. Figure 2 shows a chromatogram of the components of model mixture II. The root-mean-square deviations (RMSD) for the retention times and peak areas of the mixture II components do not exceed 0.3 and 4.2%, respectively (Table 2). The data indicate the satisfactory repeatability of the experimental results, as well as the stable operation of this column.
The detection limit, Cmin (g/s), for hydrocarbons and methanol analyzed on PTMSP032 and detected by the FID (see Table 1) was calculated by formula (1) as defined by the relevant Russian national standard [39]:
where σ is the FID’s background signal (mV); σav = 0.152 mV; <S> is the average peak area for the component (mV s); G is the component’s weight (g), which was calculated taking into account the split ratio of the sample in the injector as per applicable GOST [39]:
where Vr is the volume of the sample (mL); P is the atmospheric pressure (Pa); M is the molecular weight of the component (g/mol); Y is the volumetric concentration of the component in the gas sample (vol %); R is the gas constant; t is the ambient temperature (°C); and K is the split ratio in the injector (in this case, K = 20).
For the FID calibration, we prepared a methanol/nitrogen gas–vapor mixture by a static method (see above). Figures 3 and 4 show the retention time, peak area, and peak shape as functions of the methanol concentration in the calibration mixtures and in the sampled model mixture II.
Methanol migration as a function of its content in mixture II in PTMSP032. Sample volume 1 mL. Temperature programming: initially hold at 40°C for 11 min, heat to 85°C at a rate of 7°C/min, hold for 0.5 min, heat to 220°C at a rate of 10°C/min, and hold at 220°C until all components are completely eluted. The carrier gas was nitrogen.
As the methanol concentration in the calibration mixture increases from 0.06×10–3 to 1.01×10–2 mg/mL, the peak maximum shifts towards lower retention times (see Figs. 3 and 4 and Table 3). The peak has a tail, which ends in about the 10th min. Given that the heating is programmed to start from the 11th min, the significant asymmetry of the methanol peak does not prevent it from being quantified within an adequate margin of error.
Based on the data obtained, a primary calibration curve was plotted. The regression coefficient for the curve built using the least squares method equaled 0.9987 (Fig. 5, Table 3).
If the calibration is performed at 40°C (Tcol = 40°C), the peak areas may differ by about 2% from the values presented.
Table 3 summarizes the precision values for the calibration gas–vapor mixtures derived from five parallel measurements of peak areas and retention times in the methanol concentration range of 0.06×10–3 to 1.01× 10–2 mg/mL.
For a concentration of 0.06×10–3 mg/mL, the root-mean-square deviation of peak area in the calibration methanol gas–vapor mixtures is 4.96%. For higher concentrations, the RMSD is somewhat lower.
Thus, over the course of the study, we developed a GC method based on simple procedures for preparing the calibration mixture (avoiding the need for an extra sample-preparation step) and calibrating the instrument. This method utilizes a 30 m × 0.32 mm capillary column with a 1.55 μm poly(1-trimethylsilyl-1-propyne) film (PTMSP032), which exhibits high separation selectivity. Consequently, the method rapidly and accurately identifies impurities in an n-butane (>99% pure) sample, including C1–C10 hydrocarbons and their structural isomers, o-, m-, and p-xylene, and methanol.
For hydrocarbons, the peak areas are measured within a margin of error of 6.4%. For methanol in the concentration range of 0.06×10–3 to 1.01×10–2 mg/mL, the peak area measurement error (from 4.96 to 0.29%) does not exceed the measurement uncertainty observed in the existing GC methods employed to identify the components of n-butane or other gaseous fluids.
REFERENCES
Drugov, Yu.S. and Rodin, A.A., Monitoring organicheskikh zagryaznenii prirodnoi sredy. 500 metodik: prakticheskoe rukovodstvo (Monitoring of Organic Pollution of the Natural Environment. 500 Techniques: A Practical Guide), Moscow: Binom. Laboratoriya Znanii, 2015. https://www.books-up.ru/ru/book/monitoring-organicheskih-zagryaznenij-prirodnoj-sredy-500-metodik-3709861
Molchanov, S.A. and Samakaeva, T.O., Complex Preparation and Processing of Multicomponent Natural Gases at Gas-Chemical Complexes, Moscow: Nedra. 2013.
Chemist’s Handbook 21. Chemistry and Chemical Technology. https://www.chem21.info/info/1159614
Istomin, V.A. and Kvon, V.G., Preduprezhdenie i likvidatsiya gazovykh gidratov v sistemakh dobychi gaza (Prevention and Elimination of Gas Hydrates in Gas Production Systems), Moscow: IRTs Gazprom, 2004.
Russian National Standard GOST 33012-2014. Propane and Butane Commercial. Determination of hydrocarbon composition by gas chromatography.
Russian National Standard GOST R 54484-2011 Liquefied petroleum gases. Methods for determining the hydrocarbon composition.
Krylov, V.A., Chernova, O.Iu., Sozin, A.Iu., Kotkov, A.P., and Pushkarev, G.V., Analyt. Control, 2013, no. 17, pp. 452–458.
Yuzhakova, T., Kovács, T., Rédey, Á., Scurtu, R., Kovács, Z., Somogyi, V., Domokos, E., Ráduly, I., and Ráduly, L., Environ. Eng. Managem. J., 2012, no. 11, pp. 1963–1968. https://doi.org/10.30638/eemj.2012.245
Russian National Standard GOST R 55997-2014 Stable gas condensate, broad fraction of light hydrocarbons, liquefied petroleum gases. Determination of methanol by gas chromatography.
Russian National Standard GOST 31371.6-2008 (ISO 6974-4:2002) Natural gas. Determination of composition with defined uncertainty by gas chromatography. Part 4: Determination of nitrogen, carbon dioxide and C1 to C5 and C6+ hydrocarbons for a laboratory and on-line measuring system using two columns.
Russian National Standard GOST 31371.5-2008 (ISO 6974-5:2002) Natural gas. Determination of composition with defined uncertainty by gas chromatography method. Part 5. Determination of nitrogen, carbon dioxide and C1 to C5 and C6+ hydrocarbons for a laboratory and on-line process application using three columns.
Russian National Standard GOST 31371.6-2008 (ISO 6974-6:2002) Natural gas. Determination of composition with defined uncertainty by gaschromatography method. Part 6. Determination of hydrogen, helium, oxygen, nitrogen, carbon dioxide and C1 to C8 hydrocarbons using three capillary columns.
Berezkin, V.G., Popova T.P, Shiryaeva, V.E., Kozlov, S.P., and Vlasenko, E.V., Diagnost. Material., 2004, no. 69, pp. 3–7.
Berezkin, V.G., Korolev A.A, Malyukova, I.V., and Popova, T.P., J. Chromatogr. A, 2002, vol. 960, nos. 1–2, pp. 151–158. https://doi.org/10.1016/S0021-9673(02)00333-3
Berezkin, V.G., Khotimsky, V.S., Sidel’nikov, V.S., and Patrushev, Yu.V., Russ. J. Phys. Chem. A, 2004, vol. 78, no. 3, pp. 432–435.
Vasilyev, G.B., Mironova, M.V., Litvinova, E.G., Volkov, V.V., Khotimsky, V.V., and Kulichikhin, V.G., Polym. Sci. Ser. A, 2013, vol. 55, no. 8, pp. 510–517. https://doi.org/10.1134/S0965545X13070067
Khotimsky, V.S., Tchirkova, M.V., Litvinova, E.G., Rebrov, A.I., and Bondarenko, G.N., J. Polym. Sci. Part A, 2003, vol. 41, no. 14, pp. 2133–2155. https://doi.org/10.1002/pola.10757
Sato, S., Suzuki, M., Kanehashi, S., and Nagai, N., J. Memb. Sci., 2010, vol. 360, nos. 1–2, pp. 352–362. https://doi.org/10.1016/j.memsci.2010.05.029
Starannikova, L.E. and Teplyakov, V.V., Polym. Sci., Ser. A, 1997, vol. 39, no. 10, pp. 1142–1147.
Baschetti, M., Ghisellini, M., Quinzi, M., Doghieri, F., Stagnaro, P., Costa, G., and Sartri, G.C., J. Mol. Struct., 2005, vol. 739, nos. 1–3, pp. 75–86. https://doi.org/10.1016/j.molstruc.,
Krylov, V.A., Berezkin, V.G., Korolev, A.A., Chernova, O.Yu., and Salganskii, Yu.M., J. Anal. Chem., 2003, vol. 58, no. 4, pp. 372–374.
Berezkin, V.G., Muhina, V.P., Korolev, A.A., Faktullina, A.F., and Seroshtan, V.A., Zavod. Lab. Diagnost. Material., 2004, no. 5, pp. 9–13.
Belotserkovskaya, V.Yu. and Yakovleva, E.Yu., Russ. J. Phys. Chem. A, 2011, vol. 85, no. 5, pp. 939–934.
Yakovleva, E.Yu. and Belotserkovskaya, V.Yu., J. Anal. Chem., 2013, vol. 68, no. 7, pp. 646–651. https://doi.org/10.1134/S1061934813070125
Yakovleva, E.Yu., Patrushev, Yu.V., and Belotserkovskaya, V.Yu., Katal. Prom-ti, 2015, vol. 15, no. 2, pp. 15–19.
Yakovleva, E.Yu. and Patrushev, Yu.V., Catal. Industr., 2020, vol. 12, no. 4, pp. 280–286. https://doi.org/10.1134/S2070050420040108
Yakovleva, E.Yu., Patrushev, Yu.V., and Pai, Z.P., Katal. Prom-ti, 2017, vol. 17, no. 6, pp. 460–464. https://doi.org/10.18412/1816-0387-2017-6-460-464
Yakovleva, E.Yu., Patrushev, Yu.V., and Pai, Z.P., Russ. J. Phys. Chem. A, 2018, vol. 92, no. 5, pp. 1018–1024. https://doi.org/10.1134/S0036024418050357
Masuda, T., Isobe, T., Higashimura, T., and Takada, K., J. Am. Chem. Soc., 1983, vol. 105, no. 25, pp. 7473–7474. https://doi.org/10.1021/ja00363a061
Masuda, T., Isobe, E., and Hamano, T., Macromolecules, 1986, vol. 19, no. 9, pp. 2448–2450. https://doi.org/10.1021/ma00163a020
Patrushev, Yu.V., Yakovleva, E.Yu., Shundrina, I.K., Ivanov, D.P., and Glasneva, T.S., J. Chromatogr. A, 2015, vol. 1406, pp. 291–298. https://doi.org/10.1016/j.chroma.2015.06.013
Yakovleva, E.Yu. and Patrushev, Yu.V., J. Phys. Chem., 2021, vol. 95, no. 7, pp. 1485–1491. https://doi.org/
RMG (Interstate Standardization Recommendations) 61-2010: State System for Ensuring the Uniformity of Measurements. Accuracy, Trueness, and Precision Measures of the Procedures for Quantitative Chemical Analysis. Methods of Evaluation, 2012.
https://www.agilent.com/en/technology/multidimensional-gas-chromatography
ASTM D7059-09(2017) Standard Test Method for Determination of Methanol in Crude Oils by Multidimensional Gas Chromatography.
Tipler, A., Marotta L, DiSanzo, F., and Grecsek, H., J. Chromatograph. Sci., 2012, vol. 50, no. 3, pp. 184–189. https://doi.org/10.1093/chromsci/bmr045
https://www.restek.com/row/products/columns/gc-columns/plot-columns/8435/
Russian National Standard GOST R 8.919-2016. State System for Ensuring the Uniformity of Measurements. Reference Complexes for Certification of Standard Samples of the Composition of Natural Gas from Mains and Natural Gas Simulators.
Funding
This work was carried out with financial support from the Ministry of Science and Higher Education of the Russian Federation within the State Assignment for BIC SB RAS (project nos. AAAA-A21-121011390007-7 and AAAA-A21-121011390053-4).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Rights and permissions
About this article
Cite this article
Yakovleva, E.Y., Patrushev, Y.V. Analysis of Residual Hydrocarbons and Methanol in n-Butane on a Porous-Layer Capillary Column with Poly(1-Trimethylsilyl-1-Propyne). Pet. Chem. 62, 112–120 (2022). https://doi.org/10.1134/S0965544122010029
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544122010029