Abstract
Maghemite–copper oxide nanocomposite catalyzed oxidative coupling of formamides with β-dicarbonyl compounds in the presence of tert-butyl hydroperoxide as an oxidant to produce the corresponding enol carbamates in excellent yields (up to 92%) under the optimized conditions. The simple preparation and the ability to be recycled and magnetically separated are salient features of this catalytic system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Coupling reactions underlie an important synthetic strategy widely used in both academia and industry for the formation of carbon–carbon and carbon–heteroatom bonds [1–5]. Several reports have appeared in the literature on direct C–H functionalization for the formation of C–C and C–X bonds (X = O, S, N, P, etc.) catalyzed by transition metals, which has become a very useful tool in organic chemistry [6].
In vivo data support the pharmacological efficacy of enol carbamates as promising anxiolytics [7]. Carbamates are mixed ester–amides of carbonic acid. Their chemical behaviour is similar to that of carbonates. Dixneuf et al. have shown that enol carbamates can be prepared by addition of carbamic acids to terminal alkynes catalyzed by Ru3(CO)12 [8] or Ru(Cl)3 [9]. However, the yields and selectivities were low for aliphatic acetylenes. Among the supports used, magnetic nanocomposites have attracted more attention because they can be easily recovered from the reaction mixture simply by using an external magnet [10–19].
We have previously reported the preparation of γ-Fe2O3@CuO nanocomposite and its application as a highly efficient and magnetically separable catalyst for the formation of amides from alcohols and amines using [20]. In continuation of our interest in using magnetic nanoparticles as a catalyst support [21], herein we report the preparation of enol carbamates by oxidative coupling of formamides with β-dicarbonyl compounds in the presence of γ-Fe2O3@CuO and tert-butyl hydroperoxide (TBHP) [22]. Various enol carbamates were obtained in good to excellent yields in a one-pot manner under our conditions (Scheme 1). Magnetic nanoparticles are easy dispersed in a solution, which facilitates catalyst recovery. Simple separation by using an external magnet and the recyclability up to five times are two virtues of this system [23, 24].
RESULTS AND DISCUSSION
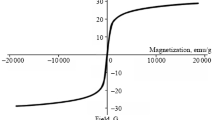
The maghemite–copper(II) oxide nanocomposite was prepared as shown in Scheme 2. The obtained heterogeneous catalyst was characterized using scanning electron microscopy (SEM), X-ray diffraction (XRD), vibrating sample magnetometry (VSM), and transmission electron microscopy (TEM). The crystalline structure of γ-Fe2O3@CuO NPs and phase purity were determined using powder XRD as shown in Fig. 1. The XRD pattern of magnetic NPs is indexed to the spinel phases (Fe2O3: 2θ = 35, 42, 51, 63, 68, and 75°) and CuO (2θ = 42, 45, and 75°). For investigating the surface, morphology, and size of γ-Fe2O3@CuO NPs, we used SEM and TEM. The resulting image (Fig. 2) shows uniform and minuscule nanoparticles. The approximate size of the particles was estimated at 30–50 nm. The magnetic properties of γ-Fe2O3@CuO were studied by vibrating sample magnetometry (VSM). Figure 3 shows the magnetization curve as a function of applied field in the range from –10000 to 10000 Oe. The saturation magnetization is 48.8 emu/g.
Initially, the oxidative coupling of ethyl acetoacetate with N,N-dimethylformamide was carried out using γ-Fe2O3@CuO as catalyst in the presence of various oxidants. When TBHP was used as an external oxidant, γ-Fe2O3@CuO promoted coupling of ethyl acetoacetate with DMF to afford the corresponding enol carbamate (Table 1, entry no. 9). The amount of the catalyst was initially optimized: it was found that 20 mg of γ-Fe2O3@CuO was sufficient to promote the reaction (entry no. 6). The effect of temperature was also evaluated. Elevated temperature was generally effective for the oxidative coupling of ethyl acetoacetate with DMF. As can be seen from Table 1, heating at 80°C proved to be the most suitable (entry no. 9), and further increasing the temperature to 100°C did not enhance the yield (entry no. 10). No desired carbamate was formed in the absence of a catalyst or oxidant (entry nos. 11 and 12).
Under the optimized conditions, the scope of this reaction was explored with various β-dicarbonyl compounds, and the results are summarized in Table 2. All products were characterized by measuring their melting points (in some cases) and recording IR and 1H and 13C NMR spectra.
Scheme 3 shows a plausible mechanism for the coupling of N,N-dimethylformamide with β-dicarbonyl compounds in the presence of γ-Fe2O3@CuO. The copper complex generated from copper salt and β-dicarbonyl compounds coordinates to DMF and undergoes internal nucleophilic addition to form copper hemiacetal species 1. Intermediate 3 is protonated to give compound 5, and the latter reacts with the radical generated by copper-catalyzed decomposition of TBHP, followed by single-electron transfer (SET) in hemiacetal radical 6, yielding gives the final enol carbamate.
The reusability of the catalyst was also evaluated. After completion of the reaction, the magnetic nanocatalyst was separated from the reaction mixture using an external magnet, washed, dried under reduced pressure at room temperature for 12 h, and used for the next reaction. The catalyst could be reused at least five times without any significant loss of catalytic activity (Fig. 4).
EXPERIMENTAL
All solvents and chemical were analytical-grade commercial products and were used without further purification. The FT-IR spectra were obtained over the range 400–4000 cm–1 with a Nicolet IR100 FT-IR spectrometer from samples pressed with spectroscopic grade KBr. The X-ray powder diffraction pattern was recorded at room temperature using a Philips X-Pert 1710 diffractometer (Co Kα radiation, λ = 1.78897 Å; voltage 40 kV, current 40 mA, scan range 10 ≤ 2θ ≤ 90°, scan speed 0.02 deg/s). The morphology of the catalyst was studied with scanning electron microscopy using Philips XL 30 and S-4160 scanning electron microscopes on gold-coated samples. The magnetic properties of γ-Fe2O3@CuO nanoparticles were measured with a vibrating magnetometer/Alternating Gradient Force Magnetometer (MDK, Iran).
Preparation of γ-Fe2O3@CuO nanoparticles. A mixture of 3.7 mmol of FeCl3·6H2O and 1.85 mmol of FeCl2·4H2O was dissolved in 30 mL of deionized water under vigorous stirring (700 rpm). Aqueous ammonia (25%, w/w, 10 mL) was added dropwise to the mixture under stirring to maintain the pH value at 11. The resulting black dispersion was continuously stirred for 1 h at room temperature and was then refluxed for 1 h. To modify the magnetic surface, 40 mL ethanol was added to the suspension, the mixture was stirred at 40°C for 1 h, 10 mL of tetraethyl orthosilicate was added, and the suspension was stirred for 24 h. The precipitate of Fe3O4 nanoparticles was separated from the aqueous solution by magnetic decantation, washed with diethyl ether and pure water several times, and dried at 140°C for 24 h to obtain γ-Fe2O3. The product was dispersed in water, 2 mmol of CuCl2·2H2O was added, and the mixture was stirred at room temperature for 24 h. The nanocatalyst was separated from the aqueous solution by magnetic decantation, washed with water, and dried at 110°C for 24 h.
REFERENCES
Monnier, F. and Taillefer, M., Angew. Chem., Int. Ed., 2009, vol. 48, p. 6954. https://doi.org/10.1002/anie.200804497
Evano, G. and Blanchard, N.M., Chem. Rev., 2008, vol. 108, p. 3054. https://doi.org/10.1021/cr8002505
Corbet, J.P. and Mignani, G., Chem. Rev., 2006, vol. 106, p. 2651. https://doi.org/10.1021/cr0505268
Correa, A., Mancheño, O.G., and Bolm, C., Chem. Soc. Rev., 2008, vol. 37, p. 1108. https://doi.org/10.1039/B801794H
Würtz, S. and Glorius, F., Acc. Chem. Res., 2008, vol. 41, p. 1523. https://doi.org/10.1021/ar8000876
Zhao, J., Fang, H., Zhou, W., and Pan, Y., J. Org. Chem., 2014, vol. 79, p. 3847. https://doi.org/10.1021/jo500192h
Gattinoni, S., Simone, C.D., Dallavalle, S., Fezza, F., Nannei, R., Amadio, D., Minetti, P., Quattrociocchi, G., Caprioli, A., Borsini, F., Cabri, W., Penco, S., Merlini, L., and Maccarrone, M. ChemMedChem, 2010, vol. 5, p. 357. https://doi.org/10.1002/cmdc.200900472
Sasaki, D. and Dixneuf, P.H., J. Chem. Soc., Chem. Commun., 1986, p. 790. https://doi.org/10.1039/C39860000790
Mahé, R., Dixneuf, P.H., and Lécolier, S., Tetrahedron Lett., 1986, vol. 27, p. 6333. https://doi.org/10.1016/S0040-4039(00)87801-6
Gagan, C., Dashan, W., and Howard, A., Chem. Commun., 2007, p. 4809. https://doi.org/10.1039/B711298J
Dalaigh, C.O., Corr, S.A., Gun’ko, Y., and Connon, S.J., Angew. Chem., Int. Ed., 2007, vol. 46, p. 4329. https://doi.org/10.1002/anie.200605216
Shin, J., Kim, H., and Lee, I.S., Chem. Commun., 2008, p. 5553. https://doi.org/10.1039/B812690A
Polshettiwar, V., Baruwati, B., and Varma, R.S., Chem. Commun., 2009, p. 1837. https://doi.org/10.1039/B900784A
Abu-Reziq, R., Alper, H., Wang, D., and Post, M.L., J. Am. Chem. Soc., 2006, vol. 128, p. 5279. https://doi.org/10.1021/ja060140u
Cano, R., Yus, M., and Ramon, D.J., ACS Catal., 2012, vol. 2, p. 1070. https://doi.org/10.1021/cs300056e
Riente, P., Yadav, J., and Pericas, M.A., Org. Lett., 2012, vol. 14, p. 3668. https://doi.org/10.1021/ol301515d
Costa, V.V., Jacinto, M.J., Rossi, L.M., Landers, R., and Gusevskaya, E.V., J. Catal., 2011, vol. 282, p. 209. https://doi.org/10.1016/j.jcat.2011.06.014
Wang, J., Xu, B., Sun, H., and Song, G., Tetrahedron Lett., 2013, vol. 54, p. 238. https://doi.org/10.1016/j.tetlet.2012.11.009
Rattanaburi, P., Khumraksa, B., and Pattarawarapan, M., Tetrahedron Lett., 2012, vol. 53, p. 2689. https://doi.org/10.1016/j.tetlet.2012.03.063
Hasani, H. and Toosi, F.S., J. Indian Chem. Soc., 2019, vol. 96, p. 1395.
Azizi, K., Esfandiary, N., Karimi, M., Kazemi, M., and Heydari, A., Appl. Organomet. Chem., 2016, vol. 31, p. 1. https://doi.org/10.1002/aoc.3658
Arfaoui, L., Janene, F., Kouass, S., Mignard, S., Touati, F., and Dhaouadi, H., Russ. J. Inorg. Chem., 2019, vol. 64, p. 1687. https://doi.org/10.1134/S003602361913006
Azizi, K., Karimi, M., and Heydari, A., Tetrahedron Lett., 2015, vol. 56, p. 812. https://doi.org/10.1016/j.tetlet.2014.12.110
Saberi, D. and Heydari, A., Tetrahedron Lett., 2013, vol. 54, p. 4178. https://doi.org/10.1016/j.tetlet.2013.05.113
ACKNOWLEDGMENTS
The authors are thankful to Pharmed Medical Industries for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hassani, H., Toosi, F.S. & Feizi, N. Oxidative Coupling of Dimethylformamide with β-Dicarbonyl Compounds Using γ-Fe2O3@CuO Nanoparticles. Russ J Org Chem 56, 1654–1659 (2020). https://doi.org/10.1134/S1070428020090249
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020090249