Abstract
The influence of preparation conditions on the size characteristics and stability of particles of aqueous dispersions of positively and negatively charged polyelectrolyte complexes based on N-succinyl chitosan and poly-N,N-diallyl-N,N-dimethylammonium chloride was studied. An increase in the concentrations of the initial polymer solutions, irrespective of the order of their mixing and the charge of the complex, makes narrower the interval of the molar ratios of the initial polyelectrolytes at which the dispersions of particles of the complexes exhibit aggregation stability. The widest interval of molar ratios at which the dispersions are stable (0.20–0.55) is observed for systems of negatively charged complexes with the initial concentration of the components of 0.05%. In the presence of 0.1 М NaCl, the initial particle radius in both types of complexes decreases to 75–80 nm. Addition of more concentrated sodium chloride solutions, especially into systems based on positively charged complexes, leads to an increase in the initial particle radius to 90–700 nm. With time, the particle size increases, and variation of the component molar ratio does not noticeably affect the particle size of the complexes. The mean particle size of polyelectrolyte complexes in the region of relative sedimentation stability of disperse systems is 75–403 nm, which opens prospects for using these particles as drug carriers for targeted delivery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The development of targeted drug delivery systems is one of the problems that can be solved using polymer nano- and microparticles. Medicines containing drugs incorporated in polymer nano- and microparticles can exhibit fewer unfavorable side effects and higher bioavailability and therapeutic performance compared to classical drug forms [1, 2]. The overwhelming majority of systems containing polymer nano- and microparticles are based on biocompatible materials such as natural polysaccharide derivatives (carboxymethyl cellulose, chitosan and its derivatives), polyesters (polylactide, polyglycolide, and their copolymers), and polyamides [2, 3].
By varying the conformational and supramolecular state of biocompatible and biodegradable polymer materials, it is possible to control their physicochemical properties and rate of release of the pharmaceutical substance, which is important for the development of medicines for targeted delivery. In this connection, particles of biocompatible polyelectrolyte complexes are of most interest as systems for encapsulation of drug molecules, because the degree of aggregation of macromolecules and hence their conformational and supramolecular state can be controlled by varying the preparation conditions.
In the development of materials based on nano- and microparticles of polyelectrolyte complexes, particular attention is paid to the thermodynamic stability and behavior of systems with variation of рН, ionic strength of the medium, temperature, magnetic field, etc. [4–6].
However, today data on the effect of specific factors on the size characteristics of particles of polyelectrolyte complexes are apparently insufficient. This problem is topical and important from both scientific and practical viewpoints for developing preparation procedures and controlling characteristics and properties of polyelectrolyte complexes.
N-Succinyl chitosan (SCTS) sodium salt polyanion is nontoxic, water-soluble, and biocompatible with tissues of a living body [7, 8]. Poly-N,N-diallyl-N,N-dimethylammonium chloride (PDADMAC) exhibits antifungal and bactericidal properties and shows promise for use in medicinal materials [9–11].
This study was aimed at determining how the preparation conditions influence the size characteristics and stability of particles of aqueous dispersions of polyelectrolyte complexes based on SCTS and PDADMAC.
EXPERIMENTAL
The following polymers were used for preparing polyelectrolyte complexes: SCTS with the molecular mass of 200 kDa, 75% degree of amino group substitution, and 82% degree of deacetylation of the initial chitosan sample from which SCTS was prepared (Bioprogress, Russia); PDADMAC with the molecular mass of 47 kDa (Bashkir Soda Company, Russia).
Samples of the initial polyelectrolytes were dissolved in purified water obtained from potable water by distillation [12]. The gravimetric concentration of SCTS and PDADMAC solutions was 0.02–0.2%.
The formation of polyelectrolyte complexes was studied by turbidimetry. The optical density of solutions was measured with a KFK-2 photocolorimeter (Zagorsk Optical and Mechanical Plant, Russia) at the absorbing layer thickness of 1 cm and wavelength of 490 nm at which all the components are optically transparent. The order of mixing the polyelectrolyte solutions was different: In the SCTS–PDADMAC system [negatively charged polyelectrolyte complex (PEC-1)], we added dropwise the polycation to the polyanion, whereas in the PDADMAC–SCTS system [positively charged polyelectrolyte complex (PEC-2)] the polyanion was added dropwise to the polycation. The composition of polyelectrolyte complexes was expressed via molar ratios of the initial polyelectrolytes z:
\( \text{PEC-1: }{{z}_{1}}=\frac{[\text{PDADMAC }]\text{ }{{V}_{\text{PDADMAC}}}}{\text{ }[\text{ SCTS }]\text{ }{{V}_{\text{SCTS}}}}, \)
\( \text{PEC-2: }{{z}_{2}}=\frac{[\text{SCTS }]\text{ }{{V}_{\text{SCTS}}}}{[\text{PDADMAC }]\text{ }{{V}_{\text{PDADMAC}}}}, \)
where [PDMADAC] and [SCTS] are the molar concentrations of the ionogenic units of PDADMAC and SCTS (M), and VPDADMAC and VSCTS are the volumes of the solutions of the corresponding components (mL).
Solutions of the mixtures were stirred at 25°С on a magnetic stirrer at 500 rpm for 10 min.
The titration result is given in the form of the dependence of the turbidity τ (τ = 2.3D, D is the optical density) on the component molar ratio z. Mixtures meeting the condition τ490 < 0.02 were considered as transparent. The reaction mixture composition starting from which τ490 becomes higher than 0.02 and visually detectable opalescence appears, suggesting the formation of optically discernible particles of the polyelectrolyte complex, was denoted as zmin. The reaction mixture composition starting from which the system loses the sedimentation stability, a precipitate is formed, and the turbidity sharply increases was denoted as zlim. To prepare polyelectrolyte mixtures containing 0.1, 0.2, and 0.5 M low-molecular-mass electrolyte, NaCl (chemically pure grade, Lenreaktiv, Russia), the corresponding weighed portion of NaCl was added to the working solution of the polyelectrolyte being titrated.
The mean radius (r, nm) of particles of polyelectrolyte complexes was determined from the turbidity spectrum [13]. The main criteria of the applicability of this method are given in [14].
RESULTS AND DISCUSSION
The formation of polyelectrolyte complexes is controlled by such factors as nature of the initial polyelectrolytes, their molecular mass, order of mixing the components, and, to a large extent, their ratio and concentration in the mixture [4]. In this connection, we determined by turbidimetric titration the component molar ratios at which the dispersed system formed does not undergo aggregation within the time comparable with that of formation of materials based on solutions and dispersions of polyelectrolyte complexes (2–3 days). Both SCTS and PDADMAC solutions were used as titrants.
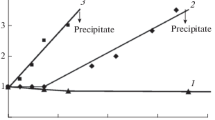
Three portions can be distinguished in the titration curves of both systems (Fig. 1). The initial portion of the turbidimetric titration curves corresponds to a homogeneous system in which PEC-1 is present in excess STCS and PEC-2 is present in excess PDADMAC. Throughout the composition range, at z ≤ zmin the system remains homogeneous, which corresponds to the formation of soluble polyelectrolyte complexes.
Curves of turbidimetric titration (1) of a solution of N-succinyl chitosan with a solution of poly-N,N-diallyl-N,N-dimethylammonium chloride and (2) of a solution of poly-N,N-diallyl-N,N-dimethylammonium chloride with a solution of N-succinyl chitosan. Initial concentrations of N-succinyl chitosan and poly-N,N-diallyl-N,N-dimethylammonium chloride solutions 0.1%.
Further increase in the titrant amount leads to the formation of visually detectable opalescence associated with the formation of sol particles. In the interval from zmin to zlim, the insoluble stoichiometric complex coexists with the soluble nonstoichiometric complex. At z ≥ zlim, both polyelectrolytes appear to be quantitatively incorporated in particles of the insoluble stoichiometric complex. Further addition of solutions of oppositely charged polymers leads to a sharp increase in the optical density and to the precipitate formation.
The formation of particles of the polyelectrolyte complex is strongly influenced not only by the molar ratios but also by the concentrations of the components. With a decrease in the concentrations of the initial polymer solutions, irrespective of the order of their mixing, the range in which particles of the complexes in aqueous dispersions are aggregatively stable becomes broader. The range in which the dispersions of the SCTS–PDADMAC polyelectrolyte complex are stable (∆z = zlim – zmin) narrows from ∆z = 0.35 for 0.05% solutions to ∆z = 0.30 for 0.1% solutions (Table 1).
Dash means that, at the initial concentrations of N-succinyl chitosan and poly-N,N-diallyl-N,N-dimethylammonium chloride of 0.02%, particles of the polyelectrolyte complexes are resistant to sedimentation, no precipitate is formed, and zlim is not reached.
In the case of dispersions of the PDADMAC–SCTS polyelectrolyte complex, the range of the existence of stable particles narrows from ∆z = 0.30 for 0.05% solutions to ∆z = 0.25 for 0.08–0.1% solutions (Table 1). Narrower intervals of the stability of the positively charged PDADMAC–SCTS complexes can be attributed to shorter length of the macrochain of the lyophilizing component (PDADMAC), which cannot provide the required amount of free ionogenic groups that are not involved in interchain ionic bonding [4]. Hence, particles of the PDADMAC–SCTS polyelectrolyte complex are more hydrophobic than those of the SCTS–PDADMAC polyelectrolyte complex and are therefore less resistant to sedimentation.
With an increase in the polymer concentration to 0.2%, the precipitate is formed in the first and second systems already on adding the first drops of both PDADMAC and SCTS solutions. This is due to the presence of excess blocking polyelectrolyte in the system.
When considering the formation of dispersed particles of polyelectrolyte complexes, we should proceed from the fact that, at a definite concentration (>0.1%) of polyelectrolytes in solutions based on relatively low-molecular-mass polymers SCTS and PDADMAC, not only separate polyelectrolyte macromolecules but also bulky macromolecular associates occurring in equilibrium with them participate in the interaction. In this case, only the charged groups on the surface of the associates are accessible to ionic bonding. Each associate has a large number of surface charges, allowing its binding with several oppositely charged associates. Apparently, specifically this factor causes the formation of relatively coarse particles of polyelectrolyte complexes, rapidly losing the stability, from SCTS and PDADMAC solutions of concentrations higher than 0.1%.
The factors affecting the complexation in polyelectrolyte solutions are the chain length and the length ratio of the interacting polymers. The molecular masses of the interacting polyelectrolytes SCTS and PDADMAC differ by a factor of approximately 4; i.e., macromolecules of these polymers significantly differ in the length, which is manifested in such characteristics of the complexes as their size and sedimentation stability and in differences in the intervals of the component molar ratios at which positively and negatively charged polyelectrolyte complexes form stable dispersions.
The aggregative stability of aqueous dispersions of polyelectrolyte complexes at component molar ratios within the stability interval was evaluated by changes in the particle size in the course of 4 days.
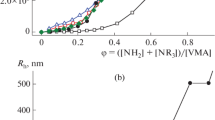
The initial (determined directly after mixing the initial polyelectrolytes) mean particle radii are 75–175 nm in PEC-1 dispersions (Fig. 2) and 85–100 nm in PEC-2 dispersions (Fig. 3); i.e., the initial particle size is virtually independent of the type of the polyelectrolyte complex. Direct dependences of the mean particle size on the component molar ratio are observed (Fig. 2). Apparently, at large deficiency of the blocking polyelectrolyte, the amount of microheterogeneous sol particles formed in relatively small, and the probability of their participation in the subsequent aggregation processes is relatively low. An increase in the lyophilizing polyelectrolyte to blocking polyelectrolyte ratio leads to an increase in the number of particles and, correspondingly, to an increase in the probability of their involvement in the formation of coarser aggregates. Variation of the order of mixing the initial polyelectrolytes does not noticeably influence the formation of polyelectrolyte complexes.
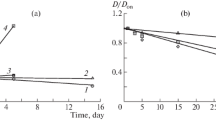
Dependence of the particle radius of polyelectrolyte complexes on the keeping time. (1) Negatively charged N-succinyl chitosan–poly-N,N-diallyl-N,N-dimethylammonium chloride complexes and (2) positively charged poly-N,N-diallyl-N,N-dimethylammonium chloride–N-succinyl chitosan complexes. Initial concentrations of succinyl chitosan and poly-N,N-diallyl-N,N-dimethylammonium chloride 0.1%.
Dependence of the particle radius of polyelectrolyte complexes on the keeping time for (a) negatively charged N-succinyl chitosan–poly-N,N-diallyl-N,N-dimethylammonium chloride complexes and (b) positively charged poly-N,N-diallyl-N,N-dimethylammonium chloride–N-succinyl chitosan complexes. Initial concentrations of succinyl chitosan and poly-N,N-diallyl-N,N-dimethylammonium chloride 0.1%. (1) Without NaCl, (2) 0.1 M NaCl, (3) 0.2 M NaCl, and (4) 0.5 M NaCl.
Aggregation processes developing in polyelectrolyte solutions are manifested in changes in the particle size with time, but within a relatively short observation period (1–2 days) the system remains resistant to aggregation, and the particle size is virtually independent of the component molar ratio (Fig. 2).
Thus, the characteristic feature of the interaction of PDADMAC and SCTS as components of polyelectrolyte complexes in a neutral aqueous solution is the formation of relatively stable systems with the dispersed phase of particles of polyelectrolyte complexes. In these systems, processes leading to changes in the particle size continuously develop in time, which ultimately leads to the phase separation with the formation of a precipitate. For example, a precipitate is formed after 4-day monitoring in both kinds of systems. Changes in the PEC particle size in time may be due in part to partial hydrolysis of carboxylate salt groups in N-succinyl chitosan sodium salt (salt of a weak acid and a strong base). As a result, a part of carboxylate groups can transform into weakly dissociating carboxyl groups and the charge density on SCTS macromolecules will decrease, which will lead to the break of a part of ionic bonds in particles of polyelectrolyte complexes and to changes in the size of supramolecular formations.
Variation of the solution ionic strength can also affect the character of the polyelectrolyte interaction. In this connection, when evaluating the possibility of preparing micro- and nanoparticles based on polyelectrolyte complexes, it is of certain interest to study their formation in aqueous solutions of low-molecular-mass salts, because the majority of biological media have relatively high ionic strength.
In the presence of 0.1 М NaCl, the initial particle size in PEC-1 and PEC-2 decreases, probably because of the break of the hydrate shell (Fig. 3). On the other hand, the addition of 0.2 and 0.5 М NaCl, especially in the case of PEC-2, leads to an increase in the initial particle size as a result of partial shielding of the macromolecule charge and, correspondingly, partial dissociation of the ion pairs of the complexes. Because the SCTS macromolecule contains, along with ionogenic groups, also acetamide and amino groups incapable of dissociation, it is quite possible that not only electrostatic interaction but also hydrogen bonds and hydrophobic interactions contribute to the formation of polymer complexes. Therefore, an increase in the solution ionic strength can lead will not necessarily lead to complete disaggregation of the complexes, and changes in the conformational and supramolecular state can lead to an increase in the particle size. With time, the particle size increases; variation of the component molar ratio does not noticeably affect the particle size of the complexes (Fig. 3), which also suggests partial shielding of the macromolecule charge with ions of the low-molecular-mass electrolyte and involvement of particles of polyelectrolyte complexes in the formation of secondary aggregates.
CONCLUSIONS
Thus, we have determined the conditions of the existence of stable dispersions and the size characteristics of different kinds of polyelectrolyte complexes based on N-succinyl chitosan and poly-N,N-diallyl-N,N-dimethylammonium chloride, which will allow the evaluation of their application field and the development of procedures for preparing various materials based on them. In the course of storage, processes leading to changes in the particle size and ultimately to precipitation of the complex continuously occur in the systems based on the polyelectrolyte complexes studied. The time to precipitate formation depends mainly on the difference between the molecular masses of the lyophilizing and blocking polyelectrolytes and on the polymer concentration in the solution. The order of mixing the components of the complexes does not noticeably affect the variation of the particle size in time. The mean particle radius of polyelectrolyte complexes in the region of sedimentation stability of the dispersed systems is 75–403 nm, which, taking into account high biological activity of the polyelectrolytes studied and their biocompatibility and biodegradability, opens prospects for using particles of the polyelectrolyte complexes as drug carriers for targeted drug delivery in a living body.
REFERENCES
Amjad, M.V., Farm. Farmakol.,, 2021, vol. 9, no. 1, pp. 4–16. https://doi.org/10.19163/2307-9266-2021-9-1-4-16
Gomzyak, V.I., Sedush, N.G., Puchkov, A.A., Polyakov, D.K., and Chvalun, S.N., Polym. Sci., Ser. B, 2021, vol. 63, no. 3, pp. 190–206. https://doi.org/10.1134/S1560090421030064
Zhao, D., Shuang Yu, Sun, B., Gao, S., Guo, S., and Zhao, K., Polymers (Basel), 2018, vol. 10, no. 4, pp. 462–477. PMID: 30966497. https://doi.org/10.3390/polym10040462
Izumrudov, V.A., Mussabayeva, B.Kh., Kassymova, Zh.S., Klivenko, A.N., and Orazzhanova, L.K., Russ. Сhem. Rev., 2019, vol. 88, no. 10, pp. 1046–1062. https://doi.org/10.1070/RCR4877.
Emmanuel, B.D., Abu-Thabit, N.Y., and Ngwuluka, N.C., Stim. Respons. Polym. Nanocarriers Drug Deliv. Appl., 2018, vol. 1, pp. 267–287. https://doi.org/10.1016/B978-0-08-101997-9.00014-X
Maiti, S., Jana, S., and Laha, B., in Design and Development of New Nanocarriers, William Andrew, 2018, pp. 223–256. https://doi.org/10.1016/B978-0-12-813627-0.00006-5
Bazunova, M.V., Sharafutdinova, L.A., Lazdin, R.Y., Chernova, V.V., Mixonov, D.N., and Zakharov, V.P., Appl. Biochem. Microbiol., 2018, vol. 54, no. 5, pp. 474–477. https://doi.org/10.1134/S0003683818050058
Skorik, Y.A., Kritchenkov, A.S., Moskalenko, Y.E., Golyshev, A.A., Raik, S.V., Whaley, A.K., Vasina, L.V., and Sonin, D.L., Carbohydr. Polym., 2017, vol. 166, pp. 166–172. https://doi.org/10.1016/j.carbpol.2017.02.097
Zhiryakova, M.V. and Izumrudov, V.A., Polym. Sci., Ser. A, 2008, vol. 50, no. 10, pp. 1057–1064. https://doi.org/10.1134/S0965545X08100064
Szafraniec-Szczęsny, J., Janik-Hazuka, M., Odrobińska, J., and Zapotoczny, S., Polymers, 2020, vol. 12, no. 9, pp. 1999–2024. https://doi.org/10.3390/polym12091999
Xian, J.L., J. Mol. Eng. Mater., 2017, vol. 5, no. 1, ID 1702001. https://doi.org/10.1142/S2251237317400019
Pharmacopoeia monograph FS.2.2.0020.15: Purified water, State Pharmacopoeia of the Russian Federation, Moscow, 2018, XIV ed.
Klenin, V.I., Termodinamika sistem s gibkotsepnymi polimerami (Thermodynamics of Systems with Flexible-Chain Polymers), Saratov: Saratovskii Univ., 1995.
Klenin, V.I., Shchegolev, S.Yu., and Lavrushin, V.I., Kharakteristicheskie funktsii svetorasseyaniya dispersnykh sistem (Characteristic Light Scattering Functions of Disperse Systems), Saratov: Saratovskii Univ., 1977.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated from Zhurnal Prikladnoi Khimii, No. 1, pp. 42–48, December, 2022 https://doi.org/10.31857/S0044461822010054
Rights and permissions
About this article
Cite this article
Bazunova, M.V., Mustakimov, R.A. & Bakirova, E.R. Conditions of Formation of Stable Polyelectrolyte Complexes Based on N-Succinyl Chitosan and Poly-N,N-diallyl-N,N-dimethylammonium Chloride. Russ J Appl Chem 95, 46–52 (2022). https://doi.org/10.1134/S1070427222010062
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427222010062