Abstract
The thermochemical activation of a juniper lignin–carbohydrate matrix was carried out using a steam explosion method in order to have an impact on the micro- and ultrastructure of coniferous wood. Changes in the chemical composition, the physico-chemical characteristics of the components and the morphological features of the wood substance during treatment were studied. The possibility of applying steam explosion as a method of directed influence upon the lignin–carbohydrate complex was shown. Thermochemical activation of lignin–carbohydrate plant matrix occurs due to the directed destruction of ester and H-bonds in the lignin–carbohydrate complex, disturbance of thermodynamic equilibrium in the system and increase of mobility of labile complexes of biopolymers. Such a directed influence allows the opening of deep layers of the cell wall and provides new data about the specific composition and structure of a wood substance and its components. Thus, the stability of the helical structures of cellulose microfibrils in the S2 layer and high mobility of intercellular lignin after the steam explosion treatment were shown.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Almost all areas of the use and processing of plant biomass have to take into account the specific features of the composition and structure of natural self-assembled plant matrices (Ansell 2015; Gorshkova et al. 2010; Bogolitsyn et al. 2010). However, the development of modern methods of complex chemical processing of wood should be based on fundamental investigations into wood composition, structure and the laws of transformation of wood components at the molecular and sub-molecular levels of organization.
Nowadays, different technologies are used for wood processing; one such technology is steam explosion (Alvira et al. 2010). Steam explosion (SE) has wide applications in modern industry as a way of producing pure cellulose (Sixta 2006) and extracting SE lignin, which can be used as an adhesive for plywood, self-binding fiber and particle board (Gravitis and Abolins 2013). SE, as a pre-treatment to increase the enzymatic accessibility of cellulose (Gravitis 1987; Jedvert et al. 2012; Muzamal and Rasmuson 2014; Muzamal et al. 2014), has been applied by the Andritz Company for bioethanol production.
The essence of steam explosion is short-term treatment of plant raw material by hot (180–260 °C) steam under pressure (1.2–3.4 MPa) with a subsequent pressure drop to atmospheric pressure (Gravitis 1987; Kallavus and Gravitis 1990; Muzamal and Rasmuson 2014; Muzamal et al. 2014; Bogolitsyn et al. 2014). It is known (Salmen et al. 2010; Bogolitsyn et al. 2010, 2015) that the lignin–carbohydrate matrix is a superposition of interpenetrating networks of lignin and hemicelluloses formed by H-bonds, C–C-bonds, ether bonds and lignin–carbohydrate bonds. The extra strength of this composition results from the mechanical bonds between macromolecules of lignin, hemicelluloses and cellulose. The impact of SE on the lignin–carbohydrate matrix consists of two components: mechanical (Tanahashi 1990; Jedvert et al. 2012; Bogolitsyn et al. 2014) and chemical (Gravitis 1987). The mechanical impact leads to the destruction of mechanical bonds between segments of lignin, cellulose and hemicellulose macromolecules because of local pressure increase. Additionally, the mechanical component of the treatment promotes the opening of deep cell wall layers (Kallavus and Gravitis 1990, 1995). The chemical impact is derived from the catalytic impact of formed acetic acid and the destruction of cell wall components (Muzamal and Rasmuson 2014; Muzamal et al. 2014; Bogolitsyn et al. 2014).
Even though steam explosion has been used for many years, there is still little knowledge of the detailed mechanisms of the process. Studies related to the steam explosion process have largely investigated chemical and physical changes in exploded wood (Tanahashi et al. 1982). However, in this author’s opinion, no studies have been carried out in order to investigate the changes taking place at the micro and nano levels of the wood matrix during its activation by the SE method. Meanwhile, steam explosion can be used not only as a method for complex processing of wood, but also as an approach for the deeper study and understanding of the morphological structure of wood. Thermochemical activation of the lignin–carbohydrate complex of wood during the SE treatment occurs due to the destruction of the thermodynamic equilibrium of the system and directed changes to the wood.
Thus, the aim of this report is to obtain new data about changes to the nano-, micro- and submolecular structure of the cell wall using SE as a method to influence the wood matrix and increase the thermochemical non-equilibrium of the lignin–carbohydrate complex. Scientific investigations into the processes taking place during SE treatment will facilitate the development of the fundamental basis of multi-stage technologies for complex processing of wood.
Juniper wood (Juniperus Communis L.) was chosen as the bio-object for this investigation as it is one of the hardest woods, in contrast to softer woods more widely used for this treatment, such as aspen (Gravitis 1987; Kallavus and Gravitis 1995), pine (Efremov and Krotova 1999), or spruce (Jedvert et al. 2012). As was noted in an earlier study (Bogolitsyn et al. 2015), juniper wood has a specific chemical composition (Zubov et al. 2012; Zubov 2013) and several specific morphological features such as a small thickness and length of tracheids, dense tracheid packing and the absence of resin ducts. These features explain juniper wood high density and resistance to different external influences. All the specific features listed above allow for decreasing the destructive constituent of steam explosion during the treatment process, performing directed thermochemical activation of the lignin–carbohydrate complex and minimizing the modification of its components.
Materials and methods
Raw material
The sampling of representative samples of juniper wood (Juniperus Communis L.) was done in the Northern taiga zone (Russia, 31°49′588″E, 61°40′058″N). The age of samples was 85 ± 5 years old.
For this investigation, the lower part of the trunk was used. Air-dried samples were ground in a laboratory rotary knife mill with water-cooling to prevent wood heating and modification. Then the sawdust was filtered through a sieve and the fraction of 1–2 mm was used.
SE treatment
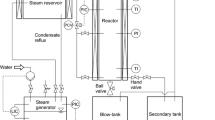
Steam explosion treatment was carried out at the Laboratory of Biomass Eco-Efficient Conversion (Latvian State Institute of Wood Chemistry). The biomass is treated with saturated steam at pressure of 3.2 MPa and temperature of 235 °C in a batch reactor with a capacity of 0.5 L in static mode. The treatment time is 3 min. After the treatment, within a split second, the biomass is decompressed (exploded) to the pressure of ambient atmosphere. The experimental steam explosion unit is shown in Fig. 1.
The steam is generated by heating water in the boiler. Upon reaching the necessary steam parameters the sample is filled into the reactor and treated by steam at needed temperature and pressure. After the treatment the sample is forced out into receiver where-from it further proceeds to the separation column with subsequent stages of water extraction, alkali extraction by 0.4 % NaOH solution and precipitation by HCl. Fractionation sequence of SE biomass is shown in Fig. 2.
Analysis of wood before and after treatment
The composition of the investigated samples (content of cellulose, lignin, ash, extractives by hot water and ethanol) was determined according to standard Tappi procedures (T222, T207, T204, T211).
UV-spectra of extracts were recorded within the range 190–400 nm using an ultraviolet spectrophotometer (UV-1800, Shimadzu, Japan).
FTIR-spectra of wood were registered using a Fourier transform infrared spectrophotometer (IRAFFINITY-1, Shimadzu, Japan) within the range 4000–400 cm−1 with resolution 4 cm−1 in KBr tablets. The intensity of a band at 1504–1515 cm−1 was chosen for characterisation of relative content of lignin (Sarkanen 1975; Karklin 1981a, b; Nuopponen et al. 2002). The band at 895 cm−1 was chosen as an internal standard.
Values of relative optical density of samples were calculated as K = D v /D vt , where Dv is optical density of component absorbance band; Dvt is optical density of internal standard absorbance band.
X-Ray analysis and determination of the degree of samples’ crystallinity were carried out using an X-ray diffractometer (XRD-7000, Shimadzu, Japan). For the investigation, we used wood tablets that were 0.6–0.8 mm thick. The tablets were made by pressing in the press-form (diameter of 25 mm). The X-ray tube parameters were as follows: accelerating voltage 50 kV; current 30 mA; target material Cu; scanning range of the 2θ angle from 10° to 70°; scanning speed 0.5°/min. The degree of crystallinity was calculated using X-ray diffractometer software.
SEM imaging
For investigation of ultramicro- and submolecular structure, longitudinal and cross sections and splits of juniper wood samples after cryomechanical sample preparation step were used. SE-treated wood samples were placed into a vacuum flask with liquid nitrogen and then freeze-dried using the Labconco (FreeZone 2.5 L) equipment. The images were obtained using a scanning electron microscope (SEM; Sigma VP ZEISS; accelerating voltage 20 kV, In Lens detector) and using an atomic-force microscope (ACM; MultiMode 8 Bruker AXS). In order to increase the image contrast the samples were sputter coated with gold. For this treatment, a Q150TES device manufactured by QUORUM was used.
Results and discussion
Changes to the chemical composition
According to current ideas, the mechanism of action of SE on the wood matrix includes deacetylation of hemicelluloses at high temperature with the formation of formic and acetic acids (Gravitis 1987; Jedvert et al. 2012). Hot steam easily penetrates into cell cavities and inner layers during SE, resulting in partial destruction of polysaccharides and extraction of low-molecular products of this destruction. This has been demonstrated by the decreased content of cellulose and hemicelluloses following SE (Table 1).
The destruction of hemicelluloses and extractives leads to the formation of low-molecular terpenes, alcohols, acids, phenols, etc. The main part of these compounds is removed with the steam-flue gas mixture at pressure drop and by washing with water in the first stage of extraction after SE treatment. The total amount of carbohydrates removed during SE treatment is 60 % of their initial content.
According to Gravitis (1987), the removal of hemicelluloses from diffuse regions in cellulose microfibrils leads to the breakage of cellulose macromolecules and therefore to a decrease in the degree of polymerization and an increase in cellulose crystallinity. This has been shown by X-ray diffractometry. In the case of juniper, SE also has an influence on the degree of crystallinity of cellulose, which increases from 30 to 39 % (Fig. 3). This process results in an increase in thermodynamic incompatibility due to the destruction of the network between lignin and carbohydrates, cell wall component stratification and phase changes.
According to the concept of the thermodynamic state of the lignin–carbohydrate matrix (Erinsh 1977; Bogolitsyn et al. 2010, 2015), the lignin of intercellular substance looks like spherical domains made of a solid solution of hemicelluloses in lignin and bonded to the carbohydrate matrix via H-bonds. These bonds can be easily destroyed even by weak organic acids. Therefore, the lignin in this a solid solution in comparison with the lignin of the cell wall is more mobile following external impacts on wood.
Organic acids (HCOOH and CH3COOH) formed during the acid hydrolysis of polysaccharides promote the destruction of ester and residual H-bonds between cellulose and the solid solution of hemicelluloses in lignin. Thus, the SE process occurs together with partial depolymerization of lignin with the formation of compounds with low molecular weight and lignin extraction from the cell wall. These processes are indicated by the data presented in Table 1.
Assumptions about the increased heterogeneity of the system and chemical and mechanical bond destruction during this treatment have been confirmed by the IR spectra of wood samples before and after SE (Fig. 4).
The experimental data show a decrease in the relative content of lignin in the wood matrix during SE treatment. Significant differences in the relative optical density were found when comparing untreated and SE-treated wood due to a decrease in the relative content of lignin (the K value is 0.498 for the sample after SE and 0.649 for the untreated sample).
Water and alkali extraction after steam explosion treatment
The further coalescence of fragments of cell wall layers and their individual components (products of the destruction of lignin and extractives) on the surface of fibrils takes place when the treatment stops (with a pressure drop) (Kallavus and Gravitis 1987, 1990; Gravitis 1987, 2005). Figure 5a confirms this effect.
The application of two-stage extraction of SE-treated wood by water and 0.4 % NaOH allows the complete removal of all precipitated components and fragments of cell wall layers from the surface of wood samples (Fig. 5b) and prepares the surface for further morphological investigations.
The analysis of the UV spectra of water and alkali extracts from two stages of extraction after SE treatment shows the presence of a significant amount of phenolic components in these extracts, which was confirmed by the characteristic peak in the region of 280 nm on the UV spectrum, corresponding to the lignin of conifer species (Fig. 6).
Previously, the lignin nature of such precipitated particles was reported in Kallavus and Gravitis (1987, 1990) and Gravitis (1987, 2005). After the SE-treatment lignin becomes softened and deposits in the sphere-like forms (size up to 1 µm) in the middle lamellae, lumen, and on the fibrils surface. The amount of lignin removed from juniper wood by the SE method under the given conditions is 50 % of its initial content in wood and corresponds to the data presented for deciduous and coniferous species (Efremov and Krotova 1999).
Effect of steam explosion treatment in the ultra-micro structure of juniper wood
For an estimation of the impact of SE and a visualization of the changes taking place in juniper wood, a study on the micro-, nano- and submolecular structure by scanning electron microscopy was performed. It should be noted that the suggested methods for sample preparation such as cryomechanical treatment (Bogolitsyn et al. 2015) and freeze-drying (Kallavus and Gravitis 1990, 1995) allow for obtaining high-quality splits of wood without additional difficulties while sample preparation and maintaining their three-dimensional structure. Figure 7 shows images of tracheids on the longitudinal and cross-sections before and after SE.
Steam explosion treatment results in the rupture or complete destruction of the cell walls of tracheids along the entire surface of the sample (Fig. 7d). This is likely due to the mechanical constituent of the SE process. The analysis of the images confirms the visual changes that took place to the cell wall structure during SE. There were spots of significant mechanical destruction on the sample surface due to the pressure gradient and the mechanical constituent of SE treatment.
High-temperature acid hydrolysis of H-bonds and ester bonds between lignin and carbohydrates leads to the almost complete removal of the primary cell wall (P) from the sample surface (Fig. 7b) and the dissolution of the components of the middle lamellae. The basis of these elements of the cell wall is formed by components with a lignin-like nature. Thus, the images confirm the lability of intercellular lignin and its removal during SE treatment (Fig. 7f). As shown in Fig. 7c, d, the removal of the tertiary layer W (verrucous layer) covering the internal cavities of tracheids occurs during SE, and so the S3 layer can be seen. The removal of the verrucous layer together with the primary layer may be explained by its phenolic nature. As shown by Fengel and Wegener (1984), the verrucous layer consists of a lignin-like substance with an admixture of carbohydrates and pectins.
Local effects lead to the rupture of the edge of pores, splitting of separate fibrils of the S1 layer and sometimes to the complete removal of the S1 layer. S1 removal promotes opening of the helical structure of the most voluminous S2 layer (Fig. 7d). It should be noted that the helical structure remains stable and is not destroyed, even under such harsh treatment.
Conclusions
SE can be used not only as separate method of processing plant raw material, but also as method of thermochemical activation of the lignin–carbohydrate matrix. Such activation allows for performing directed modifications to the cell wall for pre-treatment and further deep processing.
The labile and stable regions of the lignin–carbohydrate complex and their distribution were experimentally determined. This study showed the high mobility of the solid solution of hemicelluloses in interfibrillar lignin, which occurs due to the hydrolysis of weak ester and H-bonds without additional chemicals, and the high stability of helical structures of cellulose microfibrils in the S2 layer.
References
Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Ansell MP (2015) Wood microstructure—a cellular composite. Wood Compos. doi:10.1016/B978-1-78242-454-3.00001-9
Bogolitsyn KG, Lunin VV, Kosyakov DS et al (2010) Physical chemistry of lignin. Akademkniga, Moscow
Bogolitsyn KG, Gravitis J, Chukhchin DG et al (2014) Study of the wood substance’s morphological structure characteristics using the methods of steam explosion and supercritical fluid extraction treatment. In: Proceedings of 13th European workshop on lignocellulosics and pulp EWLP—2014. Seville, Spain, pp 231–234
Bogolitsyn KG, Zubov IN, Krasikova AA et al (2015) Juniper wood structure under the microscope. Planta 241(5):1231–1239
Efremov AA, Krotova IV (1999) Complex processing of wood wastes using the method of steam explosion. Khimiya rastitel’nogo syr’ya 2:19–39
Erinsh PP (1977) Structure and properties of wood as a multicomponent polymer system. Wood Chem 1:8–25
Fengel D, Wegener G (1984) Wood (chemistry, ultrastructure, reactions). Walter De Gruyter, Berlin
Gorshkova TA, Mikshina PV, Guryanov OP, Chemikosova SB (2010) Formation of the supramolecular structure of the plant cell wall. Biochemistry 175(2):196–213
Gravitis JA (1987) Theoretical and applied aspects of steam explosion of plant biomass. Wood Chem 5:3–21
Gravitis J (2005) First Latvian conference on nanomaterials and nanotechnologies. Riga
Gravitis J, Abolins J (2013) Biorefinery technologies for biomass conversion into chemical and fuels towards zero emissions: a review. Latvian J Phys Tech Sci 50(5):29–43
Jedvert K, Saltberg A, Lindström ME, Theilander H (2012) Mild steam explosion and chemical pre-treatment of Norway spruce. BioResources 7(2):2051–2074
Kallavus U, Gravitis J (1987) Changes in ultra-structure of fibers and re-distribution of lignin during steam explosion. Wood Chem 6:98–101
Kallavus U, Gravitis J (1990) On the impact of steam explosion on the ultra-structure of the fibers. Wood Chem 6:66–73
Kallavus U, Gravitis J (1995) A comparative investigation of the ultrastructure of steam exploded wood with light, scanning and transmission electron microscopy. Holzforschung 49:182–188
Karklin VD (1981a) IR-spectroscopy of wood and its main components. 15. Investigation of IR-spectra of dioxan-lignins of coniferous and deciduous wood. Wood Chem 4:38–44
Karklin VD (1981b) IR-spectroscopy of wood and its main components. 15. Investigation of IR-spectra of alkaline lignins of coniferous and deciduous wood. Wood Chem 4:45–49
Muzamal M, Rasmuson A (2014) Effectiveness of the rapid release of pressure during the steam explosion pretreatment. In: Proceedings of 13th European workshop on lignocellulosics and pulp EWLP—Seville, Spain, pp 591–594
Muzamal M, Gamstedt EK, Rasmuson A (2014) Modelling wood fiber deformation caused by vapour expansion during steam explosion of wood. Wood Sci Technol 48:353–372
Nuopponen M, Vyorykka J, Vuorinen T (2002) Chemical modification in heat-treated wood studied by FTIR, FTVIS and UV resonance Raman (UVRR) spectroscopies. In: Proceedings of 11th 7th European workshop on lignocellulosics and pulp—Turku, Finland, pp 19–22
Salmen L, Olsson AM, Stevanic JS, Simonovic J, Radotic K (2010) Structural organization of the wood polymers in the wood fibre structure. Arquitetura and Engenharia, Madeira, pp 47–57
Sarkanen KV (1975) Lignins. Forest industry, Moscow
Sixta H (2006) Handbook of pulp. Wiley, Weinheim
Tanahashi M (1990) Characterisation and degradation mechanisms of wood components by steam explosion and utilization of exploded wood. Wood Res 77:49–117
Tanahashi M, Takada S, Aoki T, Goto T, Higuchi T, Hanai S (1982) Characterization of explosion wood: 1. Structure and physical properties. Wood Res 69:36–51
Zubov IN (2013) Features of the formation of the carbohydrate matrix of coniferous species by the example of juniper. Dissertation, Arkhangelsk
Zubov IN, Khviyuzov SS, Lobanova MA, Gusakova MA, Bogolitsyn KG (2012) Influence of abiotic factors on the formation of carbohydrate matrix of juniper wood. Forest J 1:113–120
Acknowledgments
This research was funded from Federal Agency of Scientific Organizations of Russia under the Project Agreement No. 0410-2014-0029, and Latvian State Program “Forest and earth entrails resources: research and sustainable utilization—new products and technologies (ResProd)”. We used the equipment of Centre of Collective Use of Scientific Equipment «Arctic» (Northern (Arctic) Federal University) with funding from Ministry of Education of Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bogolitsyn, K.G., Krasikova, A.A., Gusakova, M.A. et al. Application of steam explosion as a method of wood matrix thermochemical activation. J Indian Acad Wood Sci 13, 82–89 (2016). https://doi.org/10.1007/s13196-016-0169-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13196-016-0169-3