Abstract
A series of bis-triazole conjugates, N-aryl-2-(4-{[(1H-1,2,3-benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)acetamides, have been synthesized via copper-catalyzed azide–alkyne cycloaddition, and their antitubercular activity against the human virulent H37Rv strain of Mycobacterium tuberculosis and antimicrobial activity against some gram-positive and gram-negative bacteria and fungal strains have been evaluated. The synthesized derivatives with a 2,4-difluorophenyl and 4-nitrophenyl group on the amide nitrogen atom were the most active against M. tuberculosis with excellent MIC, IC50, and IC90 values (12, 15, 16 µM and 48, 53, 62 µM, respectively). The highest antimicrobial activity was found for halogen-substituted compounds (MIC 0.24–16 µg/mL), while derivatives with electron-donating groups showed the lowest activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Tuberculosis (TB), an infection caused by Mycobacterium tuberculosis (MTB), remains the leading cause of worldwide deaths among infectious diseases [1]. The World Health Organization (WHO) reported that more than one-third of world’s population is infected with TB. The complete sequencing of the MTB genome was completed more than 10 years ago. Concurrently, the past decade has seen major progress in the understanding of TB and, as a result, several therapeutic leads have been identified to help contain the infection [2]. Recently, TMC207 (bedaquiline) was the first new anti-TB agent to be approved over 40 years [3]. However, TB remains persistently prevalent, resulting in approximately two million deaths every year. In the same way, the development of antimicrobials has been one of the major advances in medical science [4]. However, the emergence of drug-resistant populations of microorganisms has become a considerable cause of morbidity and death worldwide [5]. The design of new compounds to deal with resistant bacteria has become one of the most important areas of antibacterial research today. Five-membered nitrogen heterocycles exhibit a wide range of medicinal applications in the treatment of various diseases [6]. Among them, benzo-fused azoles containing three heteroatoms, such as benzoxadiazole, benzothiazole and benzotriazole [7, 8], have been extensively studied for their broad range of biological activity. However, few reviews focused on a single nucleus. Indeed, this paper aims to provide an overview of benzotriazole (BT)-based systems and their relevance in medicinal chemistry [9]. Although the main interest in BT is focused in the pharmaceutical field, suitably substituted benzotriazole derivatives can boost different biological properties, including pesticides, herbicides, and pharmaceuticals [10–17].
1,2,3-Triazoles proved to be among the most important nitrogen-containing five-membered heterocycles that have a wide range of applications in pharmaceuticals, supramolecular chemistry, organic synthesis, chemical biology, and industry [18]. 1,3-Dipolar cycloaddition of a 1,3-dipole to a dipolarophile (such as acetylene or alkyne) for the synthesis of 1,2,3-triazoles is a well-known transformation in synthetic organic chemistry, broadly known as “click chemistry approach” as prescribed by Sharpless; within the past few years it has become a premier component of synthetic organic chemistry [19].
In continuation of our earlier work on the synthesis and biological properties of various heterocyclic moieties [20, 21], herein, we report the synthesis of 1H-1,2,3-benzotriazole–1H-1,2,3-triazole conjugates via the click chemistry approach and their antitubercular and antimicrobial activities.
RESULTS AND DISCUSSION
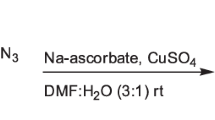
The synthetic pathway to target compounds 6a–6j is outlined in Scheme 1. Initially, compound 3 was synthesized by reacting 1H-benzotriazol-1-ol (1) with propargyl bromide (2) under drastic alkaline conditions in the presence of anhydrous potassium carbonate at 70°C for 4 h. On the other hand, N-aryl-2-azidoacetamides 5a–5j were prepared in more than 75% yield from the corresponding aromatic amines which were treated first with chloroacetyl chloride and then with sodium azide in anhydrous acetone in 2 h for each step in the temperature range between 0°C and room temperature. Finally, the cycloaddition of compounds 3 and 5a–5j was carried out in a 1:1:1 mixture of tert-butyl alcohol, water, and N,N-dimethylformamide in the presence of CuSO4 and sodium ascorbate to afford 1,2,3-triazole derivatives 6a–6j. The products were purified by standard flash column chromatography on silica gel (60–120 mesh) using hexane–ethyl acetate at a ratio of 8:2 as eluent.
The structure of compounds 6a–6j was confirmed by spectral data. The IR spectra of 6a–6j showed a strong absorption band at ~3340 cm–1 due to N–H stretching vibrations. The absorption band at ~3063 cm–1 corresponds to stretching vibrations of aromatic C–H bonds, and the band at ~1760 cm–1 was assigned to the amide carbonyl group. Moreover, the band at ~1365 cm–1 indicated the presence of triazole C–N bond. In the 1H NMR spectra of 6a–6j, the NH proton resonated as a singlet at δ ~10.52 ppm. Two signals at δ ~5.71 and ~5.34 ppm were attributed to the OCH2 and NCH2CO methylene groups, respectively. The other proton signals appeared at expected positions. The 13C NMR spectra of 6a–6j showed two peaks at δC ~51 and ~63 ppm due to methylene carbons at two sides of the triazole ring. The triazole ring carbons resonated in the aromatic region, at δC ~142 and ~128 ppm, which confirmed the cyclization. The positions of aromatic carbon signals were also consistent with the theoretical values. The mass spectra of compounds 6a–6j showed the molecular ion peaks with m/z values corresponding to their molecular weights.
The synthesized compounds were evaluated for their antitubercular activity against H37Rv strain of Mycobacterium tuberculosis as a part of contract no. HHSN272201100009I/HHSN27200002 A14 (NIAID division, USA and Department of Chemistry, Saurashtra University, Rajkot). The standard screening (primary in vitro) included determination of the MIC, IC50, and IC90 values (minimum inhibitory concentration and concentrations required for growth inhibition by 50 and 90%, respectively) [22]. As per the data in Table 1, out of ten tested compounds, 6d was found to exhibit excellent activity (MIC 12 µM, IC50 15 µM, IC90 16 µM). Compound 6j was found to be moderately active as compared to other derivatives (MIC 48 µM, IC50 53 µM, IC90 62 µM).
All newly synthesized compounds were also screened for their antimicrobial activity (Table 2) at the Department of Microbiology, School of Science, RK University, Rajkot. The minimum inhibitory concentrations (MIC, μg/mL) were determined by the microdilution method [23]. The MIC values of 6a–6j were found in the range of <0.24 to 16 µg/mL in comparison to the standard drug ampicillin (MIC 10 μg/mL). Compounds 6b, 6c, and 6e showed excellent antibacterial activity against Escherichia coli, Pseudomonas aeruginosa, and Enterococcus faecalis. Compounds 6a, 6d, and 6j showed moderate inhibition at low concentrations (0.49–16 µg/mL) compared with the standard drug in two gram-positive and one-gram negative strains. Compounds 6f–6i were not inactive against E. coli, P. aeruginosa, and E. faecalis. Compounds 6g, 6h, and 6i were efficient on one of the gram-positive strains, S. aureus, with MIC values ranging from 7.8 (6h) to 16 µg/mL (6g, 6i). In the antifungal study, compounds 6c and 6j exhibited a high activity against Candida albicans (MIC 3.9 µg/mL), while 6a and 6b showed moderate activity (MIC ~16 µg/mL). None of the other synthesized molecules was found to exhibit a good antifungal activity as compared to fluconazole as a standard drug.
We also analyzed the influence of the electronic properties of substituents on the phenyl ring on the antimycobacterial activity. As seen in Table 1, only two compounds, 6d and 6j, showed promising activity as compared to other analogs. The results of antimicrobial and antifungal screening are quite unexpected, and compounds with halogen and nitro substituents, as well as a pyridine moiety, showed fairly high activity against some specific gram-positive and gram-negative bacterial strains (E. coli, P. aeruginosa, and E. faecalis), while compounds with electron-donating groups showed comparable potency in only one bacterial strain (S. aureus). Only four compounds with a nitro group or a single halogen atom were potent in the antifungal strain (Table 2).
EXPERIMENTAL
The melting points were determined on a Büchi melting point apparatus. Thin-layer chromatography (TLC) was carried out on Eastman silica gel sheets with fluorescent indicators using hexane–ethyl acetate (3:7) as eluent; visualization of the chromatograms was done under UV light. The IR spectra were measured with a Shimadzu IR-435 spectrometer equipped with an ATR accessory. Elemental analysis was performed with a Hekatech Euro EA CHNSO analyzer, and the results were in good agreement (±0.3%) with the calculated values. Positive ion electrospray mass spectra were recorded on an Agilent 7820A/5977B GC/MS instrument (Santa Clara, CA, USA). The 1H and 13C NMR spectra were recorded in DMSO-d6 with a Bruker Avance II spectrometer at 400 and 100 MHz, respectively, using tetramethylsilane as internal reference. Chromatographic fractions were collected with a Büchi Rotavapor R-210 evaporator. Commercial reagents and solvents were not purified before use unless otherwise stated.
1-(Prop-2-yn-1-yloxy)-1H-benzotriazole (3). A dry round-bottom flask was charged with 1H-benzotriazol-1-ol (1, 1.6 g, 0.011 mol), propargyl bromide (1.67 g, 0.014 mol), anhydrous potassium carbonate (4.90 g, 0.035 mol), and DMF (6.5 mL), and the mixture was stirred at 70°C. After completion of the reaction (TLC, ethyl acetate–hexane, 3:7), the mixture (it turned to greenish black) was poured into crushed ice–water (25 g), and the solid product was filtered off with suction and dried.
N-Substituted 2-azidoacetamides 5a–5j (general procedure). A vacuum-dried round-bottom flask was charged with a solution of N-substituted 2-chloroacetamide 4a–4j (1.2 g, 0.005 mol) in 5 mL of acetone, the solution was cooled to 0°C, sodium azide (1.09 g, 0.016 mol) was added, and the mixture was stirred at room temperature for 2 h. After completion of the reaction (TLC, ethyl acetate–hexane, 3:7), the mixture was poured into crushed ice–water (10 g), and the solid product was filtered off using a vacuum pump. The formation of 4a–4j was confirmed by TLC (yellowish–reddish spot appeared in iodine vapor).
N-Substituted 2-(4-{[(1H-benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)acetamides 6a–6j (general procedure). A mixture of compound 3 (1 g, 0.0057 mol), azide 5a–5j (1.12 g, 0.0057 mol), sodium ascorbate (1.37 g, 0.007 mol), and CuSO4·5H2O (1.82 g, 0.012 mol) in H2O–t-BuOH–DMF (1:1:1, 4.5 mL) was stirred at room temperature for 3 h. After completion of the reaction (TLC), the mixture was poured into ice-cold water, and the solid product was filtered off, dried, and purified by column chromatography on silica gel (60–120 mesh) using ethyl acetate–hexane (8:2) as eluent.
2-(4-{[(1H-Benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)-N-(4-nitrophenyl)acetamide (6a). Yield 90%, light green solid, mp 180–182°C. IR spectrum, ν, cm–1: 3294 (N–H), 3093 (C–Harom), 1705 (C=O), 1573, 1350 (NO2), 1303 (C–N), 1218 (C–O). 1H NMR spectrum, δ, ppm: 11.08 s (1H, NH), 8.40 s (1H, 5-H), 8.28 d (2H, Harom), 8.05 d (1H, Harom), 7.83 d (2H, Harom), 7.53 d (2H, Harom), 7.44 d (1H, Harom), 5.74 s (2H, CH2), 5.44 s (2H, CH2). 13C NMR spectrum, δC, ppm: 170.5, 156.1, 148.9, 147.6, 140.3, 139.5, 133.4, 132.1, 129.5, 129.5, 128.1, 124.3, 124.3, 117.0, 108.7, 61.5, 52.3. Mass spectrum: m/z 394 [M]+. Found, %: C 52.70; H 3.10; N 28.20; O 16.00. C19H19N7O2. Calculated, %: C 51.78; H 3.58; N 28.42; O, 16.23.
2-(4-{[(1H-Benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)-N-(4-chlorophenyl)acetamide (6b). Yield 80%, light yellow solid, mp 190–192°C. IR spectrum, ν, cm–1: 3279 (N–H), 3124 (C–Harom), 1689 (C=O), 1365 (C–N), 1234 (C–O), 825 (C–Cl). 1H NMR spectrum, δ, ppm: 10.61 s (1H, NH), 8.39 s (1H, 5-H), 8.05 d (1H, Harom), 7.62 d (2H, Harom), 7.53 d (2H, Harom), 7.42 d (3H, Harom), 5.71 s (2H, CH2), 5.36 s (2H, CH2). 13C NMR spectrum, δC, ppm: 169.6, 158.1, 143.9, 139.2, 137.6, 134.0, 133.5, 133.5, 130.5, 129.0, 125.8, 125.8, 122.9, 112.1, 111.4, 59.2, 52.3. Mass spectrum: m/z 383 [M]+. Found, %: C 52.20; H 4.70; N 25.50; O 8.50. C17H14ClN7O2. Calculated, %: C 53.20; H 3.68; N 25.55; O 8.34.
2-(4-{[(1H-Benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)-N-(4-fluorophenyl)acetamide (6c). Yield 60%, light yellow solid, mp 184–186°C. IR spectrum, ν, cm–1: 3309 (N–H), 3070 (C–Harom), 1681 (C=O), 1365 (C–N), 1219 (C–O), 1057 (C–F). 1H NMR spectrum, δ, ppm: 10.52 s (1H, NH), 8.39 s (1H, 5-H), 8.04 d (1H, Harom), 7.60 t (2H, Harom), 7.52 d (2H, Harom), 7.43 t (1H, Harom), 7.20 t (2H, Harom), 5.72 s (2H, CH2), 5.34 s (2H, CH2). 13C NMR spectrum, δC, ppm: 171.5, 162.4, 155.3, 145.1, 136.8, 135.0, 133.9, 128.9, 127.2, 127.2, 120.3, 119.7, 119.7, 116.0, 108.3, 60.9, 51.3. Mass spectrum: m/z 367 [M]+. Found, %: C 56.20; H 3.90; N 25.79; O 8.88. C17H14FN7O2. Calculated, %: C 56.58; H 3.84; N 26.69; O 8.71.
2-(4-{[(1H-Benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)-N-(2,4-difluorophenyl)acetamide (6d). Yield 75%, light yellow solid, mp 190–192°C. IR spectrum, ν, cm–1: 3340 (N–H), 3001 (C–Harom), 1705 (C=O), 1334 (C–N), 1242 (C–O), 1095 (C–F). 1H NMR spectrum, δ, ppm: 10.52 s (1H, NH), 8.38 s (1H, 5-H), 8.05 s (1H, Harom), 7.89 s (1H, Harom), 7.52 s (2H, Harom), 7.41 t (2H, Harom), 7.03 s (1H, Harom), 5.73 s (2H, CH2), 5.46 s (2H, CH2). 13C NMR spectrum, δC, ppm: 168.3, 164.9, 157.6, 154.0, 142.2, 137.3, 133.8, 129.1, 128.3, 124.9, 120.8, 117.6, 115.0, 113.4, 110.1, 61.9, 53.1. Mass spectrum: m/z 385 [M]+. Found, %: C 53.98; H 2.30; N 26.45; O 8.07. C17H13F2N7O2. Calculated, %: C 52.99; H 3.40; N 25.45; O 8.30.
2-(4-{[(1H-Benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)-N-(3-chlorophenyl)acetamide (6e). Yield 50%, yellow solid, mp 182–184°C. IR spectrum, ν, cm–1: 3309 (N–H), 3063 (C–Harom), 1760 (C=O), 1365 (C–N), 1265 (C–O), 833 (C–Cl). 1H NMR spectrum, δ, ppm: 10.67 s (1H, NH), 8.38 s (1H, 5-H), 8.05 d (1H, Harom), 7.78 s (1H, Harom), 7.52 s (2H, Harom), 7.42 t (3H, Harom), 7.17 d (1H, Harom), 5.73 s (2H, CH2), 5.37 s (2H, CH2). 13C NMR spectrum, δC, ppm: 170.5, 160.9, 144.5, 141.6, 135.0, 135.7, 133.9, 132.6, 129.4, 127.2, 124.9, 123.5, 122.0, 117.8, 107.2, 63.3, 51.6. Mass spectrum: m/z 383 [M]+. Found, %: C 52.40; H 4.25; N 25.55; O 8.55. C17H14ClN7O2. Calculated, %: C 53.20; H 3.68; N 25.55; O 8.34.
2-(4-{[(1H-Benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)-N-(4-bromophenyl)acetamide (6f). Yield: 52%, yellow solid, mp 188–190°C. IR spectrum, ν, cm–1: 3317 (N–H), 3063 (C–Harom), 1681 (C=O), 1396 (C–N), 1242 (C–O), 740 (C–Br). 1H NMR spectrum, δ, ppm: 10.60 s (1H, N–H), 8.38 s (1H, 5-H), 8.04 d (1H, Harom), 7.54 d (6H, Harom), 7.42 t (1H, Harom), 5.72 s (2H, CH2), 5.35 s (2H, CH2). 13C NMR spectrum, δC, ppm: 171.2, 156.3, 145.3, 138.9, 136.6, 134.8, 134.8, 134.5, 127.7, 126.5, 126.5, 123.2, 121.1, 114.8, 110.1, 59.9, 52.5. Mass spectrum: m/z 427 [M]+. Found, %: C 47.99; H 3.99; N 20.98; O 7.90. C17H14BrN7O2. Calculated, %: C 47.68; H 3.30; N 22.90; O 7.98
2-(4-{[(1H-Benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)-N-(3,5-dimethylphenyl)acetamide (6g). Yield 70%, crimson solid, mp 168–188°C. IR spectrum, ν, cm–1: 3243 (N–H), 3020 (C–Harom), 1717 (C=O), 1344 (C–N), 1262 (C–O). 1H NMR spectrum, δ, ppm: 10.68 s (1H, NH), 8.43 s (1H, 5-H), 8.05 t (2H, Harom), 7.64 d (2H, Harom), 7.43 s (2H, Harom), 7.05 s (1H, Harom), 5.57 s (2H, CH2), 5.41 s (2H, CH2), 3.38 s (6H, CH3). 13C NMR spectrum, δC, ppm: 171.4, 154.8, 145.1, 141.3, 141.3, 139.2, 137.9, 134.6, 132.0, 129.9, 120.2, 115.6, 115.6, 112.6, 106.2, 62.9, 51.3, 27.7, 27.2. Mass spectrum: m/z 377 [M]+. Found, %: C 59.43; H 5.79; N 24.79; O 9.99. C19H19N7O2. Calculated, %: C 60.47; H 5.07; N 25.98; O 8.48.
2-(4-{[(1H-Benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)-N-(4-methoxyphenyl)acetamide (6h). Yield 77%, off-white solid, mp 200–202°C. IR spectrum (ATR), ѵ, cm-1: 3317 (NH), 3124 (CH), 1678 (C=O), 1379 (CN), 1274 (C–O). 1H NMR spectrum, δ, ppm: 10.65 s (1H, NH), 8.39 s (1H, 5-H), 8.34 d (2H, Harom), 8.05 d (1H, Harom), 7.90 d (2H, Harom), 7.52 d (2H, Harom), 7.35 d (1H, Harom), 5.71 s (2H, CH2), 5.22 s (2H, CH2), 4.17 s (3H, OCH3). 13C NMR spectrum, δC, ppm: 171.8, 163.4, 158.0, 145.8, 138.6, 136.1, 133.3, 128.5, 124.1, 124.1, 123.3, 117.0, 115.8, 115.8, 110.6, 60.5, 51.3, 49.8. Mass spectrum: m/z 379 [M]+. Found, %: C 57.99; H 3.52; N 26.50; O 12.00. C18H17N7O3. Calculated, %: C 56.99; H 4.52; N 25.84; O 12.65
2-(4-{[(1H-Benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)-N-(pyridin-2-yl)acetamide (6i). Yield 90%, light yellow solid, mp 202–204°C. IR spectrum, ν, cm–1: 3365 (N–H), 3042 (C–Harom), 1702 (C=O), 1324 (C–N), 1216 (C–O). 1H NMR spectrum, δ, ppm: 10.89 s (1H, NH), 8.45 s (1H, 5-H), 8.28 s (1H, Harom), 8.05 d (2H, Harom), 7.69 s (1H, Harom), 7.54 d (1H, Harom), 7.38 d (2H, Harom), 7.21 d (1H, Harom), 5.83 s (2H, CH2), 5.32 s (2H, CH2). 13C NMR spectrum, δC, ppm: 172.6, 159.2, 156.9, 150.4, 143.9, 140.5, 135.9, 133.3, 128.2, 123.6, 119.0, 117.5, 114.3, 106.8, 62.2, 51.3. Mass spectrum: m/z 350 [M]+. Found, %: C 55.85; H 3.03; N 32.98; O 8.13. C16H14N8O2. Calculated, %: C 54.85; H 4.03; N 31.98; O 9.13
2-(4-{[(1H-Benzotriazol-1-yl)oxy]methyl}-1H-1,2,3-triazol-1-yl)-N-(5-nitropyridin-2-yl)acetamide (6j). Yield 92%, yellow solid, mp 210–212°C. IR spectrum, ν, cm–1: 3298 (N–H), 3162 (C–Harom), 1718 (C=O), 1580, 1317 (NO2), 1331 (C–N), 1245 (C–O). 1H NMR spectrum, δ, ppm: 10.62 s (1H, NH), 8.37 s (1H, 5-H), 8.10 d (1H, Harom), 7.96 t (1H, Harom), 7.65 d (2H, Harom), 7.58 s (1H, Harom), 7.34 d (2H, Harom), 5.68 s (2H, CH2), 5.32 s (2H, CH2). 13C NMR spectrum, δC, ppm: 171.3, 161.0, 154.1, 148.0, 145.9, 139.6, 137.5, 134.1, 133.6, 128.2, 122.6, 117.0, 114.7, 111.6, 61.2, 53.3. Mass spectrum: m/z 395 [M]+. Found, %: C 47.64; H 4.28; N 32.89; O 15.19. C16H13N9O4. Calculated, %: C 48.61; H 3.31; N 31.89; O 16.19.
REFERENCES
Singhania, A., Wilkinson, R.J., Rodrigue, M., Haldar, P., and O’Garra, A., Nat. Immunol., 2018, vol. 19, p. 1159. https://doi.org/10.1038/s41590-018-0225-9
Nikolayevskyy, V., Kranzer, K., Niemann, S., and Drobniewski, F., Tuberculosis, 2016, vol. 98, p. 77. https://doi.org/10.1016/j.tube.2016.02.009
Mirsaeidi, M., Int. J. Mycobacteriol., 2013, vol. 2, p. 1. https://doi.org/10.1016/j.ijmyco.2013.01.004
Abimbola, T.O., Marston, B.J., Date, A.A., Blandford, J.M., Sangrujee, N., and Wiktor, S.Z., JAIDS, J. Acquired Immune Defic. Syndr., 2012, vol. 60, p. 1. https://doi.org/10.1097/QAI.0b013e318246538f
Davies, J. and Davies, D., Microbiol. Mol. Biol. Rev., 2010, vol. 74, p. 417. https://doi.org/10.1128/MMBR.00016-10
Bello-Vieda, K.F., Nunez-Dallos, N.J., Pastrana, N.G., Celis, H.F., Restrepo, A.M.S., and Avila, A.G., J. Braz. Chem. Soc., 2016, vol. 27, p. 2334. https://doi.org/10.5935/0103-5053.20160130
Piccionello, A. and Guarcello, A., Curr. Bioact. Compd., 2010, vol. 6, p. 266. https://doi.org/10.2174/157340710793237308
Briguglio, I., Piras, S., Corona, P., Gavini, E., Nieddu, M., Boatto, G., and Carta, A., Eur. J. Med. Chem., 2015, vol. 97, p. 612. https://doi.org/10.1016/j.ejmech.2014.09.089
Kattimani, P.P., Kamble, R.R., Kariduraganavar, M.Y., Dorababu, A., and Hunnur, R.K., Eur. J. Med. Chem., 2013, vol. 62, p. 232. https://doi.org/10.1016/j.ejmech.2013.01.004
Davis, D., Science, 1954, vol. 120, p. 989. https://doi.org/10.1126/science.120.3128.989
Yurdakul, O., Kose, D.A., Şahin, O., and Avcı, G.A., J. Mol. Struct., 2020, vol. 1203, article ID 127265. https://doi.org/10.1016/j.molstruc.2019.127265
Boido, A., Boido, C.C., and Sparatore, F., Il Farmaco, 2001, vol. 56, p. 263. https://doi.org/10.1016/S0014-827X(01)01033-3
Paglietti, G., Sanna, P., Carta, A., Sparatore, F., Vazzana, I., Peana, A., and Satta, M., Farmaco, 1994, vol. 40, p. 693. PMID: 7832971
Kaushik, C.P., Kumar, K., Lal, K., Narasimhan, B., and Kumar, A., Monatsh. Chem., 2016, vol. 147, p. 817. https://doi.org/10.1007/s00706-015-1544-2
Lopez-Vallejo, F., Castillo, R., Yepez-Mulia, L., and Medina-Franco, J., J. Biomol. Screening, 2011, vol. 16, p. 862. https://doi.org/10.1177/1087057111414902
Borowski, P., Deinert, J., Schalinski, S., Bretner, M., Ginalski, K., Kulikowski, T., and Shugar, D., Eur. J. Biochem., 2003, vol. 270, p. 1645. https://doi.org/10.1046/j.1432-1033.2003.03540.x
Beauchard, A., Jaunet, A., Murillo, L., Baldeyrou, B., Lansiaux, A., Cherouvrier, J.R., Domon, L., Picot, L., Bailly, C., Besson, T., and Thiery, V., Eur. J. Med. Chem., 2009, vol. 44, p. 3858. https://doi.org/10.1016/j.ejmech.2009.04.012
Nagaraju, K., Lalitha, G., Maddila, S., Gangu, K., and Jonnalagadda, S., Molecules, 2020, vol. 25, article no. 1909. https://doi.org/10.3390/molecules25081909
Kapadiya, K., Jadeja, Y., and Khunt, R., J. Heterocycl. Chem., 2018, vol. 55, p. 199. https://doi.org/10.1002/jhet.3025
Pandya, M., Kapadiya, K., Pandit, C., and Purohit, D., J. Sci. Ind. Res., 2017, vol. 76, p. 173. http://nopr.niscair.res.in/handle/123456789/40646
Kapadiya, K.M., Kavadia, K.M., Manvar, P.A., and Khunt, R.C., Anti-Infect. Agents, 2015, vol. 13, p. 129. https://doi.org/10.2174/2211352513666150915235745
Sun, W., Weingarten, R.A., Xu, M., Southall, N., Dai, S., Shinn, P., and Zheng, W., Emerging Microbes Infect., 2016, vol. 5, p. 1. https://doi.org/10.1038/emi.2016.123
Radia, A.J., Lalpara, J.N., Modasiya, I.J., and Dubal, G.G., J. Heterocycl. Chem., 2021, vol. 58, p. 612. https://doi.org/10.1002/jhet.4200
ACKNOWLEDGMENTS
The authors are thankful to the Chemistry Division and Department of Microbiology, School of Science, RK University, Kashturbadham, Rajkot, for their great support in the laboratory and antimicrobial screening. The authors are also thankful to NFDD, Rajkot, for performing spectral analyses. Sincere thanks to NIAID division, USA, for antitubercular study in a very short time.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Piludiya, R.I., Dholaria, P.V., Jivani, A.J. et al. Bis-triazole Heterocycles as Antitubercular and Antimicrobial Agents: Synthesis Using Copper-Catalyzed Click Chemistry Approach. Russ J Org Chem 58, 1280–1286 (2022). https://doi.org/10.1134/S1070428022090135
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022090135