Abstract
A new series of pyrazole-based 1,2,3-triazole derivatives (6a–x) were synthesized by employing click reaction using a 2:1 mixture of PEG-400 and water as green solvent. The synthesized intermediate and final compounds were characterized by 1H NMR, 13C NMR, and mass spectra and elemental analysis techniques. The structure of one of the final compounds, 6a was evidenced by single crystal X-ray diffraction study. Among the twenty-four compounds, five compounds (6a, 6b, 6d, 6f, and 6g) showed significant antitubercular activity against Mycobacterium tuberculosis H37Rv with a minimum inhibitory concentration (MIC) ≤ 6.25 µg/mL. The 4-(((5-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1-cyclohexyl-1H-1,2,3-triazole (6g) was the most potent compound of the series, which showed a MIC of 3.13 µg/mL. The cytotoxicity study of active anti-TB compounds on normal Vero cells revealed that the compounds are non-toxic with a high selectivity index (>37). Most of the pyrazole-1,2,3-triazole derivatives with a 4-chlorophenyl substitution at position-5 of the pyrazole ring showed a better anti-TB activity than the corresponding 4-bromophenyl or 4-methoxyphenyl substituted derivatives. The target compounds were also evaluated for their in vitro antibacterial activity and six compounds (6a, 6c, 6d, 6e, 6l, and 6w) showed promising inhibition activity against four tested strains.
Graphical Abstract
A new series of pyrazole-1,2,3-triazole derivatives were synthesized by employing a multicomponent one pot click reaction. The most potent anti-TB agent of the series showed a MIC of 3.13 µg/mL.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is one of the most dangerous and deadly microbial diseases. It is caused by a bacterium called Mycobacterium tuberculosis (MTB) and is the second greatest killer disease after HIV/AIDS among the diseases caused by a single infectious agent. According to the World Health Organization (WHO) report released in the year 2014, 9 million people fell ill with TB and 1.5 million died, among which 0.3 million were HIV-positive [1]. The standard medication for the short course treatment of tuberculosis includes a mixture of four drugs: isoniazid, rifampicin, pyrazinamide, and ethambutol for 2 months, then isoniazid and rifampicin alone for a further 4 months. These drugs are known as first line anti-TB drugs [2]. However, extensively drug-resistant tuberculosis (XDR-TB) or multidrug resistant tuberculosis (MDR-TB) even show resistance to first line therapy. In such cases the second-line drugs are implemented for the treatment. But these drugs are less preferred due to one of the possible three reasons: it may be less effective than the first-line drugs (e.g., p-aminosalicylic acid); it may have toxic side-effects (e.g., cycloserine); or it may be expensive and unavailable in many developing countries (e.g., fluoroquinolones). All the antitubercular medicines including the first line drugs show some side effects and also the course for the treatment is too long. Furthermore, except for the recently introduced bedaquiline, no new TB drugs have been introduced over the last four decades, which testifies to the lack of significant research in this area of pharmaceutical science. These major issues prompted us to search for new lead molecules to act against TB.
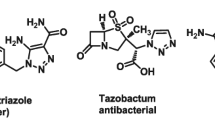
Azole-based heterocycles, especially pyrazole and 1,2,3-triazole derivatives, gained great significance in medicinal chemistry research due to their wide range of biological activities. A number of pyrazole derivatives have shown significant activities, such as antimicrobial [3, 4], analgesic [5], anti-inflammatory [6], anticancer [7], and antitubercular [8–14]. These results gave a great momentum to the search for potential pharmacologically active drugs carrying a pyrazole substituent. On the other hand, with the invention of click chemistry of cycloaddition reactions, several 1,2,3-triazole derivatives have been synthesized and are found to possess a broad spectrum of pharmacological effects [15] such as antimicrobial [16], anticancer [17], antifungal [18], antiviral [19], and anti-inflammatory [20] activities. Some N-substituted 1,2,3-triazole derivatives have shown remarkable antitubercular [21–27] properties among which derivative I-A09 [28, 29] is presently in preclinical trials. Additionally, the derivatives of 1,2,3-triazole possess remarkable metabolic stability and are proven to be amide surrogates in various bioactive compounds [30]. Further, recent studies on azoles demonstrated their ability of forming hydrogen bonding and dipole interactions with biomolecular targets, which results in improving their solubility in biological systems [31, 32]. In recent years, the molecular hybridization concept has received significant importance in drug design and development, which involves a combination of pharmacophoric moieties of two bioactive elements to produce a new hybrid lead compound with improved efficiency and efficacy, when compared to the parent compounds. It is also found that this strategy resulted in developing compounds with a modified selectivity profile, different and/or dual modes of action, and reduced undesired side effects [33, 34]. With this background and pertaining to the literature reports on promising antitubercular and antimicrobial activities of a number of pyrazole and 1,2,3-triazole derivatives (Fig. 1), we have planned to amalgamate these two structural units in a single molecular framework and synthesized a library of pyrazole-1,2,3-traizole hybrid derivatives (Fig. 2).
Results and discussion
Chemistry
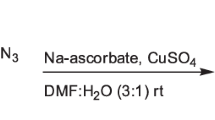
The synthetic route of intermediates 5a–c is shown in Scheme 1. The protocol involves the Claisen condensation of 4-substituted acetophenones (1a–c) with diethyl oxalate in the presence of sodium ethoxide in ethanol at room temperature to yield a corresponding sodium salt of α,γ-diketoesters (2a–c). Esters of 5-aryl-1-phenyl-1H-pyrazole-3-carboxylic acids (3a–c) were synthesized in quantitatively good yield by the conventional cyclization reaction between sodium salt of α,γ-diketoesters 2a–c and arylhydrazines in the presence of acetic acid. The ester derivatives were then reduced to corresponding alcohols (4a–c) in good yields by using LiAlH4 as the reducing agent. The propargylated scaffolds, 5-(4-aryl)-1-phenyl-3-((prop-2-ynyloxy)methyl)-1H-pyrazoles (5a–c) were obtained by the alkylation of alcohols 4a–c with propargyl bromide in the presence of sodium hydride (NaH) (60 %) in tetrahydrofuran (THF). The targeted regioselective 1,4-substituted 1,2,3-triazole derivatives (6a–x) were synthesized using a multicomponent one pot click chemistry approach by treating the propargylated scaffolds (5a–c) with alkyl bromides or benzyl bromides in presence of a catalytic amount of copper sulfate pentahydrate and sodium ascorbate in a 2:1 mixture of polyethylene glycol-400 (PEG-400) and water at 50 °C (Scheme 2). We tried using different solvent systems for the click reaction and the optimization was carried out with the reaction between 5a and 4-fluorobenzyl bromide (Table 1). Among the solvents used, a 2:1 mixture of PEG-400 and water was the most effective system, which yielded a product (6a) yield of 84 %. The reaction using a mixture of t-butanol and water as the solvent also resulted in comparable product yield. PEG-400 is an interesting solvent in synthetic chemistry as it is non-toxic and inexpensive. Also, it is soluble in water but shows poor solubility in a number of organic solvents. The greener and easy removable nature of PEG-400 prompted us to choose this solvent in the click reaction protocol.

The structures of all the synthesized compounds were confirmed by 1H NMR, 13C NMR, and mass spectral analysis as well as by elemental analysis. Cyclization of sodium (E)-1-(4-substituted)-4-ethoxy-3,4-dioxobut-1-en-1-olate (2a–c) to ethyl 5-(aryl)-1-phenyl-1H-pyrazole-3-carboxylate (3a–c) was evident by the presence of a singlet at δ 7.17 ppm due to the pyrazole ring proton, in the 1H NMR spectrum. Also, the spectrum displayed an additional five aromatic protons confirming the presence of the N-phenyl ring. The reduction of the ester group in 3a–c to give (5-(4-substituted)-1-phenyl-1H-pyrazol-3-yl)methanol (4a–c) was confirmed by the appearance of a signal corresponding to the -OH group at δ 5.25 ppm in the 1H NMR spectrum. The absence of ethyl carboxylate peaks in the spectrum further confirms the reduction of the ester group. The alkylation of 4a–c using propargyl bromide to yield scaffolds 5a–c was evidenced by the disappearance of –OH signal in the 1H NMR spectrum of 5a–c. Further, appearance of two new peaks at around δ 4.58 ppm for –OCH2 and δ 4.25 ppm for alkyne –CH protons clearly confirmed the formation of the required products. The formation of the triazole ring in 6a–x was supported by the characteristic 1,2,3-triazole ring (–CH) signal in the range δ 8.16–8.29 ppm in the 1H NMR spectra. For instance, in compound 6a, the triazole ring (–CH) signal appears as a sharp singlet at δ 8.22 ppm, all other aromatic –CH signals are in the range δ 7.18–7.44. The sharp singlet at δ 6.69 ppm is due to pyrazole ring –CH and the benzyl –CH2 appears at δ 5.59 ppm. The –CH2 signals of the methylenedioxy linkage appear at δ 4.64 and 4.56 ppm, the higher chemical shift value is for the –CH2 group attached to the pyrazole ring. The electrospray ionization mass spectrum (ESI–MS) of 6a showed a molecular ion peak at m/z 474.3, which tallies with its molecular formula C26H21ClFN5O. Further 13C NMR spectrum and elemental analysis results clearly confirms the formation of 6a. The structural parameters, yield, and melting point data of the target compounds (6a–x) are presented in Table 2.
X-ray crystallographic analysis of 6a
The single crystal of final compound 6a was grown by the slow evaporation of a 1:1 mixture of methanol-chloroform solution of the compound at room temperature. The 3-dimensional structure of the molecule was established by carrying out single crystal X-ray diffraction (SCXRD) analysis using a Bruker SMART APEXII DUO CCD diffractometer. The suitable crystal required for the analysis was selected by using a microscope and the single crystal was mounted on a goniometer tip for X-ray exposure. The structure of the crystal was solved by the full matrix least-squares method using the SHELXL-2013 package. All the atoms were positioned in different Fourier maps and refined isotropically, using a riding model and all the projections were generated using the Oak Ridge Thermal Ellipsoid Plot Program (ORTEP). It was found that the compound 6a crystallizes in triclinic space group P-1 with cell parameters; a (Ǻ) = 5.6750(3), b (Ǻ) = 10.7105(5), c (Ǻ) = 19.9112(9), Volume (Ǻ3) = 1163.87(10). The number of molecules in the unit cell (Z) was observed to be two. The ORTEP diagram (50 % probability) of 6a is depicted in Fig. 3. The details of bond lengths and bond angles of the compound are given in the supplementary information.
ORTEP diagram showing the X-ray crystal structure of 6a. (Crystal data: Chemical formula = C26H21ClFN5O, Formula weight = 473.93, Crystal system = Triclinic, Space group = P-1, a (Ǻ) = 5.6750(3), b (Ǻ) = 10.7105(5), c (Ǻ) = 19.9112(9), Volume (Ǻ3) = 1163.87(10), α = 79.5270(19), β = 85.8390(19), γ = 78.1330(19), Z = 2, F000 = 492.0, µ (mm−1) = 0.201, Temperature (T) = 296 K, Radiation wavelength (Ǻ) = 0.71073, Radiation type = Mo Kα, R-Factor (%) = 4.3, CCDC No. = 1032622)
Pharmacology
Antitubercular studies
The antimycobacterial activity of the pyrazole-1,2,3-triazole hybrids (6a–x) against MTB H37Rv (ATCC27294) was evaluated by the agar dilution method and the minimum inhibitory concentration (MIC) values are given in Table 2. Isoniazid, Ethambutol, and Pyrazinamide were used as positive drug standards. MIC is defined as the minimum concentration of compound required to completely inhibit the bacterial growth. Among twenty-four compounds screened against MTB, 13 compounds (6a, 6b, 6c, 6d, 6e, 6f, 6g, 6k, 6l, 6m, 6p, 6v, and 6w) showed MIC in the range 3.13–12.5 µg/mL. Out of eight 4-chlorophenyl substituted pyrazole derivatives (6a–h), seven compounds showed significant activity against MTB, which suggests that 4-chlorophenyl substitution at position-5 of the pyrazole (R 1) ring is a decisive factor for the activity. Compound 6g, which contain a cyclohexyl substitution on the 1,2,3-triazole ring (R 2), was found to be the most active compound of the series with a MIC of 3.13 µg/mL. The inhibition activity of 6g is comparable (in terms of MIC value) with that of the standard drug Ethambutol. All the 4-chlorophenyl substituted target compounds (6a–h), except 6h, are either equipotent or more potent when compared with their respective 4-methoxyphenyl substituted (6i–p) and 4-bromophenyl substituted (6q–x) analogues. This structure–activity relationship further confirms the significant contribution of the 4-chlorophenyl substituent towards the inhibition activity of the molecules. The nature of the substituent on the 1,2,3-triazole ring (R 2) also affected the inhibition activity. However, we did not observe a general trend in the variation of the activity with respect to electron donating or withdrawing nature of these substituents. But, in case of 4-chlorophenyl substituted derivatives (6a–h), those compounds, which carry an electron-withdrawing functional group at R 2, showed better inhibition activity than those compounds containing an electron releasing functionality. That is, compounds 6a, 6b, 6d and 6f, which contain 4-F, 4-CN, 4-NO2 and 2-F groups (at R 2) respectively exhibited MIC of 6.25 µg/mL, whereas compounds 6c (which contain an electron-donating 4-OCH3 group) and 6e (which has no substituent on the phenyl ring) showed a higher MIC (12.5 µg/mL) value.
Cytotoxicity
The in vitro cytotoxicity studies of the active anti-TB compounds with MTB MIC ≤ 6.25 µg/mL were carried out by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay [35] against a normal Vero cell line. The results of the cytotoxicity study are presented in the Table 3. All the active antitubercular compounds 6a, 6b, 6d, 6f, and 6g exhibited low toxicity and the inhibition values were between 13.22 and 29.91 % at 65 µg/mL. Further, the high selectivity index of these derivatives (>37) clearly implies the suitability of the compounds for drug development as new anti-TB agents.
Antibacterial studies
Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella typhi are the common pathogens, which cause harmful effects on human health. Some pyrazole [36] and 1,2,3-triazole [24] derivatives showed promising activity against these pathogens. Hence, we thought it was worthwhile to evaluate the antimicrobial activity of the new pyrazole-1,2,3-triazole derivatives (6a–x) against these microorganisms. The antibacterial activity of the compounds was studied following the well plate method (zone of inhibition) by using Streptomycin, the first class of antibiotic drug, as the standard. Compounds 6a, 6c, 6d, 6e, 6l, and 6w showed promising activity against tested bacterial strains (Table 4). It is interesting to note that four of these compounds (6a, 6c, 6d, and 6e) contain a 4-chlorophenyl substitution at position-5 of the pyrazole ring. The antibacterial activity of derivatives 6d and 6l against P. aeruginosa is very close to that of Streptomycin, the presence of the nitro group in these molecules could be responsible for the observed activity. It is evident from Table 4 that the target molecules show a better activity against the P. aeruginosa strain as compared to the other three bacterial strains.
Conclusions
We described the synthesis of a new series of pyrazole-1,2,3-triazole hybrids (6a–x) using a multistep synthetic route in which a substituted 1,2,3-triazole ring was constructed in the final step through the click reaction protocol. The solvent system for the click reaction was optimized in terms of the product yield and the green solvent system, a 2:1 mixture of PEG-400 and water, was the most effective among the solvent systems used. All the target compounds were characterized by 1H NMR, 13C NMR, mass spectral and elemental analyses. The in vitro antitubercular screening of the molecules against MTB H37Rv strain revealed the better anti-TB activity of 4-chlorophenyl substituted derivatives as compared to their 4-methoxyphenyl and 4-bromophenyl substituted analogues. The derivative (6g) with a 4-chlorophenyl substitution on the pyrazole ring and a cyclohexyl moiety on the 1,2,3-triazole ring is the best antitubercular agent of the series with MIC of 3.13 µg/mL. In 4-chlorophenyl substituted derivatives, an electron-withdrawing substituent on the 1,2,3-triazole ring enhanced the anti-TB activity. The cytotoxicity study on active antitubercular compounds also suggested that the compounds are nontoxic and have a high selectivity index (>37). Further, derivatives 6a, 6c, 6d, 6e, 6l, and 6w exhibited significant antibacterial activity against the tested bacterial strains. With regard to the relationship between the structure of pyrazole-1,2,3-triazole hybrids and the detected anti-TB and antimicrobial properties, it can be concluded that the chlorophenyl substituted pyrazole scaffolds are ideally suited for further structural modifications to obtain efficient antibacterial leads. These findings suggest that pyrazole-1,2,3-triazole hybrids could be considered as promising molecular patterns for potential antitubercular and antimicrobial agents and can be used for further chemical modifications for improving activity, which will be the main goal of our future research.
Experimental protocols
Analysis and instruments
All the reagents were purchased from leading reagent suppliers like Sigma Aldrich (Germany), Merck (India), Loba Chemie Pvt. Ltd., and Spectrochem Chemicals Pvt. Ltd. All the solvents were extra dried by the distillation method. Thin layer chromatography (TLC) was carried out on coated aluminum sheets with 60 F254 silica gel (Merck KGaA). The intermediate and the final compounds were purified by the column chromatography technique using silica gel 230–400 mesh size (Merck). A Stuart SMP3 melting point apparatus was used for the determination of the melting points of the newly synthesized compounds and are uncorrected. The X-Ray diffraction studies were worked out by a Bruker APEX II DUO CCD diffractometer. 1H and 13C NMR spectra of all the compounds were recorded on 400 MHz spectrometers using TMS as an internal standard. A Thermo electron corporation EA-112 series C,H,N,S analyzer was used for the elemental analysis. Molecular mass of the compounds was determined using a Waters Q-Tof micro mass spectrometer with an ESI source.
General procedure for the synthesis of final compounds (6a–x)
A mixture of appropriate scaffold (5a–c) (0.1 g), bromo compound (1.1 eq), NaN3 (1.1 eq), copper sulfate pentahydrate (0.2 eq), and sodium ascorbate (0.3 eq) was placed in a 2:1 mixture of PEG-400 and water (3 mL). The mixture was stirred at 50 °C for 12 h. Completion of the reaction was confirmed by TLC. The reaction mixture was then filtered through Celite; the residue was washed with 25 mL of ethyl acetate. The filtrate was extracted with ethyl acetate (25 × 3 mL). The organic layer was separated and washed twice with ammonia solution, then with water followed with brine solution, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure and the crude product was purified by flash column chromatography (230–400 size mesh) using 60 % ethyl acetate in pet ether to afford the pure final products (6a–x).
Spectral data of final derivatives (6a–x)
1-(4-Fluorobenzyl)-4-(((5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6a)
Off white solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.22 (s, 1H, triazole-CH), 7.38–7.44 (m, 7H, ArH), 7.18–7.29 (m, 6H, ArH), 6.69 (s, 1H, pyrazole-CH), 5.59 (s, 2H, CH2), 4.64 (s, 2H, CH2), 4.57 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 163.55, 161.12, 150.37, 144.66, 142.65, 139.89, 133.65, 132.83, 132.80, 130.76, 130.67, 130.64, 129.65, 129.26, 129.14, 128.33, 125.70, 124.58, 116.17, 115.95, 108.18, 65.62, 63.48, 52.45; ESI–MS (m/z) 474.3 (M + H)+; Anal. calculated for C26H21ClFN5O; C, 65.89; H, 4.47; N, 14.78. Found: C, 65.86; H, 4.45; N, 14.81.
4-((4-(((5-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)benzonitrile (6b)
Off white solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.28 (s, 1H, triazole-CH), 7.85 (d, 2H, ArH, j = 8.4 Hz), 7.36–7.46 (m, 7H, ArH), 7.22–7.27 (m, 4H, ArH), 6.69 (s, 1H, pyrazole-CH), 5.73 (s, 2H, CH2), 4.66 (s, 2H, CH2), 4.58 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 150.36, 144.78, 142.65, 142.02, 139.89, 133.66, 130.64, 129.65, 129.25, 129.14, 129.11, 128.34, 125.71, 125.06, 119.00, 111.38, 108.19, 65.63, 63.45, 52.62; ESI–MS (m/z) 481.3 (M + H)+; Anal. calculated for C27H21ClN6O; C, 67.43; H, 4.40; N, 17.47. Found: C, 67.39; H, 4.41; N, 17.50.
1-(4-Methoxybenzyl)-4-(((5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6c)
Pale yellow solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.16 (s, 1H, triazole-CH), 7.38–7.22 (m, 11H, ArH), 6.91 (d, 2H, ArH, J = 8.8 Hz), 6.69 (s, 1H, pyrazole-CH), 5.51 (s, 2H, CH2), 4.63 (s, 2H, CH2), 4.56 (s, 2H, CH2), 3.72 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d 6) δ ppm 159.59, 150.39, 144.58, 142.64, 139.90, 139.79, 133.65, 130.63, 130.04, 129.92, 129.64, 129.56, 129.26, 129.14, 128.47, 128.32, 125.70, 124.32, 114.58, 114.48, 108.18, 65.61, 63.51, 55.58, 52.81; ESI–MS (m/z) 486.3 (M + H)+; Anal. calculated for C27H24ClN2O2; C, 66.73; H, 4.98; N, 14.41. Found: C, 66.67; H, 5.00; N, 14.43.
1-(4-Nitrobenzyl)-4-(((5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6d)
Pale yellow solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.29 (s, 1H, triazole-CH), 8.23 (d, 2H, ArH, j = 8.8 Hz), 7.52 (d, 2H, ArH, J = 8.0 Hz), 7.38–7.44 (m, 5H, ArH), 7.22–7.27 (m, 4H, ArH), 6.69 (s, 1H, pyrazole-CH), 5.79 (s, 2H, CH2), 4.66 (s, 2H, CH2), 4.57 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 150.36, 147.69, 144.81, 143.97, 142.65, 139.88, 133.66, 130.66, 129.65, 129.45, 129.14, 128.34, 125.70, 125.11, 124.39, 108.19, 65.64, 63.46, 52.35; ESI–MS (m/z) 501.3 (M + H)+; Anal. calculated for C26H21ClN6O3; C, 62.34; H, 4.23; N, 16.78. Found: C, 62.39; H, 4.26; N, 16.81.
1-Benzyl-4-(((5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6e)
Pale yellow Syrup; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.20 (s, 1H, triazole-CH), 6.89–7.38 (m, 14H, ArH,), 6.69 (s, 1H, pyrazole-CH), 5.63 (s, 2H, CH2), 4.66 (s, 2H, CH2), 4.57 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 161.12, 144.77, 144.64, 143.45 141.5, 139.89, 132.95, 132.83, 132.79, 130.76, 130.64, 130.61, 129.65, 129.26, 129.21, 128.33, 125.69, 124.57, 116.11, 115.90, 108.16, 65.62, 63.50, 52.42; ESI–MS (m/z) 456.4 (M + H)+; Anal. calculated for C26H22ClN5O; C, 68.49; H, 4.86; N, 15.36. Found: C, 68.55; H, 4.87; N, 15.40.
1-(2-Fluorobenzyl)-4-(((5-(4-chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6f)
Off white solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.22 (s, 1H, triazole-CH), 7.18–7.40 (m, 13H, ArH), 6.69 (s, 1H, pyrazole-CH), 5.60 (s, 2H, CH2), 4.64 (s, 2H, CH2), 4.58 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 162.58, 162.12, 150.40, 143.66, 142.95, 139.91, 133.55, 132.93, 132.78, 130.66, 130.62, 130.60, 129.88, 129.64, 129.24, 128.25, 125.70, 124.68, 116.16, 115.90, 108.17, 65.63, 63.49, 52.44; ESI–MS (m/z) 474.3 (M + H)+; Anal. calculated for C26H21ClFN5O; C, 65.89; H, 4.47; N, 14.78. Found: C, 65.93; H, 4.48; N, 14.80.
4-(((5-(4-Chlorophenyl)-1-phenyl1H-pyrazol-3-yl)methoxy)methyl)-1-cyclohexyl-1H-1,2,3-triazole(6g)
Pale yellow syrup; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.20 (s, 1H, triazole-CH), 7.23–7.44 (m, 9H, ArH), 6.70 (s, 1H, pyrazole-CH), 4.63 (s, 2H, CH2), 4.58 (s, 2H, CH2); 4.47 (t, 1H, CH, J = 11.2 Hz), 2.04 (d, 2H, CH2, J = 11.2 Hz), 1.69–1.83 (m, 8H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 150.51, 144.01, 142.88, 139.94, 132.17, 130.88, 129.67, 129.60, 128.36, 125.21, 122.76, 122.32, 108.11, 65.66, 63.66, 59.42, 33.32, 25.17, 25.11; ESI–MS (m/z) 447.1 (M + H)+; ESI–MS (m/z) 448.1 (M + H)+; Anal. calculated for C25H26ClN5O; C, 67.03; H, 5.85; N, 15.63. Found: C, 67.09; H, 5.87; N, 15.65.
4-(((5-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1-cyclopentyl-1H-1,2,3-triazole(6h)
Pale yellow syrup; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.20 (s, 1H, triazole-CH), 7.17–7.55 (m, 9H, ArH), 6.70 (s, 1H, pyrazole-CH), 4.95 (p, 1H, CH, J = 6.8 Hz) 4.63 (s, 2H, CH2), 4.58 (s, 2H, CH2), 1.68–2.19 (m, 8H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 151.01, 143.98, 142.36, 139.83, 132.16, 130.01, 130.51, 129.98, 128.36, 124.67, 123.98, 122.31, 108.15, 65.69, 63.58, 59.40, 33.33, 25.17, 25.08; ESI–MS (m/z) 434.1 (M + H)+; Anal. calculated for C24H24ClN5O; C, 66.43; H, 5.57; N, 16.14. Found: C, 66.39; H, 5.59; N, 16.17.
1-(4-Fluorobenzyl)-4-(((5-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6i)
Off white solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.22 (s, 1H, triazole-CH), 7.35–7.42 (m, 5H, ArH), 7.18–7.35 (m, 6H, ArH), 6.90 (d, 2H, ArH, J = 8.8 Hz), 6.56 (s, 1H, pyrazole-CH), 5.70 (s, 2H, CH2), 4.63 (s, 1H, CH2), 4.54 (s, 2H, CH2), 3.74 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d 6) δ ppm; 163.55, 161.12, 159.68, 150.14, 144.70, 143.75, 140.25, 132.82, 132.79, 130.76, 130.67, 130.23, 129.50, 128.04, 125.62, 125.52, 124.57, 122.70, 116.16, 115.95, 114.51, 107.36, 65.73, 63.45, 55.62, 52.46; ESI–MS (m/z) 470.3 (M + H)+; Anal. calculated for C27H24FN5O2; C, 69.07; H, 5.15; N, 14.92. Found: C, 69.02; H, 5.17; N, 14.94.
4-((4-(((5-(4-Methoxyphenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)benzo nitrile(6j)
Off white solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.27 (s, 1H, triazole-CH), 7.84 (d, 2H, ArH, J = 7.6 Hz), 7.37–7.46 (m, 5H, ArH), 7.24 (d, 2H, ArH, J = 7.6 Hz), 7.14 (d, 2H, ArH, J = 8.4 Hz), 6.90 (d, 2H, ArH, J = 8.8 Hz), 6.57 (s, 1H, pyrazole-CH), 5.73 (s, 2H, CH2), 4.64 (s, 2H, CH2), 4.55 (s, 2H, CH2); 3.74 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d 6) δ ppm 159.68, 150.13, 144.83, 143.75, 142.03, 140.25, 133.20, 130.23, 129.51, 129.11, 128.04, 125.63, 125.05, 122.70, 119.00, 114.51, 111.37, 107.38, 65.74, 63.43, 55.63, 52.63; ESI–MS (m/z) 477.3 (M + H)+; Anal. calculated for C28H24N6O2; C, 70.57; H, 5.08; N, 17.64. Found: C, 70.51; H, 5.10; N, 17.68.
1-(4-Methoxybenzyl)-4-(((5-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6k)
Yellow syrup; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.16 (s, 1H, triazole-CH), 6.90–7.43 (m, 13H, ArH,), 6.57 (s, 1H, pyrazole-CH), 5.51 (s, 2H, CH2), 4.62 (s, 2H, CH2), 4.54 (s, 2H, CH2); 3.75 (s, 3H, OCH3), 3.73 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d 6) δ ppm 159.67, 159.58, 150.15, 144.61, 143.74, 140.24, 130.23, 130.04, 129.50, 128.47, 128.04, 125.63, 124.31, 122.70, 114.58, 114.50, 107.36, 65.70, 63.46, 55.62, 55.59, 52.80; ESI–MS (m/z) 482.1 (M + H)+; Anal. calculated for C28H27N5O3; C, 79.84; H, 5.65; N, 14.54. Found: C, 79.86; H, 5.66; N, 14.56.
1-(4-Nitrobenzyl)-4-(((5-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6l)
Pale yellow solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.29 (s, 1H, triazole-CH), 8.23(d, 2H, ArH, J = 8.8 Hz), 7.53(d, 2H, ArH, J = 8.0 Hz), 7.35–7.42 (m, 3H, ArH,), 7.24 (d, 2H, ArH, J = 6.9 Hz), 6.90 (d, 2H, ArH, J = 8.8 Hz), 6.57 (s, 1H, pyrazole-CH), 5.79 (s, 2H, CH2), 4.65 (s, 2H, CH2), 4.56 (s, 2H, CH2); 3.74 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d 6) δ ppm 159.68, 150.13, 147.69, 144.85, 143.98, 143.75, 140.25, 130.22, 129.50, 129.45, 128.04, 125.62, 125.09, 124.39, 122.69, 114.51, 107.38, 65.75, 63.44, 55.62, 52.35; ESI–MS (m/z) 497.3 (M + H)+; Anal. calculated for C27H24N6O4; C, 65.31; H, 4.87; N, 16.93. Found: C, 65.37; H, 4.89; N, 16.90.
1-Benzyl-4-(((5-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole (6m)
Pale yellow solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.20 (s, 1H, triazole-CH), 7.17–7.42 (m, 11H, ArH), 6.90 (d, 2H, ArH, J = 8.8 Hz), 6.56 (s, 1H, pyrazole-CH), 5.70 (s, 2H, CH2), 4.62 (s, 1H, CH2), 4.52 (s, 2H, CH2), 3.73 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d 6) δ ppm 159.67, 150.18, 144.95, 144.61, 143.71, 141.26, 130.25, 130.14, 129.78, 128.77, 128.34, 125.53, 124.32, 122.72, 114.58, 114.53, 107.35, 65.69, 63.46, 55.64, 52.81; ESI–MS (m/z) 452.3 (M + H)+; Anal. calculated for C27H25N5O2; C, 71.82; H, 5.58; N, 15.51. Found: C, 71.80; H, 5.50; N, 15.53.
1-(2-Fluorobenzyl)-4-(((5-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6n)
Off white solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.20 (s, 1H, triazole-CH), 6.69–7.42 (m, 13H, ArH), 6.57 (s, 1H, pyrazole-CH), 5.71 (s, 2H, CH2), 4.62 (s, 1H, CH2), 4.53 (s, 2H, CH2), 3.73 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d 6) δ ppm 163.58, 161.61, 159.72, 150.09, 144.68, 143.76, 140.25, 132.81, 132.83, 130.73, 130.70, 130.23, 129.48, 128.14, 125.63, 125.54, 124.57, 122.70, 116.19, 115.96, 114.51, 107.36, 65.72, 63.46, 55.63, 52.45; ESI–MS (m/z) 470.3 (M + H)+; Anal. calculated for C27H24FN5O2; C, 69.07; H, 5.15; N, 14.92. Found: C, 69.10; H, 5.18; N, 14.91.
1-Cyclohexyl-4-(((5-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6o)
Pale yellow syrup; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.19 (s, 1H, triazole-CH), 6.98–7.47 (m, 13H, ArH), 6.69 (s, 1H, pyrazole-CH), 4.96 (p, 1H, CH, J = 6.8 Hz), 4.64 (s, 2H, CH2), 3.73 (s, 3H, OCH3), 1.68–2.21 (m, 8H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 159.70, 150.92, 141.97, 140.86, 132.47, 131.79, 129.97, 129.83, 128.72, 125.60, 122.94, 122.33, 108.20, 65.69, 63.72, 59.42, 55.88, 33.33, 25.16, 25.09; ESI–MS (m/z) 444.1 (M + H)+; Anal. calculated for C26H29N5O2; C, 70.41; H, 6.59; N, 15.79. Found: C, 70.36; H, 6.57; N, 15.83.
1-Cyclopentyl-4-(((5-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6p)
Pale yellow syrup; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.20 (s, 1H, triazole-CH), 7.14–7.43 (m, 7H, ArH,), 6.91 (d, 2H, ArH, J = 8.0 Hz) 6.58 (s, 1H, pyrazole-CH), 4.90 (p, 1H, CH, J = 7.2 Hz), 4.63 (s, 2H, CH2), 4.56 (s, 2H, CH2); 3.74 (s, 3H, OCH3), 1.67–2.21 (m, 8H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 159.67, 150.21, 144.21, 143.72, 140.25, 130.22, 129.51, 128.04, 125.61, 123.14, 122.71, 114.51, 107.38, 65.73, 63.59, 61.38, 55.63, 33.30, 24.07; ESI–MS (m/z) 430.1 (M + H)+; Anal. calculated for C25H27N5O2; C, 69.91; H, 6.34; N, 16.31. Found: C, 70.00; H, 6.36; N, 16.33.
1-(4-Fluorobenzyl)-4-(((5-(4-bromophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6q)
Off white solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.20 (s, 1H, triazole-CH), 7.18–7.44 (m, 13H, ArH), 6.70 (s, 1H, pyrazole-CH), 5.59 (s, 2H, CH2), 4.63 (s, 2H, CH2), 4.57 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 163.54, 161.10, 149.57, 144.68, 142.73, 141.99, 133.73, 132.91, 132.78, 130.78, 130.71, 130.54, 129.63, 129.11, 129.20, 128.30, 124.99, 124.62, 116.19, 116.06, 107.02, 65.63, 63.47, 52.45; ESI–MS (m/z) 519.1 (M + H)+; Anal. calculated for C26H21BrFN5O; C, 60.24; H, 4.08; N, 13.51. Found: C, 60.21; H, 4.10; N, 13.53.
4-((4-(((5-(4-Bromophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazol-1yl)methyl)benzonitrile (6r)
Yellow syrup; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.27 (s, 1H, triazole-CH), 7.83 (d, 2H, ArH, j = 8.4 Hz), 7.38–7.44 (m, 7H, ArH), 7.21–7.25 (m, 4H, ArH), 6.69 (s, 1H, pyrazole-CH), 5.73 (s, 2H, CH2), 4.65 (s, 2H, CH2), 4.58 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 150.37, 144.77, 142.58, 141.89, 139.96, 137.88, 130.59, 129.65, 129.45, 129.04, 129.13, 128.31, 127.32, 125.03, 118.99, 111.37, 108.17, 65.64, 63.46, 52.64; ESI–MS (m/z) 526.1 (M + H)+; Anal. calculated for C27H21BrN6O; C, 61.72; H, 4.03; N, 16.00. Found: C, 61.72; H, 4.01; N, 16.03.
1-(4-Methoxybenzyl)-4-(((5-(4-bromophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6s)
Off white solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.18 (s, 1H, triazole-CH), 6.92–7.38 (m, 13H, ArH), 6.69 (s, 1H, pyrazole-CH), 5.50 (s, 2H, CH2), 4.63 (s, 2H, CH2), 4.57 (s, 2H, CH2), 3.73 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d 6) δ ppm 159.57, 150.38, 144.88, 142.64, 140.02, 139.89, 133.63, 130.62, 130.14, 129.96, 129.64, 129.55, 129.24, 129.14, 128.57, 128.30, 125.69, 124.30, 114.55, 114.38, 108.22, 65.60, 63.52, 55.57, 52.80; ESI–MS (m/z) 531.1 (M + H)+; Anal. calculated for C27H24BrN5O2; C, 61.44; H, 4.56; N, 13.20. Found: C, 61.49; H, 4.58; N, 13.23.
1-(4-Nitrobenzyl)-4-(((5-(4-bromophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6t)
Off white solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.29 (s, 1H, triazole-CH), 8.23 (d, 2H, ArH, j = 8.8 Hz), 7.52 (d, 2H, ArH, J = 8.0 Hz), 7.38–7.44 (m, 5H, ArH), 7.22–7.27 (m, 4H, ArH), 6.69 (s, 1H, pyrazole-CH), 5.79 (s, 2H, CH2), 4.66 (s, 2H, CH2), 4.57 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 150.33, 147.30, 143.98, 143.90, 142.85, 140.86, 133.88, 130.76, 128.85, 129.65, 129.12, 128.44, 125.75, 125.19, 124.45, 108.19, 65.62, 63.45, 52.34; ESI–MS (m/z) 546.1 (M + H)+; Anal. calculated for C26H21BrN6O3; C, 57.26; H, 3.88; N, 15.41. Found: C, 57.21; H, 3.90; N, 14.37.
1-Benzyl-4-(((5-(4-bromophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6u)
Off white solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.20 (s, 1H, triazole-CH), 7.18–7.44 (m, 11H, ArH), 6.89 (d, 2H, ArH, J = 8.8 Hz), 6.57 (s, 1H, pyrazole-CH), 5.69 (s, 2H, CH2), 4.62 (s, 1H, CH2), 4.52 (s, 2H, CH2), 3.73 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d 6) δ ppm 161.14, 144.73, 144.61, 143.39, 141.51, 140.39, 132.64, 132.22, 132.09, 130.89, 130.74, 130.52, 129.53, 129.26, 129.20, 128.30, 125.71, 124.54, 116.14, 115.91, 108.20, 65.63, 63.48, 52.41; ESI–MS (m/z) 501.1 (M + H)+; Anal. calculated for C27H21BrN6O; C, 62.41; H, 4.43; N, 14.00. Found: C, 62.42; H, 4.43; N, 14.03.
1-(2-Fluorobenzyl)-4-(((5-(4-bromophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1H-1,2,3-triazole(6v)
Pale yellow solid; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.20 (s, 1H, triazole-CH), 7.16–7.56 (m, 13H, ArH), 6.69 (s, 1H, pyrazole-CH), 5.67 (s, 2H, CH2), 4.64 (s, 2H, CH2), 4.57 (s, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 161.88, 160.90, 150.44, 143.59, 142.84, 140.01, 133.56, 132.92, 132.79, 130.68, 130.64, 130.61, 129.77, 129.69, 129.36, 128.29, 125.79, 124.67, 116.15, 115.94, 108.18, 65.62, 63.52, 52.43; ESI–MS (m/z) 519.1 (M + H)+; Anal. calculated for C26H21BrFN5O; C, 60.24; H, 4.08; N, 13.51. Found: C, 60.29; H, 4.11; N, 13.53.
4-(((5-(4-Bromophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1-cyclohexyl-1H-1,2,3-triazole(6w)
Yellow syrup; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.19 (s, 1H, triazole-CH), 7.16–7.57 (m, 13H, ArH), 6.70 (s, 1H, pyrazole-CH), 4.62 (s, 2H, CH2), 4.57 (s, 2H, CH2), 4.35(p, 1H, CH), 2.04 (d, 2H, CH2, J = 12 Hz), 1.23–1.82 (m, 8H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 150.47, 143.95, 142.67, 139.90, 132.07, 130.88, 129.67, 129.63, 128.35, 125.70, 122.51, 122.31, 108.18, 65.65, 63.68, 59.43, 33.32, 25.15, 25.08; ESI–MS (m/z) 493.1 (M + H)+; Anal. calculated for C25H26BrN5O; C, 60.98; H, 5.32; N, 14.22. Found: C, 60.94; H, 5.32; N, 14.23.
4-(((5-(4-Bromophenyl)-1-phenyl-1H-pyrazol-3-yl)methoxy)methyl)-1-cyclopentyl-1H-1,2,3-triazole(6x)
Yellow syrup; 1H NMR (400 MHz, DMSO-d 6) δ ppm 8.19 (s, 1H, triazole-CH), 7.16–7.57 (m, 13H, ArH), 6.70 (s, 1H, pyrazole-CH), 4.96 (p, 1H, CH, J = 6.8 Hz), 4.63 (s, 2H, CH2), 1.67–2.19 (m, 8H, CH2); 13C NMR (100 MHz, DMSO-d 6) δ ppm 150.47, 150.20, 144.17, 142.67, 139.88, 132.06, 130.87, 129.66, 129.61, 129.54, 129.06, 128.86, 128.34, 127.98, 125.69, 123.16, 122.31, 108.18, 107.89, 65.61, 63.61, 63.03, 61.96, 60.28, 33.30, 33.01, 24.53, 24.40, 24.07; ESI–MS (m/z) 479.3 (M + H)+; Anal. calculated for C24H24BrN5O; C, 60.26; H, 5.06; N, 14.64. Found: C, 60.24; H, 5.04; N, 14.63.
Antitubercular studies
Twofold serial dilutions of each test compound/drug were prepared and incorporated into Middlebrook 7H11 agar medium with oleic acid, albumin, dextrose, and catalase (OADC) growth supplement to get final concentrations of 50, 25, 12.5, 6.25, 3.13, 1.56, and 0.78 µg/mL. Inoculum of MTB H37Rv ATCC 27294/XDR-TB was prepared from fresh Middlebrook 7H11 agar slants with OADC (Difco) growth supplement adjusted to 1 mg/mL (wet weight) in Tween 80 (0.05 %) saline diluted to 10−2 to give a concentration of ~107 cfu/mL. Five microliters of this bacterial suspension was spotted onto 7H11 agar tubes containing different concentrations of the drug as discussed above. The tubes were incubated at 37 °C, and final readings (as MIC in μg/mL) were determined after 28 days. This method is similar to that recommended by the National Committee for Clinical Laboratory Standards for the determination of MIC in triplicate.
Cytotoxicity
Vero (African green monkey kidney) cell line was procured from the National Centre for Cell Sciences (NCCS), Pune, India. Stock cells were cultured in MEM supplemented with 10 % inactivated Fetal Bovine Serum (FBS), penicillin (100 IU/mL), streptomycin (100 µg/mL), and amphotericin B (5 µg/mL) in an humidified atmosphere of 5 % CO2 at 37 °C until confluent. The cells were dissociated with TPVG solution (0.2 % trypsin, 0.02 % EDTA, 0.05 % glucose in PBS). The stock cultures were grown in 25 cm2 culture flasks and all experiments were carried out in 96 microtitre plates (Tarsons India Pvt. Ltd., Kolkata, India). For cytotoxicity studies, each weighed test drugs were separately dissolved in distilled DMSO and volume was made up with MEM supplemented with 2 % inactivated FBS to obtain a stock solution of 1 mg/mL concentration and sterilized by filtration. Serial twofold dilutions were prepared from this for carrying out the MTT assay. The monolayer cell culture was trypsinized, and the cell count was adjusted to 1.0 × 105 cells/mL using MEM containing 10 % FBS. To each well of the 96 well microtitre plate, 0.1 mL of the diluted cell suspension (approximately 10,000 cells) was added. After 24 h, when a partial monolayer was formed, the supernatant was flicked off, the monolayer was washed once with medium, and 100 µL of different test concentrations of test drugs were added on to the partial monolayer in microtitre plates. The plates were then incubated at 37 °C for 3 days in 5 % CO2 atmosphere, and microscopic examination was carried out and observations were noted every 24 h interval. After 72 h, the drug solutions in the wells were discarded and 50 µL of MTT in PBS was added to each well. The plates were gently shaken and incubated for 3 h at 37 °C in a 5 % CO2 atmosphere. The supernatant was removed and 100 µL of propanol was added, and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader at a wavelength of 540 nm. The percentage growth inhibition was calculated using the following formula, and concentration of test drug needed to inhibit cell growth by 50 % (IC50) values was generated from the dose–response curves for each cell line. Selectivity index (SI = IC50 against Vero cells/MIC against M. tuberculosis) values were also determined. The compound that exhibited selectivity index (SI) values >10 against the Vero cell line could be considered as nontoxic [37].
Antibacterial studies
Newly synthesized organic compounds 6a–x were tested against a panel of pathogenic microorganisms, which were maintained on nutrient agar medium at 37 °C. The cultures were inoculated into 10 mL fresh nutrient broth to yield an initial suspension of approximately 10–100 cfu/mL. All broths were incubated statically at the aforementioned temperature for 24 h. Susceptibility of the test organism to the organic compound was determined by the well plate technique [38, 39]. The bacterial suspensions were serially diluted in saline and 0.1 mL from the appropriate dilution was spread on nutrient agar. The wells were dug using a sterile cork borer, and the organic compounds dissolved in DMSO were added (1.0 and 0.5 mg/mL). The same procedure was repeated for all microorganisms, and the petri plates were incubated for 18 h at 37 °C. After the incubation, the inhibition zone was measured and the values for DMSO solvent were subtracted to get the actual values.
References
World Health Organization, Global tuberculosis report 2014. http://www.who.int/tb/publications/global_report/en/
World Health Organization Treatment of Tuberculosis: Guidelines for National Programmes WHO/TB/97.220, 2nd ed., (WHO, Geneva, 1997)
N.J. Thumar, M.P. Patel, Med. Chem. Res. 21, 1751 (2012)
A. Vijesh, A.M. Isloor, P. Shetty, S. Sundershan, H.K. Fun, Eur. J. Med. Chem. 62, 410 (2013)
D. Dressen, A.W. Garofalo, J. Hawkinson, D. Hom, J. Jagodzinski, J.L. Marugg, M.L. Neitzel, M.A. Pleiss, B. Szoke, J.S. Tung, D.W.G. Wone, J. Wu, H. Zhang, J. Med. Chem. 50, 5161 (2007)
N. Gökhan-Kelekçi, S. Yabanoğlu, E. Küpeli, U. Salgın, Ö. Özgen, G. Uçar, E. Yeşilada, E. Kendi, A. Yeşilada, A.A. Bilgin, Bioorg. Med. Chem. 15, 5775 (2007)
P. Puthiyapurayil, B. Poojary, C. Chikkanna, S.K. Buridipad, Eur. J. Med. Chem. 53, 203 (2012)
P. Horrocks, M. Pickard, H. Parekh, S. Patel, R.B. Pathak, Org. Biomol. Chem. 11, 4891 (2013)
U. Pandit, A. Dodiya, Med. Chem. Res. 22, 3364 (2013)
D. Castagnolo, A. De Logu, M. Radi, B. Bechi, F. Manetti, M. Magnani, S. Supino, R. Meleddu, L. Chisu, M. Botta, Bioorg. Med. Chem. 16, 8587 (2008)
P. Gunasekaran, S. Perumal, P. Yogeeswari, D. Sriram, Eur. J. Med. Chem. 46, 4530 (2011)
R.C. Khunt, V.M. Khedkar, R.S. Chawda, N.A. Chauhan, A.R. Parikh, E.C. Coutinho, Bioorg. Med. Chem. Lett. 22, 666 (2012)
P. Aragade, M. Palkar, P. Ronad, D. Satyanarayana, Med. Chem. Res. 22, 2279 (2013)
R. Manikannan, R. Venkatesan, S. Muthusubramanian, P. Yogeeswari, D. Sriram, Bioorg. Med. Chem. Lett. 20, 6920 (2010)
G.C. Tron, T. Pirali, R.A. Billington, P.L. Canonico, G. Sorba, A.A. Genazzani, Med. Res. Rev. 28, 278 (2008)
B.S. Holla, M. Mahalinga, M.S. Karthikeyan, B. Poojary, P.M. Akberali, N.S. Kumari, Eur. J. Med. Chem. 40, 1173 (2005)
Y.-C. Duan, Y.-C. Ma, E. Zhang, X.-J. Shi, M.-M. Wang, X.-W. Ye, H.-M. Liu, Eur. J. Med. Chem. 62, 11 (2013)
N.G. Aher, V.S. Pore, N.N. Mishra, A. Kumar, P.K. Shukla, A. Sharma, M.K. Bhat, Bioorg. Med. Chem. Lett. 19, 759 (2009)
L. Zhou, A. Amer, M. Korn, R. Burda, J. Balzarini, E. De Clercq, E.R. Kern, P.F. Torrence, Antivir. Chem. Chemother. 16, 375 (2005)
A.C. Cunha, J.M. Figueiredo, J.L.M. Tributino, A.L.P. Miranda, H.C. Castro, R.B. Zingali, C.A.M. Fraga, M.C.B.V. de Souza , V.F. Ferreira, E.J. Barreiro, Bioorg. Med. Chem. 11, 2051 (2003)
D. Addla, A. Jallapally, D. Gurram, P. Yogeeswari, D. Sriram, S. Kantevari, Bioorg. Med. Chem. Lett. 24, 1974 (2014)
D. Addla, A. Jallapally, D. Gurram, P. Yogeeswari, D. Sriram, S. Kantevari, Bioorg. Med. Chem. Lett. 24, 233 (2014)
H.N. Nagesh, K.M. Naidu, D.H. Rao, J.P. Sridevi, D. Sriram, P. Yogeeswari, K.V.G. Chandra Sekhar, Bioorg. Med. Chem. Lett. 23, 6805 (2013)
A. Kamal, S.M.A. Hussaini, S. Faazil, Y. Poornachandra, G. Narender Reddy, C.G. Kumar, V.S. Rajput, C. Rani, R. Sharma, I.A. Khan, Bioorg. Med. Chem. Lett. 23, 6842 (2013)
S.R. Patpi, L. Pulipati, P. Yogeeswari, D. Sriram, N. Jain, B. Sridhar, R. Murthy, A.T. Devi, S.V. Kalivendi, S. Kantevari, J. Med. Chem. 55, 3911 (2012)
G. Surineni, P. Yogeeswari, D. Sriram, S. Kantevari, Med. Chem. Res. 24, 1298 (2015)
T. Yempala, J.P. Sridevi, P. Yogeeswari, D. Sriram, S. Kantevari, Eur. J. Med. Chem. 71, 160 (2014)
B. Zhou, Y. He, X. Zhang, J. Xu, Y. Luo, Y. Wang, S.G. Franzblau, Z. Yang, R.J. Chan, Y. Liu, Proc. Natl. Acad. Sci. 107, 4573 (2010)
L.P. Tan, H. Wu, P.-Y. Yang, K.A. Kalesh, X. Zhang, M. Hu, R. Srinivasan, S.Q. Yao, Org. Lett. 11, 5102 (2009)
A. Brik, J. Alexandratos, Y.C. Lin, J.H. Elder, A.J. Olson, A. Wlodawer, D.S. Goodsell, C.H. Wong, Chem. Bio. Chem. 6, 1167 (2005)
W.S. Horne, M.K. Yadav, C.D. Stout, M.R. Ghadiri, J. Am. Chem. Soc. 126, 15366 (2004)
K. Kushwaha, N. Kaushik, Lata, S.C. Jain, Bioorg. Med. Chem. Lett. 24, 1795 (2014)
C. Viegas-Júnior, A. Danuello, V. da Silva Bolzani, E.J. Barreiro, C.A.M. Fraga, Curr. Med. Chem. 14, 1829 (2007)
J. Ramprasad, N. Nayak, U. Dalimba, P. Yogeeswari, D. Sriram, S.K. Peethambar, R. Achur, H.S.S. Kumar, Eur. J. Med. Chem. 95, 49 (2015)
L.-L. Gundersen, J. Nissen-Meyer, B. Spilsberg, J. Med. Chem. 45, 1383 (2002)
B. Ramesh, C.M. Bhalgat, Eur. J. Med. Chem. 46, 1882 (2011)
N.C. Desai, A.R. Trivedi, H.C. Somani, K.A. Bhatt, Chem. Biol. Drug Des. (2015). doi:10.1111/cbdd.12502
B.A. Arthington-Skaggs, M. Motley, D.W. Warnock, C.J. Morrison, J. Clin. Microbiol. 38, 2254 (2000)
L. Rocha, A. Marston, O. Potterat, M.A.C. Kaplan, H. Stoeckli-Evans, K. Hostettmann, Phytochemistry 40, 1447 (1995)
Acknowledgments
NN and JR gratefully acknowledge the financial support provided by NITK, Surathkal. We are thankful to MIT Manipal and Dr. Reddy’s Institute of Life Sciences, University of Hyderabad Campus, Hyderabad for NMR and Mass spectrometer facility.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nayak, N., Ramprasad, J., Dalimba, U. et al. Synthesis of new pyrazole-triazole hybrids by click reaction using a green solvent and evaluation of their antitubercular and antibacterial activity. Res Chem Intermed 42, 3721–3741 (2016). https://doi.org/10.1007/s11164-015-2241-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2241-9