Abstract
From the point of view of aggregative stability, aqueous dispersions of particles of polyelectrolyte complexes based on chitosan hydrochloride and sodium salt of N-succinyl-chitosan were obtained and characterized. It is shown that, in the entire range of experimental conditions considered, these polyelectrolytes form an aggregately unstable disperse system. At the same time, the relative aggregative stability of the systems (the time before the formation of a precipitate of the polyelectrolyte complex) increases with an increase in the molecular weights of the polymers, with an increase in the molar ratio of the components, and with an increase in the concentration of polymers in the solution and reaches 30 days. The average particle size in the region of the relative aggregative stability of dispersed systems is 100–380 nm, which is promising for their use as lysed drug carriers for their targeted transport in the body.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In modern technologies of pharmaceuticals, much attention is paid to the development of safe systems for targeted drug delivery. Significant improvements in passive targeted drug delivery for pharmacological purposes, such as tumor cells and tissues, is effectively achieved through the use of polymer nanoparticles. The objectives of the development and application of nano- and microparticles in a biomedical application are to overcome tissue barriers, reduce the side effects of medicinal compounds (toxicity, allergenicity), and increase the efficiency by prolonging the action and, most importantly, achieving their targeted delivery to the cells and tissues of the body. Thus, nanoparticles with sizes of about 10–400 nm tend to accumulate in tumors owing to an increase in blood circulation and a decrease in lymphatic drainage of tumor tissues. Among the many types of nanoparticles as carriers for passive delivery of drug compounds, polymer particles based on natural polysaccharides have undoubted advantages. This is due to the biocompatibility, biodegradability, diverse intrinsic biological activity, and nontoxicity of the polysaccharides themselves, as well as the wide possibilities of their chemical modification and the presence of relatively simple methods for producing nanoparticles based on them [1–5]. Nanoparticles based on chitosan and its derivatives are noted among the most promising carriers in connection with a wide spectrum of its biological activity.
Many ways to produce chitosan-based nanoparticles are described [5–22]

for example, ionotropic gelation [8, 10–12], layering of polymers on preformed polymeric or inorganic nanoparticles [9, 13, 14], etc. At the same time, interest in self-organizing chitosan-based nanomaterials (microparticles, gels, films, etc.) continues unabated [11–13, 16–18, 20, 22]. Their advantages are associated with simpler implementation and no need to use previously obtained nanoparticles or additional physical effects, in particular, with ultrasonic dispersion. The self-assembled nano-objects also include polyelectrolyte complexes (PEC) of chitosan with oppositely charged polyions, which are formed in a joint solution by the mechanism of electrostatic interaction of oppositely charged ionogenic groups with the formation of saline PEC. Here we studied the influence of the nature of polyelectrolytes, their molecular characteristics, and the molar ratio of polymers in the total solution and the influence of the medium on the phase behavior of systems and determined the conditions in which PEC is formed as a dispersed system of nano- and microparticles [2, 23].
In the series of chitosan PECs studied with polysaccharides, complexes with such polyanions as Na alginate, hyaluronic acid, polyglutamic acid, carrageenans, and functional derivatives of chitosan itself, for example, sulfated chitosan, are described. From this point of view, of course, an affordable and practically significant product, the Na-salt of N-succinyl-chitosan, is interesting as a component of PEC.

The biological activity of N-succinyl-chitosan and the possibility of its use for immobilization of medicinal compounds are studied quite widely. It has been shown [24, 25] that N-succinyl-chitosan is safe when administered intravenously, which is positively different from chitosan, which causes hemolysis [26]. It has been established that it is nontoxic [27, 28] and more resistant to biodegradation [29] and possesses anticoagulant and antiplatelet and antioxidant activity [30]. N-succinyl-chitosan has been studied in the development of delivery systems for anticancer drugs—mitomycin C [27, 31, 32], taxanes [33].
As a rule, in the works devoted to the study of disperse systems of nano- and microparticles, their dimensional characteristics are given, more often the average sizes and the range of particle sizes, less often the curves of particle size distribution. At the same time, for dispersed PEC systems, the question of the dynamics of changes in the dimensional characteristics of particles with time is no less relevant. This is important from the point of view of permissible periods of storage and use of systems in the case of their practical application.
The purpose of this study was to obtain nano- and microdispersed interpolymer complexes of chitosan and N-succinyl-chitosan and to assess the aggregative stability of systems depending on the molecular characteristics of polyelectrolytes.
The tasks of the study included an assessment of the phase behavior of polyelectrolyte mixtures in aqueous solution over time and the determination of average particle sizes of complexes and changes in particle sizes depending on the ratios of molecular masses, the molar concentrations of components in the mixture, and the mass concentration of polymers, as well as the aggregative stability of systems.
EXPERIMENTAL
Samples of chitosan and sodium salt of N-succinyl-chitosan (M = 207 × 103) produced by Bioprogress (Shchelkovo, Russia) (TU 9289-067-00473124-03 and TU 9284-027-11734126, respectively) were used in the work. The sample of N-succinyl-chitosan with a molecular weight of 26 × 103 was obtained by the method of oxidative cleavage of high-molecular-weight polymer with a weight of 207 × 103 by hydrogen peroxide in aqueous solution, as described in [34]. It was believed that the content of acetamide, amino, and N-succinyl groups with the oxidative cleavage of macrochains did not change according to the law of the case. The degree of substitution of N-succinyl-chitosan was determined by ninhydrin analysis [35].
The degree of deacetylation of β chitosan was evaluated by acid-base titration with potentiometric determination of equivalence points on a TK100-B coulometric titrator according to the formula [36]
where m is the mass of chitosan in the sample (g), cNaOH is the concentration of sodium hydroxide solution consumed for titration of amino groups (mol/L), 203.2 is the molecular weight of the acetylated monomer unit of the polysaccharide, and 42.0 is the difference in molecular masses of acetylated and glucosamine monomer units. The pH of the solutions was determined using an ANION-410B pH meter.
Chitosan hydrochloride was obtained as a film from hydrochloric acid solution of chitosan (2 g of chitosan in 50 mL of 1% aqueous solution of hydrochloric acid) by drying in air and then under vacuum to constant weight. The molar ratio of hydrochloric acid : chitosan is 1.07.
The molecular weight of the studied polymers was determined by viscometry using a Ubbelohd viscometer at 25 ± 0.1°C in acetate buffer (pH 4.5). The viscosity-average molecular weight was calculated using the Mark–Kuhn–Houwink equation [η] = 1.38 × 10–4M0.85 [37]. The molecular weight of the polymers was also found by the method of sedimentation equilibrium and was calculated as follows:
Here R = 8.3 J/mol K; T is the absolute temperature; the angular velocity of rotation of the ultracentrifuge rotor ω = 2πn rad/s (n is the number of revolutions of the rotor per minute); tanα is the slope angle of dependence Z/X on solution concentration c, where X is the distance from the axis of rotation of the rotor to the point of the curve in the picture and Z = dс/dX is the height of the gradient curve (concentration gradient) at a given point; (\(1 - v{{\rho }_{0}}\)) is the Archimedean multiplier (\(v\) is the specific partial volume of the polymer and ρ0 is density of the solvent). Characteristics of the samples used are given in Table 1.
Aqueous dispersions of PEC were obtained by adding to aqueous solutions of N-succinyl-chitosan dropwise an aqueous solution of chitosan hydrochloride with vigorous stirring (500 rpm) at a temperature of 25°C with an interval of 5 min between the introduction of portions. The composition of the reaction mixture was expressed by the molar ratio z

where [X] is the molar concentration of chitosan ionic units calculated taking into account the degree of deacetylation, [CX] is the molar concentration of N‑succinyl-chitosan ionic units calculated taking into account the degree of deacetylation and the degree of modification of amino groups, and VX and VCX are the volume of solutions of the corresponding components.
The composition of the reaction mixture starting from which the formation of PEC particles occurs and the growth of optical density begins was taken as zlimit. The mass concentration of chitosan and N-succinyl-chitosan in the solution was found by the ratio of the mass of the solute to the total mass of the solution taking into account the moisture content of the polysaccharides. The humidity of polysaccharides was 4–5%.
The phase behavior of the mixtures was assessed visually by the appearance of opalescence of the mixture of solutions of polyelectrolytes and by the formation of a precipitate of the complex. The particle size of the opalescent PEC systems was determined by laser scattering using a Sald 7101 (Shimadzu) instrument. The wavelength of the semiconductor laser is 375 nm. The working range of measurement of particle diameters is 10 nm–300 μm. The measurements were carried out in special quartz Sald-BC cells with mechanical vertical mixing in an inert atmosphere of argon. The result of calculation of the particle size is presented in the form of particle diameter D. The measurement error is 3%. The particle size for freshly prepared disperse systems was determined 24 h after preparation. In order to estimate particle sizes in time, samples were taken at regular intervals from the volume of the reaction vessel and the average particle size was found. The time until the formation of a visually observed PEC precipitate in the systems was taken as the time of stability.
The analyses were performed on the equipment of the Center for Collective Use Chemistry of the Ufa Institute of Chemistry of the Ufa Federal Research Center of the Russian Academy of Sciences.
RESULTS AND DISCUSSION
The choice of a pair of polysaccharides of chitosan hydrochloride—Na salt of N-succinyl-chitosan—in addition to their practical significance for biomedical applications is due to the fact that both components, being salts, are well soluble in a neutral aqueous medium. Chitosan–N-succinyl-chitosan aqueous mixtures have a pH of 6.5–7.0. This favorably distinguishes this system from many polysaccharide pairs, including chitosan, which has solubility and acts as a polycation only in an acidic medium, as, for example, in [38].
The addition to the polyanion solution of even small portions of the polycation solution was accompanied by phase separation, which was detected by the appearance of a stable opalescence of the overall system. The range of existence of dispersions of PEC particles of these polysaccharides is limited to the molar ratio zlimit = 0.8, above which, already in the process of mixing the components, precipitation of the complex is observed. This was the case for all investigated systems with different ratios of molecular masses of polymers. Another common characteristic of all systems was their thermodynamic instability. In all systems studied, even with z < zlimit, after some time, phase decomposition with precipitation of PEC was observed. However, the time to phase decomposition and the change in the average particle size during this time substantially depend on the ratio of the molecular masses of the polyanion and the polycation, the molar ratio of ionogenic groups, and the total concentration of the polymer solution. The reasons for this are undoubtedly of interest.
When considering the interaction of the studied polysaccharides upon mixing their solutions, it must be borne in mind that, depending on the concentration, the solutions of individual polymers may have a complex supramolecular structure. Thus, when studying solutions of high-molecular chitosan samples in thermodynamically good solvents, it was shown that already diluted solutions with polymer concentrations below the crossover point C* = 1/[η] are structured systems [39]. In the case of chitosan with M = 100 × 103 (X-2), the crossover point is 0.3 wt %; for N-succinyl-chitosan with M = 207 × 103 (CX-2) in aqueous solution, C* = 0.27 wt %, i.e., solutions of these polymers with a concentration of more than 0.3 wt % are systems of interacting macromolecules. In the case of relatively low-molecular-weight polysaccharides, the crossover points for solutions of individual polymers are 1.7 wt % for N-succinyl-chitosan with M = 26 × 103 (CX-1) and 1.4 wt % for chitosan with M = 30 × 103 (X-1). In solutions of nonionic polymers after exceeding C*, the formation of a three-dimensional fluctuation mesh of links begins, in which the nodes represent links of macromolecular sections. However, a feature of solutions of charged macromolecules is the presence of an extended concentration region of semidilute solutions, in which the macromolecular coils are already in contact, and there are no meshes of links [40, 41], which is caused by electrostatic repulsion of like-charged chains. Accordingly, when considering the processes of formation of particles of the dispersed PEC phase, one should proceed from the fact that, even in dilute solutions of relatively low-molecular-weight polymers CX-1 and X-1, not only individual PE macromolecules participate in the interaction with each other, but also bulk macromolecular associates that are in equilibrium with them. In this case, the salt bonds form only the charged groups accessible to the interaction on the surface of the associates. Each associate has a large number of surface charges, which makes it possible for it to bind with several oppositely charged associates. Obviously, this is precisely what causes the formation of rather large particles rapidly losing sedimentation stability. In solutions of mixtures of CX-1 and X-1 with a total mass concentration of polymers w = 0.17–0.56 wt % at z ≤ zlimit, PEC precipitation is observed already after 24 h from the moment of their receipt. The particle sizes of PEC determined within 1–2 h from the moment of mixing are in the range Dbeginning = 200–281 nm (Table 2). A weak direct dependence of the particle size on the molar ratio of polyelectrolytes in solution is observed.
An increase in the concentration of polymers in the solution, which increases the total number of interacting objects, should also lead to a similar result. However, the effect of the concentration of a solution of charged macromolecules is more complex. It was shown in [42] that the introduction of negatively charged colloidal particles (AgI sol) into a chitosan solution leads to an earlier formation of a network of entanglements and the disappearance of the concentration region corresponding to the contacting but not chained macromolecules owing to the neutralization of the charge of chitosan during the formation of interchain ionic bonds. The formation of the fluctuation mesh of links, i.e., structuring of the solution due to the appearance of an additional grid with PEC nodes, leads to a change in the mass transfer mechanism in the system from the translational to the reptational one.
Obviously, for this reason, the time of mass transfer in the process of formation of sedimentation unstable particles should increase and during this time PEC microparticles will be detected in the volume of the liquid phase. With increasing concentration of the polymer solution of a mixture of CX-1 and X-1 to ≈2.0 wt %, the time before the PEC precipitation increases to 3 days, and the initial particle size increases to 350–450 nm (Table 3).
In mixtures of CX-2 and X-2, differing in molecular weight, with z < zlimit, turbid systems are also formed immediately, which quickly disintegrate with the formation of precipitation, as well as mixtures of low-molecular-weight polyelectrolytes, if the partial concentration of each component in the solution is less than C* corresponding to it. However, if at least one component is used in the form of a semidilute solution, then, owing to the structuring of the solution because of the appearance of an additional grid with PEC nodes, the time until precipitation increases, in this case to 7 days (Table 4). In this case, a direct dependence of the initial particle size on the molar ratio of polyelectrolytes in the mixture is traced.
The CX-1–X-2 system has the greatest difference in molecular weights. This probably led to the possibility of obtaining the highest relative aggregative stability in the series of systems studied (up to 30 days). For example, systems CX-1–X-2 manifest a strong dependence of the aggregative stability of systems on the conditions of their production. The processes developing in time occur not only in the size of the interval until the PEC precipitates but also in the relative change in particle size with time depending on the molar ratio of the components of the mixture and the concentration regime of the total solution. Particle sizes .specific to systems derived from z < zbefore and aged 24 h from the moment of receipt (Table 5) are taken as Dbeginning. As can be seen, the initial average particle sizes lie in the range of 110–360 nm. In this case, as in the systems described above, direct dependences of the average particle size on the molar ratio of the components are observed, but the dependences for systems with different concentrations of high-molecular-weight CX-2 in solution are different. In dilute CX-2 solutions of polysaccharides with w = 0.08 wt %, average particle size increases linearly with z in the range of 0.5–0.8. Obviously, with a significant lack of chitosan as a blocking polyelectrolyte, a relatively small number of dispersed particles are formed and the probability of their participation in subsequent aggregation processes is relatively small. An increase in the chitosan: N-succinyl-chitosan ratio leads to an increase in the number of particles and, accordingly, to the probability of their involvement in the formation of larger aggregates.
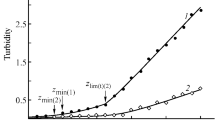
In solutions of any concentration with the same value of z over time, processes develop that manifest themselves in a relative change in particle size D/Dbeginning up to the moment of sedimentation (Fig. 1). In a dilute solution of polysaccharides with a significant lack of chitosan (z = 0.5) as a blocking polyelectrolyte, particles appear with the smallest size in this system, and over time the particle size increases by 3 times by the time the precipitate falls out (Fig. 1a) in a relatively short time before the formation of a precipitate (5 days). With an increase in the chitosan content, particles of a relatively large size form immediately, and the relative size of particles in the volume of the solution ceases to vary with time (z = 0.6) or slightly decreases (z = 0.7–0.8), while the time to precipitation increases to 15 days. In concentrated CX-2 solutions, only a relative decrease in particle size with time is observed (Fig. 1b), and the stability of the systems increases to 30 days.
The relative change in particle size of the polyelectrolyte complex X-2–CX-1 depending on the exposure time. The molar ratio of the components in the mixture z = 0.8 (1), 0.7 (2), 0.6 (3), 0.5 (4), 0.4 (5), and 0.2 (6); total mass concentration of polymers in solution w = 0.10–0.11 (a) and 0.92–0.96 wt % (b).
Thus, characteristic of the interaction of chitosan hydrochloride and the Na salt of N-succinyl-chitosan in an aqueous solution is the formation of aggregatively unstable systems with a dispersed phase of PEC particles, in which processes occur continuously leading to a change in the particle size and ultimately precipitation of the complex. The time to formation of precipitate mainly depends on the difference in molecular weights of lyophilizing and blocking polyelectrolytes and the total concentration of polymers in the solution. With a significant difference in molecular weights and total concentration ≥0.5 wt %, it is possible to obtain systems in which the dispersed phase of microparticles in a solution lasts up to 30 days. The average particle size in the region of relative aggregative stability of disperse systems is 100–380 nm, which is promising for their use as lysed carriers of medicinal media for their targeted transport in the body.
REFERENCES
M. A. Krayukhina, N. A. Samoilova, and I. A. Yamskov, Russ. Chem. Rev. 77, 799 (2008).
V. A. Izumrudov, I. F. Volkova, E. S. Grigoryan, and M. Yu. Gorshkova, Polym. Sci., Ser. A 53, 281 (2011).
M. Ishihara, H. Hattori, and S. Nakamura, Int. J. Pharma Bio Sci. 6, 162 (2015).
Q. Yuan, J. Shah, S. Hein, and R. D. K. Misra, Acta Biomater. 6, 1140 (2010).
J. Yang, S. Han, H. Zheng, H. Dong, J. Liu, Carbohydr. Polym. 123, 53 (2015).
A. Grenha, J. Drug Targeting 20, 291 (2012).
J. Venkatesan, S. Anil, S.-K. Kim, and M. S. Shim, Polymers 8 (2), 30 (2016).
X. Z. Shu and K. J. Zhu, J. Microencapsulation 18, 237 (2001).
N. Lia, C. Zhuang, M. Wang, X. Sun, S. Nie, and W. Pan, Int. J. Pharm. 379, 131 (2009).
A. A. Gubaidullina, G. I. Smagina, A. I. Melent’ev, and M. M. Alsynbaev, Biotekhnologiya, No. 5, 45 (2010).
N. G. Balabushevich, V. A. Izumrudov, and N. I. Larionova, Polym. Sci., Ser. A 54, 540 (2012).
E. A. Kirzhanova, M. A. Pechenkin, N. G. Balabushevich, and N. G. Balabushevich, Moscow Univ. Chem. Bull. (Engl. Transl.) 71, 127 (2016).
R. Guo, L. Chen, and S. Cai, J. Mater. Sci.: Mater. Med. 24, 2093 (2013).
M. Khorram, M. Samimi, A. Samimi, and H. Moghadam, J. Appl. Polym. Sci. 132, 2093 (2015).
A. Skorik, A. A. Golyshev, A. S. Kritchenkov, E. R. Gasilova, D. N. Poshina, A. J. Sivaram, and R. Jayakumar, Carbohydr. Polym. 162, 49 (2017).
Y. Yang, S. Wang, Y. Wang, X. Wang, Q. Wang, and M. Chen, Biotechnol. Adv. 32, 1301 (2014).
K. Barck and M. F. Butler, J. Appl. Polym. Sci. 98, 1581 (2005).
S. Boddohi, N. Moore, P. A. Johnson, and M. J. Kipper, Biomacromolecules 10, 1402 (2009).
A. Bernkop-Schnurch and S. Dunnhautpt, Eur. J. Pharm. Biopharm. 81, 463 (2012).
M. S. Gurina, R. R. Vil’danova, L. A. Badykova, N. M. Vlasova, and S. V. Kolesov, Russ. J. Appl. Chem. 90, 219 (2017).
S. B. Patil and K. K. Sawant, Colloids Surf., B 84, 384 (2011).
A. Rafiee, M. H. A. Taraneh Gazori, and F. Riazi-rad, Asian Pac. J. Trop. Dis. 4, 372 (2014).
A. B. Zezin and V. A. Kabanov, Rus. Chem. Rev. 51, 833 (1982).
Y. Kato, H. Onishi, and Y. Machida, Biomaterials 21, 1579 (2000).
A. A. Panevin, A. A. Golyshev, Y. A. Skorik, S. G. Zhuravskii, and D. L. Sonin, Pharm. Chem. J. 50, 711 (2017).
B. C. Dash, G. Rethore, M. Monaghan, K. Fitzgerald, W. Gallagher, and A. Pandi, Biomaterials 31, 8188 (2010).
Y. Song, H. Onishi, and T. Nagai, Int. J. Pharm. 98, 121 (1993).
M. Izume, Chitin Chitosan Res. 4, 12 (1998).
K. Kamiyama, H. Onishi, and Y. Machida, Biol. Pharm. Bull. 22, 179 (1999).
Y. A. Skorik, A. S. Kritchenkov, Y. E. Moskalenko, A. A. Golyshev, S. V. Raik, A. K. Whaley, L. V. Vasina, and D. L. Sonin, Carbohydr. Polym. 166, 166 (2017).
M. Sato, H. Onishi, J. Takahara, Y. Machida, and T. Nagai, Biol. Pharm. Bull 19, 1170 (1996).
Y. Song, H. Onishi, Y. Machida, and T. Nagai, J. Controlled Release 42, 93 (1996).
Y. A. Skorik, A. A. Golyshev, A. S. Kritchenkov, E. R. Gasilova, D. N. Poshina, A. J. Sivaram, and R. Jayakumar, Carbohydr. Polym. 162, 49 (2017).
N. I. Kupreev, D. V. Bykovskii, and V. A. Kuznetsov, RF Patent No. 2417088 (2011).
S. V. Nemtsev, V. M. Bykova, E. A. Ezhova, and S. A. Lopatin, in Proceedings of VIII International Conference “Modern Perspectives in Investigation of Chitin and Chitosan”, Moscow, Russia, 2006 (VNIRO, Moscow, 2006), p. 109.
Yu. A. Kuchina, N. V. Dolgopyatova, V. Yu. Novikov, V. A. Sagaidachnyi, and N. N. Morozov, Vestn. MGTU 15, 107 (2012).
A. I. Gamzazade, V. M. Slimak, A. M. Skljar, E. V. Stykova, S.-S. A. Pavlova, and S. V. Rogozin, Acta Polym. 36, 420 (1985).
L. A. Badykova, R. Kh. Mudarisova, I. M. Borisov, and M. S. Gurina, Russ. J. Appl. Chem. 89, 1126 (2016).
E. I. Kulish, V. V. Chernova, V. P. Volodina, and S. V. Kolesov, Polym. Sci., Ser. A 57, 508 (2015).
A. V. Dobrynin, M. Rubinshtein, and R. H. Colby, Macromolecules 28, 1859 (1995).
M. Muthukumar, J. Chem. Phys. 107, 2619 (1997).
M. V. Bazunova, D. R. Valiev, V. V. Chernova, and E. I. Kulish, Polym. Sci., Ser. A 57, 675 (2015).
Funding
The work was performed in the Institute of Chemistry UFRC RAS within the State task for 2017–2019 (AAAA-A17-117011910026-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolesov, S.V., Gurina, M.S. & Mudarisova, R.K. On the Stability of Aqueous Nanodispersions of Polyelectrolyte Complexes Based on Chitosan and N-Succinyl-Chitosan. Polym. Sci. Ser. A 61, 253–259 (2019). https://doi.org/10.1134/S0965545X19030076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965545X19030076