Abstract—Some results of studying the effect of the thermochemical activation of biomass on the degree and efficiency of the extraction of phenolic compounds from Juniperus communis L. wood with supercritical CO2 and various cosolvents are presented. Phenolic components in the obtained extracts were identified by high-performance liquid chromatography. It has been shown that the use of protonic cosolvents provides the extraction of much higher amounts of phenolic compounds, in particular, vanillin and vanillic acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Renewable plant feedstocks are rich in bioactive compounds (BACs), among which phenolic compounds are worth noting. These are characterized by a broad spectrum of bioactivity and have a favorable effect on the physiological state of a human organism. Their most important properties are antimicrobic (bactericidal, anticeptic) and anti-inflammatory [1]. The antioxidant activity of medical-grade feedstocks, which is successfully applied for the creation of both pharmaceutical preparations and bioactive supplements (BASs), is caused by the presence of phenolic compounds. The search for the sources of phenolic compounds characterized by different types of bioactivity is one of an urgent problem in pharmaceutical research. Some approaches to solving these problems are based on the study of plants that have been used in folk medicine for a long time. One such plant is Juniperus communis L., which is known for a high content of extractive compounds [2, 3] and, consequently, is of interest for the production of valuable BASs, including supplements of phenolic nature. Juniper extracts are traditionally used in folk medicine as a diuretic, antiseptic, antivirus, and anti-inflammatory remedy and also for the treatment of dermatological diseases [4]. Benzoic acid and eugenol contained in juniper extracts may be included into the composition of acesodyne, antitumour, cardioprotective, and biocidal preparations and anticeptics [5, 6].
In addition, juniper wood extracts can be used as an alternative to synthetic fungicides for the impregnation of other wood species susceptible to external effects, thus providing resistance to damage by white and brown rot and insects [7, 8]. It has been noted that hexane core wood extracts have more antimicrobial activity than bark and sapwood extracts, and ethanol extracts have a higher antifungal activity than the extracts obtained with the use of other solvents, such as methanol and hexane [7, 9].
One of the best known and widely used phenolic compounds is vanillin produced on an industrial scale. Among the earlier methods of vanillin production are the catalytic oxidation of wood, lignin, and lignin-containing wood-derived products, e.g., lignosulfonates [10–12]. At the present time, the synthesis of vanillin is generally performed on the basis of products of petrochemical plants [13], so the production of vanillin from renewable plant feedstocks without the use of hazardous and toxic reagents is an urgent scientific and practical problem.
The listed phenolic compounds are of undoubted interest from the viewpoint of different industry branches, thus explaining an increase in the number of efficient methods of their extraction from plant feedstocks that have been developed and procedures for their identification and quantitative determination with the engagement of contemporary analytical methods. Traditionally, BACs are extracted from plant feedstocks with the use of organic solvents, most of which are inflammable, explosive, and toxic, as well as not always being selective. For this reason, new approaches that would provide the possibility to manage without environmentally hazardous reagent and meet the main principles of green chemistry, have appeared. One such approach is based on the application of supercritical (SC) fluid extraction (SFE) with carbon dioxide, which provides the mild extraction of BACs due to the deep penetration of SC fluids into plant feedstocks [14, 15]. To increase the extraction selectivity, a binary solvent representing SC–CO2 with a cosolvent is often used, and the degree of the extraction of active natural compounds and the composition of obtained extracts depend on the type of cosolvent [16]. Many studies have been devoted to the effect of a cosolvent on the extraction of a target component from different materials, but the mechanism of this effect has not been completely clarified.
For the more efficient extraction of BAC, in particular, phenolic compounds, it is necessary to apply new extraction methods and technologies, which imply the stages of additional physiochemical effect on the capillary porous structure of plant biomass. In earlier studies of the fine structure of the wood matrix [3, 17], it has been shown that some specific features of juniper wood, such as its increased density and its absence of resin ducts necessitate the application of both chemical and thermochemical activation effects promoting the penetration of an extragent deep into the cell wall and the destruction of weak bonds in the lignin–carbohydrate complex. Such an effect may be the treatment by steam explosion (SE). This method consists in the short-term treatment of feedstocks with heated steam within a temperature range of 180–260°C at a saturated vapor pressure of 12–34 atm with an abrupt decrease in pressure to the atmospheric level (SE). The effect produced in this process on the lignin–carbohydrate matrix is determined by two components: mechanical (due to a local increase in pressure) and chemical (due to the interaction of formed organic acids with the cell wall components).
This paper studied the effect of the thermochemical activation of wood feedstock and the modifying additives incorporated into the binary solvent on the degree of the extraction of phenolic components and the quantitative and qualitative composition of Juniperus communis L. wood extracts.
EXPERIMENTAL
Object of Study
The selection of representative juniper (J. communis L.) wood samples was performed in compliance with GOST (State Standard) 16128-70 in the northern taiga zone; the age of the studied samples was 85 ± 5 years. Wood chips were obtained from juniper wood, which was preliminarily dried to an air-dried state and peeled from the bark and bast. The samples were chopped in a LM-201 laboratory rotary knife mill with a water cooling system preventing the wood from heating and modification. An average 1–2-mm fraction of sawdust was used for analyses.
Equipment and Treatment Conditions
The SC fluid extraction of wood samples with a binary solvent (SC–CO2 with a cosolvent) was performed on a SFE-5000 setup (Thar Process, United State). The treatment parameters were selected on the basis of the analysis in the literature [17, 18]. Extraction was conducted in the dynamic regime with the addition of protic (ethanol, acetic acid) and aprotic (dimethylsulfoxide or DMSO) cosolvents at the following process parameters: 120°C; 250 atm; treatment time, 1 h. The CO2 (liquid) flow rate was 25 mL/min, and the cosolvent flow rate was 5 mL/min; the volumetric flow rates were determined at a treatment pressure of 250 atm and a CO2 flow rate gauge temperature of 2.5°C.
The thermochemical activation of the wood matrix by SE was performed in the Laboratory of Ecoefficient Biomass Conversion of the Latvian State Institute of Wood Chemistry in the batch regime in a periodic apparatus with a volume of 0.5 L at a pressure of 31.5 atm and a temperature of 235°C for 3 min with further aqueous and alkaline extraction with a 0.4% NaOH solution [19].
Analysis of Supercritical Wood Extracts
The chromatographic analysis of phenolic compounds was performed on a Nexera X2 LC30AD system for superfast chromatography (Shimadzu, Japan). A Nukleodur PolarTec reverse phase column (150 × 3.0 mm, 3 μm) (Macherey-Nagel, Germany) was used for separation. A mixture containing 20 vol % of acetonitrile, 79.5 vol % of water, and 0.5 vol % of HCOOH was used as an eluent. Highly pure water of reagent grade with a specific resistance of 18.2 MΩ cm was obtained immediately before experiment in the Simplicity UV system (Millipore, France). The calibration solutions of analyzed compounds in acetonitrile were prepared via the consecutive dilution of the primary solution with a concentration of 0.90–1.00 g/L to concentration within a range of 0.1–50.0 mg/L. Chromatographic analysis conditions were selected in the previous studies [17]. The gradient elution regime was primarily used. However, all experiments in this work were performed in the isocratic elution regime due to the need to attain the ultimate simplicity and speed of analysis. The optimal conditions of chromatography have proven to be close to the conditions used in the work [20]. The identification and quantitative analysis of the components incorporated into the obtained extracts were performed against the reference samples with a quality of 98–99% (Sigma–Aldrich, Fluka).
The overall content of phenolic compounds was determined colorimetrically using the Folin–Ciocalteu reagent. The calibration curve was plotted against a reference compound (gallic acid). The absorbance was measured in 1 h after the calibration solutions, the analyzed sample of extracts, and the Folin–Ciocalteu reagent were mixed together on a UV-1800 spectrophotometer (Shimadzu) at 725–730 nm.
RESULTS AND DISCUSSION
Most of SC fluid extraction methods are based on the use of SC–CO2 cosolvent binary solvents. In these methods, a cosolvent is introduced into the system to make the process more selective and efficient. However, to understand the mechanisms of its interaction with the extracted compounds, it is necessary to take into account the following factors. First, CO2 is an aprotic solvent characterized by a low dielectric constant, a zero dipole moment, and a strong quadrupole moment, due to the two opposite dipoles of C=O bonds [21]. Quadrupole forces may be an important component of interaction between CO2 and other molecules in the system. Thus, when SC–CO2 is introduced as a protic cosolvent of alcohols, the polarity and basicity of the binary solvent grows, whereas its acidity remains almost unchanged. When aprotic cosolvents are used at almost unchanged basicity and acidity, the degree of the extraction of compounds containing highly polarizable functional groups is observed to grow [22].
Second, the introduction of acids, bases, or aprotic compounds into SC–CO2 as cosolvents changes the composition of the solvate shells of extracted compounds and, as a consequence, their reactivity.
Thus, according to Table 1, pKa of phenolic compounds grows by 5–8 points upon the transition from water to DMSO and by 5 points upon the transition to ethanol due to an increase in the free dissociation energy of phenols ΔGdis, determined as follows:
where \(\Delta G_{{{\text{dis}}}}^{{{\text{vac}}}}\) and ΔGdis are the change in the free dissociation energy of phenol in a vacuum and a given solvent, and \(\Delta G_{{\text{c}}}^{{{\text{PhOH}}}}\), \(\Delta G_{{\text{c}}}^{{{\text{Ph}}{{{\text{O}}}^{ - }}}}\), and \(\Delta G_{{\text{c}}}^{{{{{\text{H}}}^{ + }}}}\) are the change in the free energy of phenol in the transfer of phenol, a phenolate anion, and a proton from a vacuum into a solvent.
In [23, 24] it is shown that the solvation of phenolate anions is abruptly weakened with an increase in the DMSO content in the system, whereas the solvation of non-ionized phenol groups becomes stronger, thus leading to the growth of pKa for monomeric phenols. A similar effect is also observed when protic solvents, such as ethanol and acetic acid, are used. Hence, the introduction of cosolvents (DMSO, ethanol, acetic acid) weakens the protolytic properties of the phenols, which strengthens the solvation of relatively low-polar non-ionized phenolic forms, thus producing an increase in the efficiency and a change in the selectivity of their extraction. These facts are confirmed by the results given in Table 2.
It has been demonstrated that the traditional extraction with ethanol in a Soxhlet apparatus is least efficient with regard to the completeness of the extraction of phenolic compounds.
It is noteworthy that the studied solvents have an affinity to SC–CO2, but treatment is performed at a temperature below their critical point [2, 26]. For this reason, it is possible to presume that SC–CO2 acts a transporting agent in the binary solution due to its high penetrability and provides the better access of a cosolvent to the deep layers of the cell walls of plant feedstocks, thus appreciably broadening the spectrum of extracted compounds in comparison with traditional extraction in the Soxhlet apparatus (Table 2).
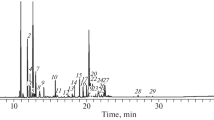
The introduction of protic solvents promotes the extraction of a much greater amount of phenolic compounds. Thus, the maximum extraction of vanillin (6.11 mg/L), vanillic acid (1.79 mg/L), and acetovanillone (1.75 mg/L) is attained in the process of SC extraction with the use of acetic acid as a cosolvent (Fig. 1). The use of ethanol as a cosolvent has proven to be less efficient. The use of DMSO in this guise leads generally to the extraction of vanillin, vanillic acid, and acetovanillon in the amounts of 2.20–2.36 mg/L (Fig. 2).
Experimental data show that the change in the extraction selectivity by the SC–CO2–cosolvent system is produced by some distinctions in solvation interactions. The obtained results agree with the literature data. Thus, the studies [2, 27, 28] of preferential solvation effects have demonstrated appreciable changes in the composition of solvate shells for guaiacil phenols after the change in the solvent polarity. It has been shown that a higher content of DMSO (the most basic solvent) is observed in the solvate shells of the non-ionized forms of the most polar compounds, such as vanillic acid, vanillin, and acetovanillon. Hence, the preferential solvation of these compounds with DMSO leads to their selective extraction. In the case of ethanol and acetic acid, the highest preferential solvation parameters are typical for vanillin, thus indicating its predominant extraction with their application as cosolvents.
It has also been demonstrated that the overall content of phenols is maximal for the extract obtained by SC extraction with the use of DMSO as a cosolvent. This may be explained by the presence of a great amount of high-molecular-weight compounds and lignin–carbohydrate complex destruction products, which are extracted from a sample, as DMSO is a good delignifying reagent [14].
As already mentioned [3, 17], the specific structural features of juniper wood necessitate preliminary activating effects. The method used for the preparation of wood for SC extraction is based on the structural transformation of the initial matrix by means of not only chemical, but also mechanical effect of SE. It has been shown that the mechanical component of wood matrix activation increases the amount of extracted compounds of phenolic nature by several times due to the modification of the capillary porous structure of the wood matrix and the appearance of a great number of cell wall areas accessible for extraction. In addition, SE is accompanied by the acidic hydrolysis of polysaccharides with the formation of organic acids (HCOOH and CH3COOH), which catalyze the cleavage of ester and residual hydrogen bonds of cellulose with a solid solution of hemicelluloses in lignin. These processes lead to the depolymerization of intercellular space lignin with the formation of low-molecular phenolic compounds and their further extraction from the cell wall [19]. It has also been noted that the compounds incorporating carbonyl and carboxyl groups, such as aldehydes and acids (vanillin and vanillic acid), are revealed in maximal amounts because of the oxidative processes occurring in the course of steam explosion due to the presence of atmospheric oxygen.
It has been demonstrated that the extract after the stagewise treatment of wood with ethanol contains a maximal amount of vanillin for all treatments.
Hence, the performed studies have shown that the preliminary modification of the capillary porous structure of the wood matrix is required to improve the efficiency of SC extraction, and the selection of an optimal cosolvent allows the selectivity of the extraction to be increased, along with the targeted control over the composition of obtained extracts. The stagewise treatment meeting the principles of green chemistry with the application of steam-explosion at the first stage and the SC extraction with the binary SC–CO2–ethanol solvent at the second stage promotes the recovery of a maximum vanillin amount.
REFERENCES
O. M. Andersen and K. R. Markham, Flavonoids. Chemistry, Biochemistry and Applications (CRC, Boca Raton, FL, 2006), p. 471.
R. P. Adams and A. N. Tasnev, Phytologia 95, 302 (2013).
K. G. Bogolitsyn, I. N. Zubov, M. A. Gusakova, et al., Planta 241, 1231 (2015).
G. Stanic, I. Samarzija, and N. Blazevic, Phytother. Res. 12, 494 (1998).
J. A. Baur and D. A. Sinclair, Nat. Rev. Drug Discov. 5, 493 (2006).
A. A. D’yakov, V. N. Perfilova, and I. N. Tyurenkov, Vestn. Aritmol., No. 39, 49 (2005).
I. Tumen, F. J. Eller, C. A. Clausen, and J. A. Teel, Bioresources 8, 12 (2012).
S. P. Mun and L. Prewitt, Forest Products J. 6, 443 (2011).
A. M. Clark, J. D. McChesney, and R. P. Adams, Phytother. Res. 4, 15 (1990).
V. E. Taraban’ko and N. V. Koropachshskaya, Khim. Rastit. Syr’ya, No. 1, 5 (2003).
O. D. Kamaldina and Ya. A. Massov, Production of Vanillin from Lignosulfonates (TsBTI TsINIS, Moscow, 1959) [in Russian].
US Patent No. 2692291A (1954).
R. Kumar, R. K. Sharma, and P. S. Mishra, Int. J. PharmTech Res. 4, 266 (2012).
K. G. Bogolitsyn, V. V. Lunin, D. S. Kosyakov, et al., Physical Chemistry of Lignin, Ed. by K. G. Bogolitsyn and V. V. Lunin (Akademkniga, Moscow, 2010) [in Russian].
C. G. Pereira and M. A. A. Meireles, Food Bioprocess Technol. 3, 340 (2010).
J. Shi, L. S. Kassama, and Y. Kakuda, in Functional Food Ingredients and Nutraceuticals: Processing Technologies (CRC, Boca Raton, FL, 2007), Vol. 13, p. 3.
K. G. Bogolitsyn, M. A. Gusakova, A. A. Krasikova, A. D. Ivakhnov, S. S. Khviuzov, D. G. Chukhchin, and I. N. Zubov, Russ. J. Phys. Chem. B 11, 1089 (2017).
A. M. Aliev, G. K. Radjabov, and A. M. Musaev, J. Supercrit. Fluids 102, 66 (2015).
K. Bogolitsyn, A. Krasikova, M. Gusakova, J. Gravitis, S. Khviuzov, D. Chukhchin, and I. Zubov, J. Indian Acad. Wood Sci. 13, 82 (2016).
D. V. Ovchinnikov, D. S. Kosyakov, and K. V. Ul’ya-novskii, Anal. Kontrol’ 18, 302 (2014).
E. J. Beckman, J. Supercrit. Fluids 28, 121 (2004).
V. T. Wyatt, D. Bush, J. Lu, J. P. Hallett, C. L. Liotta, and C. A. Eckert, J. Supercrit. Fluids 36, 16 (2005).
K. G. Bogolitsyn, N. S. Gorbova, and D. S. Kosyakov, Russ. J. Phys. Chem. A 77, 590 (2003).
K. G. Bogolitsyn, D. S. Kosyakov, and N. S. Gorbova, Russ. J. Phys. Chem. A 77, 1745 (2003).
M. I. Ermakova, M. F. Kiryushina, and M. Ya. Zarubin, Khim. Drevesiny, No. 5, 23 (1984).
D. A. Lemenovskii and V. N. Bagratashvili, Soros. Obrazov. Zh., No. 10, 36 (1999).
S. S. Khviyuzov, D. S. Kosyakov, K. S. Gorbova, and K. G. Bogolitsyn, Zh. Fiz. Khim. 86, 1640 (2012).
D. S. Kosyakov, K. S. Gorbova, K. G. Bogolitsyn, and L. V. Gusakov, Russ. J. Phys. Chem. A 81, 1076 (2007).
ACKNOWLEDGMENTS
This work was performed on the equipment of the Scientific Shared Facilities Center Arctic at Lomonosov Northern (Arctic) Federal University and the equipment of the Scientific Shared Facilities Center Critical Technologies of the Russian Federation in the Field of Arctic Environment Safety of the Laverov Federal Center for Integrated Arctic Research of the Russian Academy of Science.
Funding
The research was financially supported by the Federal Agency for Scientific Organizations of Russia within project no. AAAA-A18-118012390231-9 “Physicochemical, Genetic, and Morphological Foundations for the Adaptation of Plant Objects under Changing High-Latitude Climate Conditions.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Krasikova, A.A., Bogolitsyn, K.G., Gusakova, M.A. et al. Analysis of Phenolic Components in Supercritical Extracts of Juniperus communis L. Wood with High-Performance Liquid Chromatography. Russ. J. Phys. Chem. B 13, 1164–1168 (2019). https://doi.org/10.1134/S1990793119070169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793119070169