Abstract
The dynamics of changes in the size of polyelectrolyte complex microparticles of chitosan hydrochloride and polyanions—the sodium salt of N-succinylchitosan and hyaluronic acid under the action of enzyme hyaluronidase—is considered. We demonstrate that polyelectrolyte complex microparticles undergo enzymatic degradation, which leads to a decrease in particle size to 16–70 nm within 2 to 10 min depending on the polymer pair. We assume that the enzymatic degradation of polyelectrolyte complex microparticles proceeds due to the enzymatic cleavage of the lyophilizing chain fragments and the release of a hydrophobic core of nanoparticles composed of densely packed ladder sequences of pairs of units of electrostatically complementary polyelectrolytes linked by salt bonds. The subsequent hydrophobic interactions between these particles lead to the rapid formation of precipitates of stoichiometric polyelectrolyte complexes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, chitosan polyelectrolyte complexes (PECs) with anionic polysaccharides and their use in medicine, pharmacy, food industry, etc., are considered [1–3]. One of the core biomedical aspects of their use is due to the biodegradability of their constituent components—the biogenic polymers. The structure and properties of chitosan PECs depend on the nature, structure of copolyelectrolytes (molecular weights, charge density, and distribution of ionogenic groups along the polymer chain), and the conditions for PEC formation (pH and ionic strength of a solution, temperature, concentration, and ratio of polymers). A change in type of a copolyelectrolyte and the conditions of a polyelectrolyte reaction makes it possible to obtain polymer materials as a gel, films, membranes, porous structures, liquid crystal dispersions, nanoparticles, and microparticles [4–10]. The nanoparticles of dispersions formed spontaneously in a solution of charge-complementary polyelectrolytes (PEs) according to the mechanism of electrostatic interaction between oppositely charged ionogenic groups to form salt interpolyelectrolyte complexes are attracting special attention among many other types of polymer nanoparticles and microparticles due to the simplicity of the process of obtaining them. The use of nanoparticles and microparticles as carriers of medicinal compounds can overcome the tissue barriers, decrease the side effects after their introduction (toxicity and allergenicity), and increase their efficiency due to the prolongation of action and, most importantly, their targeted transfer into cells.
The materials based on natural polymers for temporary functioning in a body must have a controllable degradation period, and their degradation products must be nontoxic and easily excreted from the body. Biocompatible and biodegradable polymers, such as chitosan, N-succinylchitosan, and hyaluronic acid, fully meet these requirements (Scheme 1). Numerous works showed that these biopolymers undergo degradation under the action of various nonspecific enzymes, including those of a living organism (pepsin, trypsin, collagenase, hyaluronidase, and lysozyme) [11–13]. The enzymatic degradation process may be controlled by a change in various characteristics of a material depending on its type. In the case of polysaccharide solutions, the changes in the degree of polymerization or viscosity indices are found as a rule [14, 15]. The enzymatic destruction of chitosan was studied by the accumulation of reduction sugars [16, 17]. Weight loss or a change in the quality of a material’s surface is most often studied in the case of polysaccharide films, fibers, and bulk materials (implants or matrices) [18, 19]. It seems appropriate to study the biodegradation process directly from the change in particle size in the case of biopolymer nanoparticles and microparticles. The size of particles directly determines their transport and distribution in a body, and their destruction is a factor leading to the release of biologically active compounds introduced into them.

The type of material (solution, gel or film) influences the enzymatic destruction, because there is a fundamental difference between the availability of links of individual polymer macromolecules in a solution to interact with an enzyme and their availability in a bulk sample from the topochemical point of view [14, 20]. Considering that chitosan PEC microparticles are attracting a great deal of attention as a depot to deliver biologically active compounds to organs and tissues of a living organism, the study of their behavior in an enzyme-containing medium that simulates the environment of a living organism is an important and urgent task.
The aim of this study is to assess the enzymatic stability of PEC microparticles based on polycation—chitosan hydrochloride (CHC) and some polyanions of sodium salt of N-succinylchitosan (SCH) and hyaluronic acid (HA)—in an aqueous dispersion medium under the action of an enzyme hyaluronidase and to find the features of this process.
EXPERIMENTAL
Commercial chitosan with MW = 70 kDa and the deacetylation degree of 87% (TU 9289-067-00473124-03), water-soluble sodium salt of N-succinylchitosan with MW = 207 kDa and the degree of substitution of 62% (TU 9284-027-11734126, Bioprogress, Russia), and hyaluronic acid with MW = 43 kDa (Leko Style, Russia) were used without additional purification. The hyaluronidase in a pharmaceutical form Lidaza (Mikrogen, Russia) was used as an enzyme drug.
Chitosan hydrochloride was prepared as a film from a hydrochloric acid solution of chitosan (2 g of chitosan in 50 mL of a 1% hydrochloric acid solution) by drying in air and then under a vacuum to a constant weight. The hydrochloric acid : chitosan molar ratio was 1 : 07.
Chitosan samples with MW = 30 kDa and N-succinylchitosan with MW = 67 kDa were prepared via oxidative destruction of high molecular weight polymers with hydrogen peroxide in an aqueous solution as described earlier [21]. We assumed that the amount of acetamide, amino, and N-succinyl groups did not change during the oxidative destruction of macrochains according to the law of chance.
The deacetylation degree (DD) of chitosan was assessed via acid-base titration with the potentiometric determination of the equivalence points on a TK100-B coulometric titrator according to the formula [22]:
where m is the mass of chitosan in a sample (in g), СNaOH is the concentration of the sodium hydroxide solution (in mol/L), V2 – V1 is the difference between the volumes of the second and first inflections on a potentiometric titration curve, which corresponds to the neutralization of amino groups in chitosan, V2 is the volume of the NaOH solution for titration of the HCl bound to the amino groups of chitosan, V1 is the volume of the NaOH solution to titrate the excess amount of HCl not bound to the amino groups of chitosan, 203.2 is the molecular weight of an acetylated monomer unit of a polysaccharide, and 42.0 is the difference between the molecular weights of the acetylated and glucosamine monomer units. The pH of the solutions was found on an ANION-410V pH meter.
The molecular weight (MZ) of degraded polymers was found with the sedimentation equilibrium method on a MOM-3080 ultracentrifuge and was calculated according to the formula
where R = 8.3 J/(mol K–1), T is the absolute temperature, ω = 2πn rad/s is the angular speed of rotation of the ultracentrifuge rotor, n is the number of turns of a rotor per minute, tgα is the slope of the curve of the relationship between Zi/Xi and the concentration of solution C, Xi is the distance from the axis of the rotation of a rotor to the given point of the gradient curve, Zi = (dC/dX)i is the height of the gradient curve (concentration gradient) at the given point, Xi(1 – vρ0) is the Archimedean factor, v is the specific partial volume of the polymer, and ρ0 is the density of the solvent.
Aqueous dispersions of the PECs were prepared via the titration of an aqueous solution of polyanion with an aqueous solution of chitosan hydrochloride under vigorous stirring (500 rpm) at room temperature with an interval between the introduction of portions of 5 min. The phase behavior of the systems was assessed visually after the appearance of opalescence in the mixture of the PE solutions and by the formation of a complex precipitate. The size of the particles of the opalescent PEC systems were found with laser scattering on a Sald 7101 unit (Shimadzu). The wavelength of a semiconductor laser was 375 nm. The operation of the particle diameter measurement ranged from 10 nm to 300 µm. The measurements were performed in Sald-BC special quartz cuvettes with mechanical vertical stirring at room temperature. The median particle sizes corresponding to the point on the differential distribution curve, to the left and right of which 50% of the area under the distribution curve was contained, were used for the comparative description of particles by size. The measurement error was 1%. The stability time of the system was considered as that until the formation of a visually observable PEC deposit.
The molar ratio of components z in the resulting systems, when the PEC particles were formed, was found as a ratio of the molar concentration of the ionogenic units of the CHC calculated taking into account the deacetylation degree to that of the ionogenic groups of polyanion (PA): z = [CHC]/[PA]. For SCH, the concentration of ionogenic units was calculated taking into account the deacetylation degree and the modification degree by amino groups.
The disperse systems were also characterized from the mass amount of the sum of polymers ω (in wt %). The mass concentration of CHC and PA in the solution was found from the ratio of mass of a solute to the total mass of the solution taking into account the amount of moisture of the polysaccharides. The moisture amount of the polysaccharides was 4–5%.
An enzyme drug dissolved previously in a low amount of distilled water was introduced into the aqueous dispersion of the PEC microparticles to perform the enzymatic destruction. The samples were taken from the volume of the reaction system at regular intervals to assess the dynamics of changes in the particle sizes, and the current particle sizes were found.
RESULTS AND DISCUSSION
The objects were chosen due to the following considerations. Hyaluronidase is a specific enzyme that cleaves HA. At the same time, this enzyme successfully hydrolyzes chitosan and CHC in solution [15]. The optimum activity of hyaluronidase appears within pH 5.5–7.0. All components, including the CHC and SCH salts, are also readily soluble in a neutral aqueous environment. The initial chitosan, which has рK 6.2–6.5 depending on the molecular weight [23], is almost not dissolved in the solutions with pH 6.5–7.0 because of the insufficiently high amount of charged groups. At the same time, the chitosan samples as hydrochloride salts are readily soluble in water and do not form precipitates during their long-term storage [24]. The pH values of aqueous mixtures of CHC–SCH are 6.5–7.0 and the CHC–HA mixtures have a PH of 6.9. The CHC and SCH salts are strong polyelectrolytes and are fully ionized in neutral aqueous solutions.
When the CHC solutions are mixed with polyanions in certain molar ratios, the disperse systems of PEC microparticles appear, which leads to a stable opalescence of the mixture. The particle size and the stability of the systems in the case of the formation of sedimentation unstable dispersions depend on the ratio of the molecular weights of the PEs, the z value, and the concentration of the polymer solution [25]. The systems whose parameters provided the preparation of dispersions with nanometer particles and with the stability not to form a precipitate during the enzymatic destruction were selected as the initial ones. The enzymatic destruction of chitosan in solution assessed by the decrease in the intrinsic viscosity was described by the kinetic curves reaching its constant value within 24–120 h [26].
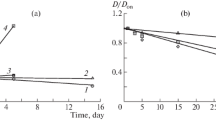
Table 1 and Fig. 1 show the sizes of particles of the initial complexes and the dynamics of the change in their size over time. The destruction of polysaccharide chains that form PEC particles proceeds in the presence of the enzyme in all disperse systems, which leads to a decrease in the particle size over time. At the same time, the enzymatic destruction process has some features for PEC types.
When the solutions of chitosan hydrochloride having MW = 70 kDa (CHC(70)) and of HA are mixed, a relatively stable (up to 360 h) disperse system with an initial particle size of 209 nm appears (Table 2). When the enzyme drug was introduced into the solution, the destruction process proceeded rapidly, and the particle size decreased down to 18 nm within 15 min and then remained unchanged for at least several hours. The particle size in the disperse system, however, increases up to 200 nm after about 20 h, which indicates that the processes of the structure’s formation start to take place in the system. The particle size increases to 450 nm within 40 h and to 500 nm after 70 h. A precipitate forms in the system after 190 h.
The destruction process for the CHC(70) and SCH PECs with MW = 67 kDA (SCH(67)) also begins very rapidly. The size of particles for a pair of polymers with comparable molecular weights decreased almost by a factor of three after 10 min (Table 2). An increase in particle size, however, was observed after 15 min despite the enzyme in the system, and a PEC precipitate appeared after 30 min. The size of particles was 769 nm after the vigorous stirring of the sedimentary suspension for 5 h and it was 784 nm the next day under the same measurement conditions. The size of the particles for the pair of polymers with sharply different molecular weights decreased much more significantly by a factor of 20 after 10 min of destruction, and a PEC precipitate appeared in the system after 20–30 min.
Therefore, the increase in size of the particles during the enzymatic destruction together with their decrease, which leads to the faster formation of PEC precipitates than that without an enzyme, was an unexpected result. It should be noted that the addition of a new portion of the enzyme did not lead to further destruction. Indeed, the size of the particles was 470 nm after 40 min in the case of fermentolyzed CHC(70)–HA PECs with a particle size of 443 nm after addition of a new portion of lidase. The further addition of lidase to the CHC(70)–SCH(67) PEC dispersion after 24 h also did not cause a significant destruction of particles.
These observations may be explained based on the PEC structure as ladder polymers. The sequences of pairs of units connected by salt bonds alternate in polymers with the defects consisting of disconnected PE hydrophilic units [26]. The scheme of the enzymatic destruction of PECs may be represented as shown in Fig. 2 according to the results obtained.
About 20% of the units in PECs are found in the defects as free chain ends and loops due to the steric mismatch between electrostatically complementary PE chains [27]. Let us assume that both glycosidic bonds in the ladder sequences available on the surface of the PEC particles and those of the free units involved in the chain defects and in the hydrophilic shell of the PEC particles are broken according to the law of chance under the action of the enzyme. In the first case, the cleavage of the chains should not affect the PEC structure in any way, because it does not change the length of the salt bond sequences and does not lead to new components in the system. In the second case, the destruction leads to additional chain fragments of free PEs in the solution probably due to their low molecular weight. The destruction of the excess PEs to form products with a lower molecular weight should be taken into account.
PEC particles in the excess polyanion are relatively stable over a long time period for the pair of strong CHC(30)–SCH(207) PEs, which differ greatly in degrees of polymerization (P) and correspond to the RPA \( \gg \) RPEC condition, probably due to the large number of lyophilizing defects. The enzyme, “cutting off” these defects, releases the hydrophobic particles with a composition similar to a stoichiometric one. Such particles are prone to the aggregation process and the appearance of a precipitate due to the hydrophobic interactions.
The CHC(70)–SCH(67) pair with comparable molecular weights possesses a low stability of the disperse system due to only the hydrophilic units of defects. When CHC short chains appear during the enzymatic destruction of the defects, these chains are probably rapidly involved in the formation of stoichiometric PECs with the excess PA to form hydrophobic particles and their subsequent rapid aggregation to form a precipitate.
The additional free chains of the CHC with a low molecular weight obtained after enzymatic destruction will lead to a complex due to an excess of polyanion. In addition, it should be noted that weak polyanions such as HA contain nonionized carboxyl groups at pH 6.5–6.9 and include in their composition insoluble PEC particles in excess [28], which participate in the formation of hydrogen bonds and hydrophobic interactions.
CONCLUSIONS
In summary, we showed that PEC nanoparticles in aqueous colloidal dispersions based on biodegradable polysaccharides—CHC and N-succinylchitosan salts and hyaluronic acid—undergo enzymatic degradation under the action of hyaluronidase, which results in a rapid decrease of their size. This is probably due to the cleavage of free lyophilizing chain fragments under the action of the enzyme and, therefore, due to the release of the hydrophobic nucleus of nanoparticles composed of densely packed ladder sequences of pairs of units of electrostatically complementary polyelectrolytes bound by salt bonds. The aggregation of the decomposed particles due to hydrophobic interactions leads to a subsequent increase in the size of the particles up to the loss of their sedimentation stability and precipitation, and it proceeds more rapidly than in the case of dispersions that have not been destroyed of the PEC nanoparticles.
REFERENCES
V. A. Izumrudov, Polym. Sci., Ser. A 54, 513 (2012).
M. A. Krayukhina, N. A. Samoilova, and I. A. Yamskov, Russ. Chem. Rev. 77, 799 (2008). https://doi.org/10.1070/RC2008v077n09ABEH003750
A. V. Iliva and V. P. Varlamov, Appl. Biochem. Microbiol. 41, 5 (2005).
V. A. Petrova, A. S. Orekhov, D. D. Chernyakov, et al., Crystallogr. Rep. 61, 945.
P. Sabitha, J. Vijaya Ratna, and K. Ravindra Reddy, Int. J. Chem. Technol. Res. 2, 88 (2010).
B. Kh. Musabaeva, K. B. Murzagulova, M. E. Kim, V. A. Izumrudov, and Z. Zh. Aripzhanova, Farm. Farmakol. 5 (2), 164 (2017).
M. G. Devi, S. Dutta, A. T. Al Hinai, and S. Feroz, Korean J. Chem. Eng. 32, 118 (2015).
D. D. Chernyakov, V. A. Petrova, Yu. G. Baklagina, I. V. Gofman, and Yu. A. Skorik, Izv. Ufimsk. Nauch. Tsentra RAN, No. 3-1, 99 (2016).
M. S. Gurina, R. R. Vil’danova, L. A. Badykova, N. M. Vlasova, and S. V. Kolesov, Russ. J. Appl. Chem. 90, 219 (2017).https://doi.org/10.1134/S1070427217020100
A. Rafiee, M. H. Alimohammadian, G. Taraneh, et al., Asian Pacif. J. Tropic. Disease 4, 372 (2014).
H. Zhang, Y. Du, X. Yu, M. Mitsutomi, and S. Aiba, Carbohydr. Res. 320, 257 (1999).
Chitin and Chitosan: Preparation, Properties and Application, Ed. by K. G. Skryabin, G. A. Vikhoreva, and V. P. Varlamov (Nauka, Moscow, 2002) [in Russian].
E. I. Kulish, I. F. Tuktarova, V. V. Chernova, V. P. Zakharov, and S. V. Kolesov, Russ. J. Phys. Chem. B 9, 237 (2015).
V. V. Chernova, A. S. Shurshina, and E. I. Kulish, Russ. J. Phys. Chem. B 12, 495 (2018).
V. V. Chernova, I. F. Tuktarova, and E. I. Kulish, Russ. J. Phys. Chem. B 11, 338 (2017).
E. I. Kulish, V. V. Chernova, A. R. Khusnutdinova, V. P. Volodina, and S. V. Kolesov, Russ. J. Appl. Chem. 85, 156 (2012). https://doi.org/10.1134/S1070427212010314
A. P. Lun’kov, B. Ts. Shagdarova, and A. V. Il’ina, Izv. Ufimsk. Nauchn. Tsentra RAN, No. 3-1, 56 (2016).
M. V. Konovalova, D. V. Kurek, E. A. Duriev, S. G. Litvinets, and V. P. Varlamov, Izv. Ufimsk. Nauchn. Tsentra RAN, No. 3-1, 42 (2016).
K. Tomihata and Y. Ikada, Biomaterials 18, 567 (1997).
I. F. Tuktarova, V. V. Chernova, R. Y. Lazdin, and E. I. Kulish, Prot. Met. Phys. Chem. Surf. 52, 297 (2016).
N. I. Kupreev, D. V. Bykovskii, and V. A. Kuznetsov, RF Patent No. 2417088 C1, Byull. Izobret., No. 12 (2011).
Yu. A. Kuchina, N. V. Dolgopyatova, V. Yu. Novikov, V. A. Sagaidachnyi, and N. N. Morozov, Vestn. MGTU 15 (1), 107 (2012).
R. N. Tharanathan and F. S. Kittur, Crit. Rev. Food Sci. Nutr 43, 61 (2003).
R. Z. Khairullin, S. N. Kulikov, V. E. Tikhonov, et al., Vestn. Kazan. Tekhnol. Univ., No. 7, 148 (2010).
S. V. Kolesov, M. S. Gurina, and R. Kh. Mudarisova, Polym. Sci., Ser. A 61, 195 (2019).
A. B. Zezin and V. A. Kabanov, Russ. Chem. Rev. 51, 833 (1982). https://doi.org/10.1070/RC1982v051n09ABEH002921
A. V. Zezin, V. B. Rogacheva, V. S. Komarova, and Ye. F. Razvodovskii, Polym. Sci. USSR 17, 3032 (1975).
A. B. Zezin, V. V. Lutsenko, V. A. Izumrudov, and V. A. Kabanov, Polym. Sci. USSR 16, 694 (1974).
ACKNOWLEDGMENTS
The samples were analyzed on the equipment of the Collective Usage Center “Khimiya” at the Ufa Institute of Chemistry (Ufa Federal Research Center, Russian Academy of Sciences).
Funding
This work was supported by program no. АААА-А17-117011910026-3 of the Federal Scientific Research of state academies for 2019–2021.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Tulyabaev
Rights and permissions
About this article
Cite this article
Kolesov, S.V., Badykova, L.A. & Mudarisova, R.K. Enzymatic Stability of Chitosan Interpolyelectrolyte Complex Nanoparticles. Russ. J. Phys. Chem. B 14, 1049–1054 (2020). https://doi.org/10.1134/S199079312006007X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199079312006007X