Abstract—
Currently, brain tumors are becoming more common and their clinical picture is aggravated by serious complications. According to the statistics of the World Health Organization (WHO), glioblastoma multiforme (GBM) is the most aggressive brain tumor with high invasive capacity, which is difficult to predict, while most cases are sporadic and do not have a genetic predisposition. Since GBM cannot be simply eliminated by operation, drug availability to penetrate the blood-brain barrier (BBB) is greatly complicated and the accumulation of chemotherapeutics in GBM is low, the therapeutic effects are poor, and there is a strong need to develop various approaches to deliver drugs to the CNS. The vector delivery of antitumor drugs is becoming more relevant, as well as various drugs that change the permeability of the BBB to facilitate the passage of antitumor drugs and their greater specific accumulation in tumor cells. The reversible short-term opening of tight junctions in brain endothelial cells and the effect on the functioning of active outflow transport systems represented by ATP-binding cassette transporters have been under serious research focus for the last few years in order to develop the appropriate drug delivery to the brain to treat GBM. A particularly promising direction in this area is the development of drugs that do not violate the integrity of the BBB and do not require the introduction of additional drugs to improve their activity and permeability. Active delivery nanoparticles are more effective than passively directed nanoparticles. Drugs that induce changes in the permeability of the BBB for various nanoparticles and other anticancer drugs are very effective, but they have a number of disadvantages and can cause complications. Therefore, before using these substances, all the risks should be evaluated, the controllability of this process, and the effectiveness of the drugs that inhibit ongoing reactions. It is considered safer and more effective to use active targeted drug delivery, which uses the attachment of site-specific ligands to the surface of nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
At present, the prevalence of brain tumors is increasing, and their clinical picture is aggravated by more and more serious complications. GBM is one of the most common types of tumor. According to the World Health Organization (WHO), it accounts for up to 52% of primary brain tumors and up to 20% of all intracranial tumors. GBM is one of the most aggressive and difficult-to-predict brain tumors; most cases are sporadic and do not have a genetic predisposition [1]. In addition, the overwhelming majority of anticancer drugs have an extremely low bioavailability, and therefore a reduced dose of the drug gets to the GBM cells compared to the administered dose. This complicates the development of new drugs, reduces the effectiveness of already known drugs. Therefore, in recent years, drugs with vector delivery are becoming more and more popular.
In this article, we will consider various options for the delivery of anticancer drugs to glioblastoma (GBM), and analyze their strengths and weaknesses.
METHODOLOGY FOR RESEARCHING NEW METHODS OF TREATMENT FOR GLIOBLASTOMA
When studying the features of targeted drug delivery to GBM cells and chemotherapeutic induction of changes in the permeability of the blood-brain barrier (BBB), published articles from the Elsevier, NCBI MedLine, Scopus, Scholar.Google, Embase, Web of Science, MedLine, the Cochrane Library, EMBASE, Global Health, CyberLeninka and RSCI databases were analyzed for better drug penetration. The following keywords were used to search for English-language articles: “Glioblastoma,” “targeted therapy,” “tight junctions,” “TJs,” “BBB,” “blood brain barrier,” “case report,” “targeted drug delivery,” “EPR-effect,” “statistics,” “ABC-transporters,” “chitosan nanoparticles,” “nps,” “nanoparticles drug.” The following keywords were used to search for Russian-language articles: “Glioblastoma,” “clinical case.” The assessment of the acceptability of the English- and Russian-language original sources was carried out in several stages: they looked through the headings, abstracts and full-text articles, then carried out an additional search for the sources indicated in the selected articles. Papers containing original studies in small groups of patients (or experimental animals) were excluded, as well as articles that provided preliminary research results or duplicated the results of previous research.
FEATURES OF VECTOR DELIVERY OF ANTITUMOR DRUGS
Since, at present, brain tumors are very common, and every year the clinical picture of oncological diseases becomes more and more difficult [2], vector delivery of anticancer drugs is becoming much more relevant. The advantages of using them are undeniable [3]:
(1) It becomes possible to use very aggressive drugs with the least harm to the body, because the dose of the drug is several times less [4].
(2) The toxicity of therapy decreases [5], therefore, the tolerability of such therapy [6] is improved since the drug is delivered directly to the tumor; its negative effect on other tissues of the body is significantly reduced.
(3) There is a high selectivity of anticancer drugs, achieved by targeted transport to target cells using protein vectors [7, 8].
This method of drug delivery to the target is based on the covalent attachment of a protein vector to the drug, which helps to deliver this conjugate into the cell via receptor-mediated endocytosis.
In this review article, we focus on the vector delivery of drugs to cells of GBM multiforme, one of the most common brain tumors [9], undirected drug delivery to which is significantly complicated [10, 11], which means that targeted delivery of chemotherapy drugs could help solve a number of problems.
VECTOR MEDICINE DELIVERY FOR GBM CELLS
Indeed, one of the most pressing problems in the treatment of glioblastoma multiforme is the relatively poor bioavailability and high doses of the drug used [12, 13]. Therefore, nanoparticles with active or passive targeted delivery are considered one of the most effective drug delivery systems [14, 15]. Passively targeted drugs are delivered through the EPR effect, an increased permeability and retention effect in which molecules of a certain size (usually liposomes, nanoparticles, and macromolecular drugs) accumulate in tumor tissue more than in normal tissues. Thus, this method takes into account and uses such features of the tumor as high vascular density, a well-developed vascular network, ineffective lymphatic drainage, as well as the unique properties of the nanoparticles themselves: their size and shape, characteristics of their surface since they influence the EPR effect [16, 17].
However, passive directional action has significant drawbacks [18]: The EPR effect depends on the diffusion of the drugs, however, only a few drugs can effectively diffuse across cell membranes. Diffusion of drugs in brain tumors is often impaired because they have a relatively weak EPR effect due to the dense matrix of the brain; an increase in the pressure of the interstitial (intercellular) fluid in the tumor due to ineffective lymphatic drainage leads to the accumulation of larger particles in the tumor and the diffusion of smaller ones. Therefore, when injected intravenously, ~95% of nanoparticles with a passive targeted delivery system accumulate nonspecifically in other organs and do not reach the tumor [14, 16].
There is at least one more neuroprotective barrier for the penetration of such drugs to brain tumor cells – the BBB [17]. Therefore, passively directed drugs cannot reach invasive tumor cells in sufficient quantities, and the EPR effect is too weak near infiltrating tumor cells [19–21]. In addition, the blood-brain tumor barrier also prevents drugs from entering the tumor mass, especially with the high activity of TIL cells (tumor-infiltrating lymphocytes; lymphocytes infiltrating the tumor), thereby promoting resistance to chemotherapy and disease recurrence [22, 23].
DIFFERENT METHODS OF DELIVERY OF ANTITUMOR DRUGS TO OVERCOME THE BBB
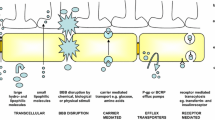
Relatively recently, a new method of delivering chemotherapy drugs to tumor cells to overcome the BBB was developed. It consists in the reversible and short-term opening of tight junctions in the endothelial cells of the brain. The stimuli of this process can be different: chemical (mannitol), biological (histamine and bradykinin), physical (ultrasound and electromagnetic waves). The principle of their action, accordingly, is also different [24, 25]. For example, mannitol reduces the volume of endothelial cells in the brain, dehydrating them, thereby changing their shape and opening tight junctions for several hours. And bradykinin acts on β2-receptors of endothelial cells of the brain, which leads to disruption of the integrity of tight junctions and, accordingly, an increase in the BBB permeability for drugs [15]. In addition, surfactants, polysorbate 80 and sodium dodecyl sulfate, can also destroy dense compounds [14, 15].
However, the application of this method is limited by a number of factors:
(1) high toxicity;
(2) violation of the neuroprotective function of the BBB;
(3) violation of the BBB integrity is not enough to achieve a significant result in patients with glioblastoma multiforme, because drugs still need to overcome other physiological barriers (e.g., brain parenchyma) to reach target cells [14];
(4) lack of direction of action [15].
Active outflow transport systems, represented by ATP-binding cassette transporters (ABC-transporters), play an important role in protecting the BBB from the penetration of anticancer drugs through it [26]. Anticancer drugs are substrates for efflux transporters, which limit the penetration of drugs to tumor cells. Therefore, new methods are being developed to increase the amount of the drug that penetrates the brain cells (without violating the integrity of the BBB), which include blocking outflow and blocking outflow transporters [15].
However, specific inhibitors of efflux transporters also have a number of disadvantages: insufficient degree of inhibition, uncontrolled degree of inhibition, and increased BBB permeability after inhibition, which increases the penetration of toxic elements into the brain. So far, no statistically significant results have been found in clinical trials of efflux transporter inhibitors.
Therefore, before use, it is necessary to take into account the required level of inhibition and the overall safety of this therapeutic strategy; it is necessary to find the most suitable “drug-inhibitor” combination in accordance with the target tissue [27, 28].
To increase the selectivity of absorption and accumulation, active targeted drug delivery is used, in which the attachment of site-specific ligands to the surface of nanoparticles is used. Affinity ligands (antibodies, peptides, or aptamers) bind on the surface of target cells with antigens or receptors that are overexpressed by tumor cells and not expressed by healthy cells. Site-specific binding of ligands leads to internalization of nanoparticles through receptor-mediated endocytosis and thereby enhances therapeutic effects. However, the main group of nanoparticles is developed without directly changing the characteristics of the receptor binding site [12, 14, 17]. It is interesting to note that upon systematic administration, nanoparticles usually form a “crown” layer. This is due to the circulation in biological fluids of the body of proteins, peptides and other cellular substances that are adsorbed on the surface of nanoparticles, usually changing their initial physicochemical properties and giving them a new biological identity, affecting the absorption of nanoparticles by cells, circulation time and bioavailability [29, 30].
CONCLUSIONS
Thus, various methods of delivering chemotherapy drugs to GBM cells are used. Active delivery nanoparticles are more efficient than passively targeted nanoparticles. Drugs that induce changes in the BBB permeability for various nanoparticles and other anticancer drugs are very effective, but they have a number of drawbacks and can lead to the development of complications, therefore, before using these drugs, one should first assess all the risks, the controllability of this process, and the effectiveness of drug-inhibitors of ongoing reactions. Active targeted drug delivery is considered safer and more effective when the attachment of site-specific ligands to the surface of nanoparticles is used.
REFERENCES
Louis, D.N., Ohgaki, H., Wiestler, O.D., Cavenee, W.K., Burger, P.C., Jouvet, A., Scheithauer, B.W., and Kleihues, P., Acta Neuropathol., 2007, vol. 114, pp. 97–109.
Wu, W., Zhong, D., Zhao, Z., Wang, W., Li, J., and Zhang, W., World J. Surg. Oncol., 2017, vol. 15, p. 231. https://doi.org/10.1186/s12957-017-1300-7
Miranda, A., Blanco-Prieto, M.J., Sousa, J., Pais, A., and Vitorino, C., Int. J. Pharm., 2017, vol. 531, pp. 389–410. https://doi.org/10.1016/j.ijpharm.2017.07.049
Touat, M., Idbaih, A., Sanson, M., and Ligon, K.L., Ann. Oncol., 2017, vol. 28, pp. 1457–1472. https://doi.org/10.1093/annonc/mdx106
Le Rhun, E., Preusser, M., Roth, P., Reardon, D.A., Bent, M., Wen, P., Reifenberger, G., and Weller, M., Cancer Treat. Rev., 2019, vol. 80, p. 101896. https://doi.org/10.1016/j.ctrv.2019.101896
Deng, S., Liu, L., Wang, D., Tong, D., and Zhao, G., World Neurosurg., 2018, vol. 110, pp. 174–179. https://doi.org/10.1016/j.wneu.2017.11.038
Shamul, J.G., Shah, S.R., Kim, J., Schiapparelli, P., Vazquez-Ramos, C.A., Lee, B.J., Patel, K.K., Shin, A., Quinones-Hinojosa, A., and Green, J.J., Int. J. Nanomed., 2019, vol. 14, pp. 10047–10060.
Goryaynov, S.A., Gol’dberg, M.F., Golanov, A.V., Zolotova, S.V., Shishkina, L.V., Ryzhova, M.V., Pitskhelauri, D.I., Zhukov, V.Yu., Usachev, D.Yu., Belyaev, A.Yu., Kondrashov, A.V., Shurkhay, V.A., and Potapov, A.A., Zh. Vopr. Neirokhir. im. N.N. Burdenko, 2017, vol. 81, pp. 5–16. https://doi.org/10.17116/neiro20178135-16
Liu, Y., Hao, S., Yu, L., and Gao, Z.Z., World J. Surg. Oncol., 2015, vol. 13, p. 142. https://doi.org/10.1186/s12957-015-0558-x
Moraes, F.Y., Lo, A., Morgan, E.R., Millar, B.A., Shultz, D.B., Maurice, C., Harlos, C., Kongkham, P., Bernstein, M., Zadeh, G., Laperriere, N., Mason, W., and Berlin, A., Can. J. Neurol. Sci., 2018, vol. 45, pp. 199–205. https://doi.org/10.1017/cjn.2017.278
Chanchotisatien, A., Xiong, J., Yu, J., and Chu, S., World Neurosurg., 2019, vol. 122, pp. 573–576. https://doi.org/10.1016/j.wneu.2018.11.113
Wang, K., Kievit, F.M., Jeon, M., Silber, J.R., Ellenbogen, R.G., and Zhang, M., Adv. Healthcare Mater., 2015, vol. 4, pp. 2719–2726. https://doi.org/10.1002/adhm.201500563
Whittle, J.R., Lickliter, J.D., Gan, H.K., Scott, A.M., Simes, J., Solomon, B.J., MacDiarmid, J.A., Brahmbhatt, H., and Rosenthal, M.A., J. Clin. Neurosci., 2015, vol. 22, pp. 1889–1894. https://doi.org/10.1016/j.jocn.2015.06.005
Wu, M., Fan, Y., Lv, S., Xiao, B., Ye, M., and Zhu, X., Drug Deliv., 2016, vol. 23, pp. 2720–2725.
Zhan, C., Li, B., Hu, L., Wei, X., Feng, L., Fu, W., and Lu, W., Angew. Chem., Int. Ed. Engl., 2011, vol. 50, pp. 5482–5485. https://doi.org/10.1002/anie.201100875
Zhang, B., Zhang, Y., Liao, Z., Jiang, T., Zhao, J., Tuo, Y., She, X., Shen, S., Chen, J., Zhang, Q., Jiang, X., Hu, Y., and Pang, Z., Biomaterials, 2015, vol. 36, pp. 98–109.
Zhang, H., Zhang, W., Zhou, Y., Jiang, Y., and Li, S., Transl. Oncol., 2017, vol. 10, pp. 229–240. https://doi.org/10.1016/j.tranon.2016.12.011
Raucher, D., Dragojevic, S., and Ryu, J., Front. Oncol., 2018, vol. 8, p. 624. https://doi.org/10.3389/fonc.2018.00624
Beauchesne, P., Blonski, M., and Brissart, H., In Vivo, 2011, vol. 25, pp. 991–993.
Luthra, P.M. and Lal, N., Eur. J. Med. Chem., 2016, vol. 109, pp. 23–35. https://doi.org/10.1016/j.ejmech.2015.11.049
Madan, J., Pandey, R.S., Jain, V., Katare, O.P., Chandra, R., and Katyal, A., Nanomedicine, 2013, vol. 9, pp. 492–503. https://doi.org/10.1016/j.nano.2012.10.003
Zhang, L., Habib, A.A., and Zhao, D., Oncotarget, 2016, vol. 7, pp. 38693–38706. https://doi.org/10.18632/oncotarget.9584
Zou, L., Tao, Y., Payne, G., Do, L., Thomas, T., Rodriguez, J., and Dou, H., Oncotarget, 2017, vol. 8, pp. 6564–6578. https://doi.org/10.18632/oncotarget.14169
Wen, L., Tan, Y., Dai, S., Zhu, Y., Meng, T., Yang, X., Liu, Y., Liu, X., Yuan, H., and Hu, F., Drug Delivery, 2017, vol. 24, pp. 1843–1855. https://doi.org/10.1080/10717544.2017.1386731
Chang, J., Mancuso, M.R., Maier, C., Liang, X., Yuki, K., Yang, L., Kwong, J.W., Wang, J., Rao, V., Vallon, M., Kosinski, C., Zhang, J.J., Mah, A.T., Xu, L., Li, L., Gholamin, S., Reyes, T.F., Li, R., Kuhnert, F., Han, X., Yuan, J., Chiou, S.H., Brettman, A.D., Daly, L., Corney, D.C., Cheshier, S.H., Shortliffe, L.D., Wu, X., Snyder, M., Chan, P., Giffard, R.G., Chang, H.Y., Andreasson, K., and Kuo, C.J., Nat. Med., 2017, vol. 23, pp. 450–460. https://doi.org/10.1038/nm.4309
Stoyanov, G.S., Dzhenkov, D., Ghenev, P., Iliev, B., Enchev, Y., and Tonchev, A.B., Med. Oncol., 2018, vol. 35, p. 27. https://doi.org/10.1007/s12032-018-1083-x
Beier, C.P., Schmid, C., Gorlia, T., Kleinletzenberger, C., Beier, D., Grauer, O., Steinbrecher, A., Hirschmann, B., Brawanski, A., Dietmaier, C., Jauch-Worley, T., Kölbl, O., Pietsch, T., Proescholdt, M., Rümmele, P., Muigg, A., Stockhammer, G., Hegi, M., Bogdahn, U., and Hau, P., BMC Cancer, 2009, vol. 9, p. 308. https://doi.org/10.1186/1471-2407-9-308
Qu, J., Zhang, L., Chen, Z., Mao, G., Gao, Z., Lai, X., Zhu, X., and Zhu, J., Drug Delivery, 2016, vol. 23, pp. 3408–3416.
Litofsky, N.S., Mix, T.C., Baker, S.P., Recht, L.D., and Smith, T.W., Surg. Neurol., 1998, vol. 50, pp. 579–585.
Yabar, A., Melendez, R., Munoz, S., Deneo, H., Freire, J., Dominguez, V., Carrasco-Navarro, R.M., Diaz, M.E., and Velarde-Lopez, R.E., Mol. Clin. Oncol., 2017, vol. 6, pp. 503–509. https://doi.org/10.3892/mco.2017.1185
Author information
Authors and Affiliations
Contributions
Authors E.E. Tyagunova and R.K. Kostin made an equal contribution to the work, equal to that of the first author.
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This study does not contain any research involving humans or animals as research objects.
Conflict of Interests
The authors declare they have no conflict of interest.
Additional information
Abbreviations: EPR effect, the effect of increased permeability and retention; TIL, tumor-infiltrating lymphocytes, GBM, glioblastoma multiforme; BBB, blood-brain barrier.
Corresponding author: phone: +7 (977) 623-15-11.
Rights and permissions
About this article
Cite this article
Shlapakova, T.I., Tyagunova, E.E., Kostin, R.K. et al. Targeted Antitumor Drug Delivery to Glioblastoma Multiforme Cells. Russ J Bioorg Chem 47, 376–379 (2021). https://doi.org/10.1134/S1068162021020254
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021020254