Abstract
Paulownia wood demand is increasing, but other parts of the tree remain underused. The leaves have medicinal properties, and their processing with a clean technology was explored. Supercritical fluid extraction (SFE) was proposed for the production of extracts from Paulownia elongata x fortunei leaves. Three isotherms (35 °C, 45 °C, and 55 °C) were studied in the pressure range of 10–30 MPa to assess their influence on the extraction yield and antiradical properties. The use of ethanol as cosolvent was also evaluated. A global extraction yield of 4 g extract /100 g of leaves was obtained at 30 MPa and 45 °C using 10% ethanol (w/w) as modifier; the last fractions reached up to 0.30 g Trolox eq./g extract. Serial extractions with different concentrations of ethanol (60, 70, 80, and 96%) were performed. The global yield obtained with 70% ethanol in three stages was 32.9 g extract/100 g leaves, and the antiradical capacity of the first stage extract was equivalent to 0.4 g Trolox/g extract. Extraction kinetics was studied, and overall extraction curves were represented using Sovová’s model.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Agricultural, food, and forest wastes have phytochemicals with commercial interest that could be recovered to provide added value to these secondary streams and has become an interesting subject of research [1]. Most species from Paulownia genus are widely known for their wood, which is light and flexible, does not crack or deform easily, and has considerable moisture resistance and flame-retardant properties. Wood industry has application in pulp and paper and manufacture of furniture, music instrument and handcrafts, or farm implements [2]. Furthermore, this genus comprises nine fast-growing species with good adaptability to poor soils and without competing for food crops [3]. Moreover, it is being evaluated as a bioenergy crop [4]. In the last years, sterile and non-invasive hybrid clones have been created to preserve their genetics and guarantee homogeneity in wood growth and quality. One of those clones is Paulownia elongata x fortunei developed by Cotevisa (Valencia) and named Paulownia Cotevisa 2®. Wood is the most used part, but valorization of other parts of the plant could contribute to sustainability following a circular economy approach. Paulownia leaves are rich in phenolic acids and flavonoids [5,6,7], and in other components, such as terpenoids and phytosterols [8]. The extractives from P. tomentosa leaves have shown metal chelating, antioxidant, antibacterial, and healing properties [6, 9]; both the extract composition and properties of the extracts are highly influenced by the extracting solvent [7].
The use of “green” solvents represents nowadays the preferred way to obtain bioactive compounds from agro-food industry wastes for food and nutraceutical applications [1]. Extraction of compounds from natural sources is the most studied application of supercritical fluids (SCF) [10], and supercritical (sc) CO2 extraction can be suitable to efficiently recover phenolics from plant materials [11]. Sustainable green technologies often involve the use of solvent extraction processes that rely on GRAS (Generally Recognized As Safe) solvents, the most commonly used being ethanol, water, and combinations of both [12]. Supercritical fluid extraction (SFE) with CO2 is considered a green technology because carbon dioxide can also be regarded a GRAS solvent due to its non-flammability, low toxicity, and availability [1, 13]. As far as the authors know, this technology has not been tried for this plant material.
The aim of this work is the valorization of leaves of the hybrid Paulownia elongata x fortunei by the extraction of bioactive compounds using supercritical CO2. A control experiment using a conventional ethanol serial extraction experiment was performed for the evaluation of extraction yield and the antiradical properties.
2 Materials and methods
2.1 Raw material
Paulownia elongata x fortunei leaves were collected in June 2017 from a plantation located in Nois (Foz, Lugo, Galicia, Spain) harvested by Maderas Álvarez Oroza. SL. The average moisture content was 74.12 ± 0.73% and was gravimetrically determined by oven-drying until reaching a constant weight (ISO 638/2008). Once collected, the leaves were dried at room temperature for 15 days with manually flip. Then, leaves were stored in airtight bags in boxes, in the absence of light. The final moisture content was 10 ± 0.39%. Leaves were milled with a grinder (Moulinex MC3001) and stored in a dark and dry place for further analysis.
2.2 Conventional ethanol extraction
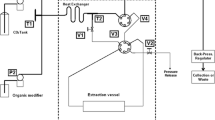
Serial kinetics assays were performed using ethanol at 96% in water as solvent. Paulownia leaves were contacted with ethanol at a liquid to solid mass ratio of 20:1 (v/w). Samples were maintained in a shaker at a 40 °C and 150 rpm for 24 h and were collected at different times (1, 2, 3, 5, 7, 9, and 24 h) and filtered, and the solids were put again in contact with 96% ethanol. This process was carried out for four times.
Paulownia elongata x fortunei conventional extraction with different concentrations of ethanol was performed. The same liquid to solid mass ratio, agitation, and temperature than in previous assays were established. The concentrations of ethanol in water tested were 60%, 70%, 80%, and 96% (v/v). After 24 h, a filtration process was carried out to separate the solid and the liquid phases. Liquid phase was reserved to analyse, and the solid was contacted again with ethanol at the same concentration in order to repeat the process. Serial extractions were carried out in five stages. All experiments were carried out in duplicate.
2.3 Supercritical extraction
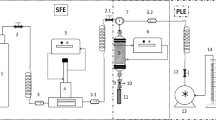
Paulownia leaves (20 g) were packed with glass beads in a 1000-mL extraction cell (Thar Process, Inc., USA) and extracted using supercritical CO2 (solvent mass flow was fixed at 25 g/min). The experiments were performed in duplicate at pressures in the range 10–30 MPa and at temperatures in the range 35–55 °C. Extraction time was fixed at 30 min. Extraction experiments with a polar modifier were also performed, using absolute ethanol at concentrations 2, 5, 10, and 15% wt%. Dynamic extractions were performed when the desired experimental conditions in the extractor were achieved.
Kinetic assays were performed during 3 h by collecting extract samples at pre-established time intervals. The overall extraction curves (OEC) obtained were evaluated using the mass transfer model described by Sovová [14]. This model is based on the assumption that part of the extractable material is easily accessible to the solvent because cell walls are broken after milling the samples, while the rest of the solutes remain trapped inside of the intact cells where the solvent have to penetrate by diffusion to dissolve the soluble compounds. Therefore, the extraction process could be divided into three different periods: (i) constant extraction rate (CER) period, where easily accessible solutes are extracted mainly by convection at a constant rate; (ii) falling extraction rate (FER) period, where mass transfer starts to be controlled by diffusion; and (iii) diffusion controlled (DC) period, in which easily extractable solutes have been removed and mass transfer is governed by diffusion. The extracted solute as a function of time is described by the following equations:
where:
being mext is the mass of extracted solute (kg), Q is the solvent flow rate (kg h−1), Ys is the solubility of the extract in the solvent (kg kg−1), x0 is the initial mass fraction of the extract in the inert material (kg kg−1), xk is the mass fraction inside unruptured cells (kg kg−1), mS is the mass of the inert solid material (kg), ρf is the density of the solvent (kg m−3), ρs is the bed density (kg m−3), ε is the bed porosity, kfa is the fluid-phase mass transfer coefficient (h−1), ksa is the solid-phase mass transfer coefficient (h−1), tCER is the extraction time at the end of the CER period (h), tFER is the extraction time at the beginning of the diffusional period (h), and YCER is the mass ratio of the extracted solute at the bed outlet during the CER period (kg kg −1).
The initial mass ratio (x0) was fixed as the asymptotic value at infinite time. YCER was employed as an initial estimation of Ys and then fitted to the experimental data along with the adjustable parameters of the model (kfa, ksa, xk) by minimizing the sum of least squares between the experimental and calculated values of mext. The fraction of broken cells r, also known as grinding efficiency and defined as
was employed as a model parameter instead xk, as it is expected to be practically constant in all the experiments since all the raw material was subjected to the same grinding process.
The absolute average relative deviation (AARD) given by Eq. (11) was used for error estimation:
where n is the experimental observation number and mexp and mcalc are the experimental and calculate extract mass, respectively.
2.4 Extracts characterization
Extraction yield was gravimetrically determined from triplicate analysis.
Total phenolic content (TPC) was determined following the Folin–Ciocalteu method described by Singleton and Rossi [15] using 0.5 mL extract or standard (gallic acid; Sigma), which were dispersed in 3.75 mL distilled water, followed by 0.25 mL Folin-Ciocalteu reagent diluted 1:1 (v/v,) and 0.50 mL sodium carbonate solution (10%, w/v). Samples were mixed and incubated for 1 h at room temperature in the darkness. The absorbance was measured at 765 nm. Results were expressed as grams of gallic acid equivalents (GAE) per gram of extract. All assays were carried out in triplicate.
Trolox equivalent antioxidant capacity (TEAC) was calculated to estimate the ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt radical cation scavenging capacity with the method proposed by Re et al., [16]. The ABTS•+ solution was diluted with phosphate buffer saline (PBS) (pH 7.4) to an absorbance of 0.70 at 734 nm. Aliquots of extract (10 μL) or 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were added to 1.00 mL diluted ABTS•+ solution. Samples were incubated in a water bath at 30 ± 2 °C for 6 min, and the absorbance was measured at 734 nm. Control samples with solvent and ABTS•+ solution were run in each assay, and all assays were carried out by triplicate. Results were expressed as Trolox equivalents antioxidant capacity (TEAC) value.
2.5 Statistical analysis
Significant differences between results were calculated by analysis of variance (ANOVA) using the software STATISTICA 8 (StatSoft. Inc. USA). The significant differences (p < 0.05) were evaluated by Tukey’s test. Mean values and their standard deviations were calculated and presented on the figure as error bars.
3 Results and discussion
3.1 Ethanol extraction
Four serial kinetics assays with ethanol at 96% were performed. Figure 1 a shows the global extraction yield obtained for each kinetic. It could be observed that in the first kinetic, the yield increased continuously for 24 h until reaching 14 g/100 g leaves. The same behaviour presented the second extraction, but, in this case, the growth was very light reaching 4.5 g/100 g leaves at 24 h. Nevertheless, extractions 3 and 4 exhibited a constant extraction yield in the time that varied between 2 and 3 g extract/100 g leaves. The phenolic extraction yield (Fig. 1b) presented a similar behaviour to the global extraction yield, increasing continuously during the first extraction until reaching values of 2.28 g GAE eq./100 g leaves. In the second extraction, the values obtained are lower than those of the first, 0.78 g GAE eq./100 g leaves, whereas in the following extractions a slight increase was observed in the first 9 h, reaching values in the range of 0.34–0.45 g GAE eq./100 g leaves. Regarding to the antioxidant activity of the extracts (Fig. 1c), the ABTS radical scavenging increased continuously in the first extraction up to a value of 56.8 g Trolox eq./100 extract. For the following extractions, the higher increase is shown in the first 9 h, reaching at 24 h values in the range of d 36–52 g Trolox eq./100 g extract.
Serial extractions at different concentrations of ethanol were tested for Paulownia leaves. Figure 2a shows that the yields obtained in first extraction were five times higher than those obtain in the second extraction. Note that maximum yield values were exhibited in extractions with ethanol at 70% and 80%, 23.2 and 23.9 g extract/100 g leaves, respectively. Second and third stages presented very similar yields that varied between 3.2 and 6.3 g extract /100 g leaves. Yields obtain in the third stage doubled the yields determined in fourth and fifth stages (0.3–1.9 g extract/100 g leaves). The total yield was maximal for 70% ethanolic extraction with 35.86 g extract/100 g leaves. In general, the total phenolic content (Fig. 2b) and the antioxidant capacity (Fig. 2c) were maximum in extractions with 80% ethanol. The total phenolic contents (TPCs) that were 2.6 and 0.5 g GAE eq./100 g leaves were reached in the first and second stages, respectively. In subsequent stages results obtain were lower than 0.5 g GAE eq./100 g leaves. The TPC values decreased with each extraction performed. Antioxidant capacity reached 43.73 and 52.10 g Trolox eq./100 g extract with ethanol at 80% in the first and second extraction, respectively. In the following extractions, values of 24.24 (3rd stage, 80% ethanol), 36.13 (4th stage, 80% ethanol), and 28.94 (5th stage, 96% ethanol) g Trolox eq./100 g extract were determined. The total phenol content in the first stage was in the range 10–15 g GAE eq./100 g extract for the different solvents and the TEAC value between 30 and 50 g Trolox eq./100 g extract, with maximum values for the highest solvent concentration.
Influence of the different ethanol concentrations (  60%,
60%,  70%,
70%,  80%,
80%,  96%) in serial extractions on the extraction yield (a), and total phenol content (b) and ABTS scavenging capacity (c) of the extracts from P. elongata x fortunei leaves. Significant differences (p < 0.05) at different ethanol concentrations for the same extraction step are denoted with different letters
96%) in serial extractions on the extraction yield (a), and total phenol content (b) and ABTS scavenging capacity (c) of the extracts from P. elongata x fortunei leaves. Significant differences (p < 0.05) at different ethanol concentrations for the same extraction step are denoted with different letters
Similar values of total phenol content and antioxidant capacity were reported in ethanol extracts obtained by conventional extraction by Ahmad-Qasem et al. [17] in olive leaves and by Jang et al. [18] in Oplopanax horridus leaves.
3.2 Supercritical extraction
3.2.1 Influence of pressure and temperature
The effect of the extraction pressure and temperature on the extraction yield is shown in Fig. 3a. The lowest extraction yields were obtained at 10 MPa, probably due to the lower density of CO2, which determines the solvent power, compared with the values at higher pressure. A positive influence of increasing pressure from 10 to 20 MPa was noticed regardless the operation temperature, whereas an increases in pressure from 20 to 30 MPa does not have significant influence on extraction yield. At constant pressure the effect of temperature on extraction yield is more complex as it can act by two opposing mechanisms: On the one hand, an increase in temperature increases solubility due to an increase in solute vapour pressure, but at the same time an increase in temperature decreases solubility due to a reduction in solvent density. At 10 MPa, an increase in temperature from 35 to 55 °C significantly decreases the extraction yield from 1.28 to 0.16 g extract/100 g leaves as a result of the decrease in solvent density, which varies from 716.6 to 348.3 kg/m3. At higher pressures there is no significant effect of the increase in temperature on the extraction performance despite the reduction in solvent density, suggesting that the effect of the solute vapour pressure is beginning to be significant.
Similar effect of pressure and temperature in the extraction of eugenol from clove was reported by Frohlich et al. [19]. At constant temperature an increase in extraction pressure resulted in a positive effect on the extraction yield whereas temperature had no significant effect on the yield in the pressure range of 18.5 to 22 MPa. In the supercritical extraction of vetiver roots, at 14 MPa an increase in temperature from 40 °C to 60 °C reduced the extraction yield from 1.63 to 1.35%, whereas at 20 MPa, the yield varied from 1.93 to 2.23% of leaves with the same increase of temperature [20].
The conditions leading to the highest extraction yields favour the extraction of compounds that are not active and the antioxidant activity is lower. The influence of the extraction pressure was more marked on the antiradical properties (Fig. 3b). At higher pressures, ABTS radical scavenging was favoured by the increase in temperature from 35 to 45 °C.
Similar behaviour was reported by Mazzutti et al. [21], who obtained the highest antioxidant activity values at 30 MPa, improving with the increase of temperature.
3.2.2 Influence of modifier concentration
Supercritical CO2 is a poor solvent for polar components and the extraction efficiency can be greatly improved by the addition of polar modifier. In the present work, ethanol was selected for its non toxic and biorenewable character. Figure 4a shows the influence of the addition of ethanol as polar modifier on the extraction yield at 45 °C. Although in Fig. 3b it is the temperature of 55 °C that obtains the best results also presents greater deviations. This would make it possible for the results at 55 and 45 °C could be similar. For this reason, the temperature of 45 °C was chosen, because it could avoid degradation of some compounds with lower energy consumption. Extreme pressure conditions have been studied for this temperature, observing the range of values obtained. According to the Tukey test performed, there are no significant differences in the values obtained with different proportions of ethanol as co-solvent neither at 10 MPa nor 30 MPa. Regarding the antioxidant activity of the extracts, the addition of a polar modifier enhanced significantly the ABTS at 30 MPa (Fig. 4b).The beneficial effect of the addition of ethanol was also reported for other leaves [20, 22].
Influence of the ethanol concentration as modifier on the extraction yield (a) and the antiradical properties, determined as TEAC value for ABTS scavenging capacity (b) of P. elongata x fortunei leaves at the conditions of 10 MPa, 45 °C (circle) and 30 MPa, 45 °C (triangle). Significant differences (p < 0.05) are denoted with different letters
3.2.3 Kinetic experiments and modelling
Kinetic experiments of the SFE of Paulownia leaves were investigated by fitting the extraction data at different operational conditions with the Sovová’s model [14]. The experimental OECs and the modelled results are shown in Fig. 5. The adjustable parameters for the model and the average absolute deviation obtained for each condition are presented in Table 1.
Overall extraction curves of paulownia leaves obtained by SFE: 10 MPa, 45 °C (circle); 30 MPa, 45 °C (triangle); and 30 MPa, 45 °C, 10% ethanol (square). Lines represents Sovová’s model fitting: (dash) Constant extraction rate period; (dots) falling extraction rate period; (dashed line) diffusion controlled extraction period. The standard deviation of the values represented is less than 10%
Three conditions for kinetics were proposed in order to compare the results between them when different conditions were modified at longer extraction time. One of these comparisons is focused on the different of pressures (10 MPa and 30 MPa). The other one consisted in observing the increase of yield when ethanol was used as cosolvent in comparison with the extraction without cosolvent.
The total yield after 360 min of extraction with pure CO2 increases from 0.17 to 0.85 g extract/100 g leaves as pressure increases from 10 to 30 MPa. The use of 10% ethanol as cosolvent caused a marked increase of the global yield of up to 4.15 g extract/100 g leaves. This indicates a positive effect of ethanol mainly due to the increased polarity of the solvent mixture enhancing the extraction of polar compounds [21]. As can be seen in Table 1, that addition of ethanol increases solubility by more than 3 times compared with pure CO2.
The OECs obtained shows the typical behaviour of SFE kinetics and can be divided in three regions: a constant extraction rate (CER), where the easily accessible solute is extracted and the mass transfer is mainly controlled by convection. Once the most easily solute has been extracted, a falling extraction rate (FER) period begins where a change in the mass transfer mechanism from convection to diffusion occurs. Finally, a diffusion-controlled rate (DC) occurs, where the mass transfer is governed only by diffusion mechanism [23].
The parameter r represents the easily accessible extract fraction and is affected by the sample pre-treatment process such as drying, grinding, or sieving [24]. In the present study the r-value remained practically constant around 0.5, for all the conditions tested, since the raw material was subjected to the same pre-treatment before supercritical extraction.
The mass transfer coefficients in the fluid phase, kfa, where higher than the mass transfer coefficients in the solid phase, ksa. The convective mass transfer mechanism is faster than the diffusion mechanism due to the difficulty associated with the extraction of solute from inside the solid compared with the extraction of solute from the surface of the particles. In general, the external mass transfer coefficient is two to three orders of magnitude greater than the internal coefficient [25]. As shown in Table 1, the effect of ethanol content on mass transfer coefficients seems to be negligible, whereas pressure affects to kfa and ksa in two opposite trends. ksa, increased with increasing pressure probably due to a decrease in solid phase resistance. Conversely, kfa decreases with increasing pressure, which may be due to a decrease in solvent velocity due to increased solubility resulting in an increase in resistance to matter transfer [26]. Similar pressure effect has been reported in the extraction of leaves and flowers of Galphimia glauca [27] or corn distiller’s dried grain [28].
The phenolic extraction yield profile of the kinetic assays is illustrated in Fig. 6a. At 10 MPa and 45 °C, the phenolic extraction yield remains practically negligible. The increase in pressure to 30 MPa leads to a slight increase in the extraction phenolic extraction yield, with a value of 0.011 g GAE eq./100 g leaves after 360 min of extraction. Regarding the impact of ethanol as a co-solvent, a great increase in the extraction yield of phenolic compounds is observed, probably due to the increased solvent power of the polar compounds given the increased polarity of the solvent by the addition of ethanol. A phenolic extraction yield of 0.16 g GAE eq./100 g leaves was obtained after 360 min.
The antioxidant activity of the fractions collected during SFE is illustrated in Fig. 6b. Antioxidant activity was not detected for the fractions collected at 10 MPa and 45 °C. In the case of extraction at 30 MPa and 45 °C the TEAC values of the fractions remained constant in the range of 1.5–4 g Trolox eq./100 g extract. Similar to the phenolic extraction yield, the antioxidant activity is strongly affected by the addition of ethanol as co-solvent. The fractions collected during the SFE with ethanol as modifier exhibited the highest antioxidant activity with an increasing trend reaching a maximum value of 31.61 g Trolox eq./100 g extract. The phenolic content of the fractions obtained using ethanol as co-solvent showed a close dependence on the ABTS radical scavenging capacity (Fig. 7), suggesting that the phenolic compounds extracted under this conditions represent a major contribution to the antioxidant activity.
4 Conclusions
The supercritical fluid extraction of compounds with antiradical properties from Paulownia elongata x fortunei leaves provided maximum extraction yields operating at 20 MPa and 35 °C, but higher temperatures and pressures favoured the ABTS radical scavenging capacities of the extracts. The addition of ethanol as a polar modifier increased the yields by 50% or more than 100% at 30 and 10 MPa, respectively, and doubled the ABTS radical scavenging at 30 MPa. The beneficial effect of increasing operation pressure and the addition of cosolvent was observed. The model proposed by Sovová [14] was used to describe the supercritical extraction kinetics. The predicted extraction curves adequately agreed with the experimental data for the investigated conditions.
Data availability
The data presented in this work is available.
References
Alexandre AMRC, Serra AT, Matias AA, Duarte CMM, Bronze MR (2020) Supercritical fluid extraction of Arbutus unedo distillate residues—impact of process conditions on antiproliferative response of extracts. J CO2 Util 37:29–38. https://doi.org/10.1016/j.jcou.2019.11.002
He T, Vaidya B, Perry Z, Parajuli P, Joshee N (2016) Paulownia as a medicinal tree: traditional uses and current advances. European J Med Plants 14:1–15. https://doi.org/10.9734/ejmp/2016/25170
Jiménez L, Rodríguez A, Ferrer JL et al (2005) Paulownia, a fast-growing plant, as a raw material for paper manufacturing | La Paulownia: Una planta de rápido crecimiento como materia prima para la fabricación de papel. Afinidad 62:100–105
Feria MJ, López F, García JC et al (2009) Energy and products by hydrolysis from forestry and industrial crops | Energía y productos de hidrólisis a partir de cultivos industriales y forestales. Afinidad 66:458–464
Singh MP, Park KH, Khaket TP, Kang SC (2018) CJK-7, a novel flavonoid from Paulownia tomentosa triggers cell death cascades in HCT-116 human colon carcinoma cells via redox signaling. Anti Cancer Agents Med Chem 18:428–437. https://doi.org/10.2174/1871520617666171026170009
Móricz ÁM, Ott PG, Knaś M, Długosz E, Krüzselyi D, Kowalska T, Sajewicz M (2019) Antibacterial potential of the phenolics extracted from the Paulownia tomentosa L. leaves as studied with use of high-performance thin-layer chromatography combined with direct bioautography. J Liq Chromatogr Relat Technol 42:282–289. https://doi.org/10.1080/10826076.2019.1585604
Pontaza-Licona YS, Ramos-Jacques AL, Cervantes-Chavez JA, López-Miranda JL, Ruíz-Baltazar ÁJ, Maya-Cornejo J, Rodríguez-Morales AL, Esparza R, Estevez M, Pérez R, Hernandez-Martínez AR (2019) Alcoholic extracts from Paulownia tomentosa leaves for silver nanoparticles synthesis. Results Phys 12:1670–1679. https://doi.org/10.1016/j.rinp.2019.01.082
Zhang D l, Li XQ (2011) Studies on the chemical constituents from the leave of Paulownia tomentosa. J Chinese Med Mater 34:232–234
Schneiderová K, Šmejkal K (2015) Phytochemical profile of Paulownia tomentosa (Thunb). Steud. Phytochem Rev 14:799–833. https://doi.org/10.1007/s11101-014-9376-y
Araújo MN, Azevedo AQPL, Hamerski F et al (2019) Enhanced extraction of spent coffee grounds oil using high-pressure CO2 plus ethanol solvents. Ind Crops Prod 141. https://doi.org/10.1016/j.indcrop.2019.111723
Díaz-Reinoso B, Moure A, Domínguez H, Parajó JC (2006) Supercritical CO2 extraction and purification of compounds with antioxidant activity. J Agric Food Chem 54:2441–2469. https://doi.org/10.1021/jf052858j
Vardanega R, Prado JM, Meireles MAA (2015) Adding value to agri-food residues by means of supercritical technology. J Supercrit Fluids 96:217–227. https://doi.org/10.1016/j.supflu.2014.09.029
Tamkutė L, Liepuoniūtė R, Pukalskienė M, Venskutonis PR (2020) Recovery of valuable lipophilic and polyphenolic fractions from cranberry pomace by consecutive supercritical CO2. J Supercrit Fluids 159:104755. https://doi.org/10.1016/j.supflu.2020.104755
Sovová H (1994) Rate of the vegetable oil extraction with supercritical CO2-I. Modelling of extraction curves. Chem Eng Sci 49:409–414. https://doi.org/10.1016/0009-2509(94)87012-8
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Ahmad-Qasem MH, Cánovas J, Barrajón-Catalán E, Micol V, Cárcel JA, García-Pérez JV (2013) Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov Food Sci Emerg Technol 17:120–129. https://doi.org/10.1016/j.ifset.2012.11.008
Jang M, Lee YC, Hong HD et al (2017) Anti-oxidative and anti-inflammatory activities of devil’s club (Oplopanax horridus) leaves. Food Sci Biotechnol 26:213–220. https://doi.org/10.1007/s10068-017-0029-y
Santos KA, Klein EJ, Fiorese ML, Palú F, da Silva C, da Silva EA (2020) Extraction of Morus alba leaves using supercritical CO2 and ultrasound-assisted solvent: evaluation of β-sitosterol content. J Supercrit Fluids 159:104752. https://doi.org/10.1016/j.supflu.2020.104752
Palsikowski PA, Besen LM, Santos KA et al (2019) Supercritical CO2 oil extraction from Bauhinia forficata link subsp. pruinosa leaves: composition, antioxidant activity and mathematical modeling. J Supercrit Fluids 153:104588. https://doi.org/10.1016/j.supflu.2019.104588
de Souza ARC, Guedes AR, Folador Rodriguez JM, Bombardelli MCM, Corazza ML (2018) Extraction of Arctium lappa leaves using supercritical CO2 + ethanol: kinetics, chemical composition, and bioactivity assessments. J Supercrit Fluids 140:137–146. https://doi.org/10.1016/j.supflu.2018.06.011
Cadena-Carrera S, Tramontin DP, Bella Cruz A et al (2019) Biological activity of extracts from guayusa leaves (Ilex guayusa Loes.) obtained by supercritical CO2 and ethanol as cosolvent. J Supercrit Fluids 152:104543. https://doi.org/10.1016/j.supflu.2019.104543
Braga MEM, Quispe-Condori S, Rosa PTV, Meireles MAA (2018) Mathematical modelling of turmeric compounds extraction using high pressurized solvents mixture. J Supercrit Fluids 140:348–355. https://doi.org/10.1016/j.supflu.2018.07.014
Confortin TC, Todero I, Canabarro NI, Luft L, Ugalde GA, Neto JRC, Mazutti MA, Zabot GL, Tres MV (2019) Supercritical CO2 extraction of compounds from different aerial parts of Senecio brasiliensis: mathematical modeling and effects of parameters on extract quality. J Supercrit Fluids 153:104589. https://doi.org/10.1016/j.supflu.2019.104589
Barzotto ILM, Santos KA, da Silva EA, Sene AC, da Silva NS, Vieira L (2019) Supercritical extraction of Eugenia involucrata leaves: influence of operating conditions on yield and Α-tocopherol content. J Supercrit Fluids 143:55–63. https://doi.org/10.1016/j.supflu.2018.08.003
Sodeifian G, Ghorbandoost S, Sajadian SA, Saadati Ardestani N (2016) Extraction of oil from Pistacia khinjuk using supercritical carbon dioxide: experimental and modeling. J Supercrit Fluids 110:265–274. https://doi.org/10.1016/j.supflu.2015.12.004
Verónico Sánchez FJ, Solis OE, Zamilpa A et al (2020) Extraction of Galphimines from Galphimia glauca with supercritical carbon dioxide. Molecules 25:477. https://doi.org/10.3390/molecules25030477
Ciftci ON, Calderon J, Temelli F (2012) Supercritical carbon dioxide extraction of corn distiller’s dried grains with solubles: experiments and mathematical modeling. J Agric Food Chem 60:12482–12490. https://doi.org/10.1021/jf302932w
Acknowledgments
The authors thank Ángel Álvarez for his help in collecting samples and Sheila Gómez for her technical assistance.
Code availability
Not applicable.
Funding
This work was funded by the Ministry of Economy, Industry and Competitiveness of Spain through the project CTM2015-68503-R and by the PIF grant BES-2016-076840.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Paula Rodríguez Seoane and Beatriz Díaz Reinoso. The first draft of the manuscript was written by Herminia Domínguez, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodríguez-Seoane, P., Díaz-Reinoso, B. & Domínguez, H. Supercritical CO2 extraction of antioxidants from Paulownia elongata x fortunei leaves. Biomass Conv. Bioref. 12, 3985–3993 (2022). https://doi.org/10.1007/s13399-020-01022-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01022-3