Abstract
The Sr0.8Ce0.2Mn1 – yCoyO3 – δ (y = 0.2, 0.3, 0.4, 0.5, and 0.6) complex oxides with the perovskite structure obtained from simple oxides by the solid–state reaction technique have been examined using structural analysis and magnetic measurements. Substitution of Co for Mn in the dilute Sr0.8Ce0.2MnO3 antiferromagnet with a Néel temperature of TN = 210 K leads to a decrease in the degree of tetragonal distortion of the crystal structure and transition to the cubic cell. The degeneracy of the antiferromagnetic interaction (TN = 138 K at y = 0.2) observed at the first stage of the substitution of Co for Mn changes for its gradual enhancement with an increase in the magnetic transformation temperature up to 239 K at y = 0.6. An increase in the Co content weakens the competition between the ferromagnetic and antiferromagnetic couplings and reduces the temperature of the transition to the spin-glass-like state. The magnetic inhomogeneity and formation of Co2+–Mn4+ ferromagnetic clusters in Sr0.8Ce0.2Mn0.4Co0.6O2.69 at 140 K have been observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Manganites and cobaltites of alkaline- and rare-earth elements with the perovskite structure attract attention of researchers due to their interesting physical and chemical properties important for application. In particular, the interest in Ln1 – xAxMnO3 (Ln is the rare-earth element and A is the alkaline-earth element) manganites is due to the colossal magnetoresistive effect caused by the optimal balance between spin, charge, and orbital orderings [1, 2]. The SrCoO3 cobaltite is a ferromagnet with a Curie temperature of TC = 270–300 K and metallic conductivity [3], while the substituted cobaltite La1 – xSrxCoO3 has a complex phase diagram with the antiferromagnetism and insulator behavior at the low Sr concentrations and ferromagnetism and conductive properties at the high Sr concentrations.
In [4–6], the effect of substitution of Co for Mn in the La1 – xAxMn1 – yCoyO3 (A = Ca, Sr) complex oxides with the hole conductivity was studied. It was established that an increase in the Co content leads to the weakening of the ferromagnetic ordering and formation of the spin-glass-like state of the cluster type inside the ferromagnetic state. In particular, the magnetism of the La1.25Sr0.75MnCoO6 perovskite was identified using two transformations: a ferromagnetic transition at TC (217 K) and a frequency-dependent transition at Tg < 100 K [6]. The high TC value is determined by the local TC values of weakly fluctuating clusters based on the valence combination of Co2+–Mn4+, while the low spontaneous moment at 2 K results from their low content.
It is interesting to investigate the effect of cobalt on the structure and properties of electron-doped manganites, including A1 – xCe4 + xMnO3 (A is Sr or Ca) solid solutions (SSs) [7–9], which are considered to be potential cathode materials for solid-state fuel cells [10]. The Ce-substituted \({{{\text{A}}}_{{1 - x}}}{\text{Ce}}_{x}^{{4 + }}{\text{Co}}{{{\text{O}}}_{3}}\) cobaltites are attractive for studying the influence of a heterovalent substitution on the Curie temperature and conductivity [11, 12].
Substitution of high-valence Ce4+ cations for strontium in SrMnO3 and SrCoO3 significantly affects their structural and charge characteristics and magnetic and transport properties. As is known [13], SrMnO3 prepared under normal conditions has a hexagonal structure and is an antiferromagnet with a Néel temperature of TN = 278 K. Embedding of high-valence Ce4+ cations in the Sr sites first (at x = 0.01–0.05) stabilizes the Sr1 – xCexMnO3 cubic structure and, at 0.1 < x ≤ 0.3, the tetragonally distorted perovskite structure (sp. gr. I4/mcm). In this case, the corresponding number of Mn4+ cations are reduced to Mn3+ ones, which leads to a strong distortion of MnO6 octahedra and an increase in the parameter a and unit cell volume. The parameter c in Sr1 – xCexMnO3 first increases, attaining its maximum value at x = 0.15, and then decreases [14].
The magnetic and electrical properties of these SSs depend, to a great extent, on the x value. The Sr0.9Ce0.1MnO3 SS is a C-type antiferromagnet with TN = 290 K, which passes from the metallic to semiconductor state at 315 K. The magnetic behavior of Sr1 – xCexMnO3 at 0.1 < x < 0.3 is caused by the strong competition between the double exchange and superexchange couplings [9], which results in the absence of a magnetically ordered state in this system and the dominance of the cluster or spin-glass-like state at low temperatures (below 20 K). The temperature dependence of the magnetic susceptibility of the Sr0.8Ce0.2MnO3 sample has a broad maximum at 150–300 K, which is interpreted in the framework of a concept of dilute antiferromagnetism with TN = 210 K. According to [8], in the paramagnetic region (x ≥ 0.2), the ferromagnetic clusters form.
The solid solutions with x = 0.25 and 0.35 [9] are characterized by the negative magnetoresistive effect based on the charge ordering, which occurs near 110 K.
Zhang et al. [15] reported on the synthesis, str-ucture, and conductive properties of the Sr0.8Ce0.2Mn0.8Co0.2O3 complex oxide. In contrast to Sr0.8Ce0.2MnO3, the octahedra in the Co-containing compound structure are regular, which is due to the absence of Jahn–Teller Mn3+ cations. Embedding of cobalt to the Mn sites reduces the electrical conductivity. The electrochemical characteristics of the Co-doped Sr0.8Ce0.2MnO3 compound as a cathode material were described in [10].
The crystal structure of the Sr1 – xCexCoO3 – δ compound obtained under high-pressure [11] remains cubic up to x = 0.4. When the synthesis is performed in sealed ampoules, the cubic phase concentration range is limited to 0 < x <0.15 [12]. This SS has a crystal structure of the high-temperature SrCoO3 – x modification and is characterized by the high oxygen exchange and electrical conductivity. The oxygen deficiency in the Sr1 – yCeyCoO3 – δ compound increases with temperature and decreases with increasing partial pressure of oxygen and Ce concentration.
In this work, we present the results of investigations of the structural characteristics and magnetic pro-perties of the double-substitution Sr0.8Ce0.2Mn1 ‒ yCoyO3 – δ SSs in a wide concentration range (y is 0.2, 0.3, 0.4, 0.5, and 0.6). In our previous study [16] of two samples in this series (y = 0.3 and 0.4), we showed that embedding of cobalt to the manganese sites in Sr0.8Ce0.2Mn1 – yCoyO3 – δ is interesting in terms of the crystal structure evolution and competition between the antiferromagnetic and ferromagnetic couplings. In [17], we analyzed the Ce, Mn, and Co oxidation states in the Sr1 – xCexMn1 – yCoyO3 – δ SSs using X-ray absorption spectroscopy and magnetic measurements.
2 EXPERIMENTAL
The Sr0.8Ce0.2Mn1 – yCoyO3 – δ samples were synthesized by the solid-phase reactions from CeO2, MnO2, Co3O4, and SrCO3 simple oxides containing no lower than 99.95% of the basic substance. The stoichiometric mixtures of these oxides and strontium carbonate were thoroughly grinded, pressed at a pressure of 3000 kg/cm2, and sintered at a temperature increased with a step of 100°C with intermediate grinding each 10 h. The calcined reaction products were cooled in a furnace to room temperature. The initial annealing temperature was 950°C and the final temperature was 1350°C. The presence of impurities in the products was monitored by X-ray diffraction on a Shimadzu XRD-7000S diffractometer using the PDF 2 database (ICDD, USA, Release 2009). The processing of diffraction patterns and refinement of the crystallochemical parameters were performed by a Rietveld full-profile analysis using the FULLPROF 2016 program.
The oxygen content in the samples was analyzed by the weight loss upon calcination in the H2 flow at 950°C for four hours. The oxygen index in the initial samples was calculated taking into account the fact that during the reaction cobalt is reduced to the metallic state and the residual oxygen is bound to divalent manganese, trivalent cerium, and SrO.
The magnetic measurements were performed at the Center of Collective Use of the Mikheev Institute of Metal Physics, Ural Branch, Russian Academy of Sciences on a QD MPMS-XL-5 SQUID magnetometer in the temperature range of 2–300 K in magnetic fields of 0.5 and 5.0 kOe (dc susceptibility). The measurements were performed upon cooling the samples in measured (FC data) and zero (ZFC data) magnetic fields. Using the dynamic magnetic susceptibility (ac susceptibility) measuring technique, the real (χ') and imaginary (χ'') susceptibility components were determined at ac magnetic field amplitudes of up to 4 Oe and a frequency of 80 Hz. The magnetization curves and magnetic hysteresis loops were obtained at 2 K on the samples cooled in zero magnetic field according to the following scheme: first, the magnetization curve was measured in a magnetic field increased from 0 to +50 kOe and then the hysteresis loops were measured upon field variation from +50 to –50 kOe and from ‒50 to +50 kOe.
3 RESULTS AND DISCUSSION
3.1 Structural Characteristics of the Sr0.8Ce0.2Mn1 – yCoyO3 – δ (y = 0.2, 0.3, 0.4, 0.5, and 0.6) SS
According to the X-ray data, all the samples with y values between 0.2–0.6 are single-phase. The composition with y = 0.7 contains, along with the main phase, CeO2. The Sr0.8Ce0.2MnO3 crystal structure parameters obtained by us are almost consistent with those reported in [8]. Table 1 gives the parameters a and c of the Sr0.8Ce0.2Mn1 – yCoyO3 (y = 0–0.6) SS and oxygen contents the samples. It was established that the SSs with y = 0.2 and 0.3, similar to Sr0.8Ce0.2MnO3, are tetragonal (sp. gr. I4/mcm). The samples with y ≥ 0.4 have a cubic cell (sp. gr. Pm3m). Thus, the SSs with y ≥ 0.4 undergo the phase transition from the tetragonal to cubic syngony stable in the SSs with y = 0.5 and 0.6. The decrease in the oxygen content in the SS upon substitution of Co for Mn is indicative of the formation of oxygen-deficient samples, which is quite natural and agrees with the Sr0.9Ce0.1CoO3.69 cobaltite composition [12].
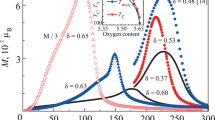
Figure 1 shows dependences of the parameters a and c and volume V of the Sr0.8Ce0.2Mn1 – yCoyO3 – δ SS unit cell on the y value. The a, c, and V values for the SSs with y = 0, 0.2, and 0.3 in Fig. 1 are presented for comparison for a simple perovskite cell. The averaged parameter V0 for the tetragonal SSs was calculated using the formula V0 = a2c/V. It can be seen that with an increase in the degree of substitution of Co for Mn, the crystal lattice parameters significantly change. Embedding of Co in the oxygen octahedron leads, first of all, to a sharp decrease in the parameter c, which is caused by a decrease in the number of Mn3+ cations. In the range from 0 to 0.3, the parameter a decreases weaker.
The unit cell volume of the SS with a tetragonal structure in this y range also decreases with increasing cobalt content. At y > 0.3 and in the cubic SSs, the parameter a and volume V increase. The solid solution with the maximum Co content (Sr0.8Ce0.2Mn0.4Co0.6O2.69) has the parameter a (3.841 Å) similar to the parameter of Sr0.9Ce0.1CoO2.74 (a = 3.843 Å [12]).
3.2 Magnetic Properties of the Sr0.8Ce0.2Mn1 – yCoyO3 – δ SS
The measured magnetic characteristics of the Sr0.8Ce0.2Mn1 – yCoyO3 – δ SS in the range of 2–300 K are shown in Figs. 2–5. The temperature dependences of the magnetic susceptibility χ for all the investigated samples at H = 5 kOe have two anomalies (Figs. 2 and 3) and demonstrate a decrease in x with the increasing Co content. The high-temperature Tmax1 anomalies at all the y values correspond to the transition from the paramagnetic to some new magnetic state. The fact that the FC magnetic susceptibility does not tend to a sharp increase at temperatures below Tmax1 allows us to conclude that there is no ferromagnetic ordering in all the investigated SSs. Below these maxima, the FC and ZFC curves start diverging; the discrepancy between them sharply increases at Tmax2 (at 20–40 K). The discrepancy between the FC and ZFC curves below the magnetic transformation point Tmax1 is typical of all the Co-containing samples. The measurements in magnetic fields of 0.5 and 5 kOe coincide, except for the SS with y = 0.6. It follows from Fig. 4 that the temperature dependence of χ for Sr0.8Ce0.2Mn0.4Co0.6O2.69 measured in a field of H = 0.5 kOe contains one more anomaly accompanied by a sharp divergence of the ZFC and FC curves. This anomaly accompanied by small maxima in the ZFC curves at 136 K in a field of 500 Oe is especially pronounced and shifted to 142 K when measured in a magnetic field of 100 Oe (inset in Fig. 4).
At the minimal substitution of Co for Mn (y = 0.2), the SS is characterized by a weaker broad maximum in the χ(T) dependence in the range of 100–160 K (Fig. 2) as compared with Sr0.8Ce0.2MnO3 [8]. The temperature dependence of the magnetic susceptibility below 138 K reveals a discrepancy between the ZFC and FC curves and the χ(T) curve has no kink typical of the AFM transition related to a decrease in the magnetic susceptibility. At the same time, the χ–1(T) dependence (Fig. 5) obeys the Curie–Weiss law far above Tmax1 (138 K) and has the positive Θ constant in the range of 250–400 K, as in Sr0.8Ce0.2MnO3 [8].
In the SSs with the higher cobalt content, the maximum at Tmax1 becomes more clear and shifts towards higher temperatures to 179, 195, 197, and 230 K at y = 0.3, 0.4, 0.5, and 0.6, respectively (Fig. 3). At y = 0.3, 0.4, and 0.5, upon further cooling in the range of 75–100 K, the magnetic susceptibility slightly increases, which is accompanied by the divergence of the FC and ZFC curves. At 28 K (y = 0.3), 20 K (y = 0.4), and 15 K (y = 0.5), the maxima in the ZFC dependences are fixed. It can be seen in Fig. 5 that at high temperatures the magnetic susceptibility of SSs with x values of 0.3, 0.4, 0.5, and 0.6 obeys the Curie–Weiss law
where C is the Curie constant (cm3) and Θ is the Curie–Weiss constant (K). The negative Θ values are indicative of the preferred antiferromagnetic couplings. The solid solution with x = 0.2 is characterized, similar to Sr0.8Ce0.2MnO3, by the positive Θ constant (Table 2).
The magnetization curves at 2 K (Fig. 6) contain narrow hysteresis loops without saturation traces in a magnetic field of 50 kOe with a residual magnetization of up to 0.02 emu/g and coercivity B of up to 350 Oe. Taking these data into account, the low-temperature anomalies for the Sr0.8Ce0.2Mn1 – yCoyO3 – δ SSs at 20–34, as in the case of Sr0.8Ce0.2MnO3 [9], should be attributed to the transitions to the spin-glass-like state.
According to the ac susceptibility measured for a number of samples (Fig. 7), the χ'(T) curves exhibit anomalies at temperatures similar to Tmax1 and Tmax2 recorded during the dc susceptibility measurements.
Tables 2 and 3 give the magnetic characteristics of all the investigated samples. The data on the sample with y = 0.2 were obtained from the χ–1(T) dependence in the range of 250–400 K [16]. According to the RAS and XANES data from [15, 17], cerium in these SSs has the form of Ce4+ cations; therefore, the total magnetic moment corresponds to cobalt and manganese cations. The Mn and Co valence and spin states in Sr0.8Ce0.2Mn1 – yCoyO3 – δ (y = 0.2–0.6) were calculated taking into account the data on the oxygen nonstoichiometry of the samples (Table 1) and the experimental effective magnetic moments. In the calculations, we used the following configurations of cations in the octahedral environment: Mn4+ (\(t_{{2g}}^{3}e_{g}^{0}\), S = 3/2), Mn3+ (\(t_{{2g}}^{3}e_{g}^{1}\), S = 2), Co2+ (\(t_{{2g}}^{5}e_{g}^{2}\), S = 3/2), and Co3+ (\(t_{{2g}}^{4}e_{g}^{2}\), S = 2 – the high-spin state). In addition, the spin–orbit contribution to the Co2+ effective magnetic moment was taken into account.
It follows from the data given in Table 2 that the experimental effective magnetic moment μexp (4.76μB) for the Sr0.8Ce0.2Mn0.8Co0.2O3 compound is much higher than the μcalc value calculated assuming manganese to have the form of Mn4+ cations and cobalt to have the Co2+ form. Meanwhile, as was shown in study [15] by the XANES technique, Mn in Sr0.8Ce0.2Mn0.8Co0.2O3 has the higher valence than in Sr0.8Ce0.2MnO3. This means that the larger Mn fraction in this SS has the form of Mn4+ cations and Co is in the form of Co2+ cations. This conclusion agrees with the results of investigations of other Mn and Co complex oxides, including those with the perovskite [6] and spinel structures [18], where the stable Mn4+‒Co2+ valence configuration, rather than Mn3+–Co3+, was also fixed. The μexp value higher than the calculated one can be attributed, as in Sr0.8Ce0.2MnO3, to the formation of ferromagnetic clusters in the paramagnetic region.
As follows from Table 2, with a further increase in the Co content in the SS, the number of Mn3+ cations decreases (to 16% at y = 0.3) and, at y = 0.4 and 0.5, they completely vanish. As the oxygen content in these samples decreases, the bivalent cobalt content grows.
It is interesting to compare the examined Co and Mn valence state evolution with the available data on the SrMn1 – xCoxO3 system. In this system, in contrast to Sr0.8Ce0.2Mn1 – xCoxO3, the substitution of Co for Mn leads to the formation of a number of homologous compounds with the quasi-one-dimensional structure, including Sr14Mn8Co3O33, Sr9Mn5Co2O21, and Sr4Mn2CoO9 [19]. In these compounds, the d elements are ordered in different structural positions in the form of Mn4+ and Co2+ cations. The presence of Mn4+ cations is considered to be the most probable situation in the SrCo1 – xMnxO3 – δ (0 ≤ x ≤ 0.30) SS [20]. It follows from Table 3 that in the Sr0.8Ce0.2Mn1 – yCoyO3 SS with increasing y, there is a trend to a decrease in the number of Mn3+ cations and preferred formation of the Mn4+–Co2+ valence combination.
Based on the data from Table 3, we can explain the change in the unit cell parameters in the series of Sr0.8Ce0.2Mn1 – yCoyO3–δ (y = 0–0.6) SSs. The decrease in the tetragonal distortion and parameters a and c at the first stage is consistent with a decrease in the content of Jahn–Teller Mn3+ cations with an ionic radius of 0.645 Å [21], which are replaced by Co3+ cations with a smaller size (0.61 Å). Further, Co2+ cations with an ionic radius of 0.745 Å make a decisive contribution to the unit cell size and transition to the cubic structure.
To estimate the nature of the magnetic interactions in the investigated solid solutions, we used the data from [8, 9] that Sr0.8Ce0.2MnO3 is characterized as a dilute antiferromagnet (TN = 210 K) with a spin-glass-like state below 20 K and ferromagnetic clusters or a microscopic inhomogeneous magnetic phase in the paramagnetic region. Analysis of the Mn and Co valence and spin states in Sr0.8Ce0.2Mn1 – yCoyO3 – δ (y = 0–0.6) on the basis of the magnetic measurements suggests that the possible antiferromagnetic (AFM) couplings in the investigated compositions are Mn3+–O–Mn3+, Mn4+–O–Mn4+, and Co3+–O–Mn4+ and the ferromagnetic (FM) couplings are Co2+–O–Mn4+ and Mn3+–O–Mn4+. In Sr0.8Ce0.2MnO3, the magnetism is determined by the competition between the Mn3+–O–Mn3+ and Mn4+‒O–Mn4+ couplings and the Mn3+–O–Mn4+ couplings. The shift of the broad maximum in Sr0.8Ce0.2Mn0.8Co0.2O2.95 to 138 K is indicative of the weakening of the short-range antiferromagnetic correlations at the retained complex χ–1(T) dependence above this temperature. The positive constant Θ for this sample and a significant discrepancy between the ZFC and FC curves below 35 K point out the conservation of the competing ferromagnetic and antiferromagnetic couplings, which result in the spin-glass-like state at low temperatures. An increase in Tmax1 with the Co content (up to 239 K at y = 0.6) and the growth of the negative constant Θ are apparently due to the enhancement of the AFM Mn4+–O–Mn4+ couplings against the background of the weakened FM couplings. The occurrence of a χ jump in the FC curve and a weak maximum in the ZFC χ(T) curve at 140 K in the Sr0.8Ce0.2Mn0.4Co0.6O2 sample when measured in magnetic fields of 500 and 100 Oe evidence for the magnetic inhomogeneity of this sample and are caused by the high content of Co2+ cations and formation of FM Co2+–Mn4+ clusters. This conclusion can be confirmed by the results of investigations of the SrCo1 – xMnxO2.74 SS, in which the ferromagnetism with TC = 150 K detected at the minimum Mn content (x = 0.05–0.10) is broken at the higher content of this element [20].
4 CONCLUSIONS
The results of crystal structure investigations and magnetic measurements of the electron-doped Sr0.8Ce0.2Mn1 – yCoyO3 – δ (y = 0.2, 0.3, 0.4, 0.5, and 0.6) SSs allowed us to draw the following conclusions. The solid solution with y ≥ 0.4 undergoes a concentrational phase transition from the tetragonal syngony (sp. gr. I4/mcm) to the cubic one (sp. gr. Pm3m), which is stable up to y = 0.7. The observed structural transformation is related to the substitution of Co2+ and Co3+ cations for Jahn–Teller Mn3+ ions and a decrease in the oxygen content. The evolution of the magnetic properties of the SSs was analyzed using the available data on the Sr0.8Ce0.2MnO3 manganite, which is characterized as a dilute antiferromagnet with TN = 250 K and a spin-glass-like behavior and contains ferromagnetic clusters in the paramagnetic region at low temperatures. Upon the minimum substitution of cobalt for manganese (у = 0.2) in Sr0.8Ce0.2MnO3, we observed a sharp decrease in TN to 138 K, a positive Θ constant in the Curie–Weiss law, and a spin-glass-like behavior at T < 35 K. The behavior of the samples with the high cobalt content is caused by a further decrease in the content of Mn3+ cations (at y = 0.3) and their disappearance (at y = 0.4 and 0.5). As a result, the magnetic transformation point shifts to the high-temperature region, apparently due to the enhancement of the AFM Mn4+–O–Mn4+ couplings at the retained spin-glass-like state at low temperature. In the sample with the maximum cobalt content, when measured in fields of 100 and 500 Oe, the region of ferromagnetic clusters was observed against the background of possible antiferromagnetism (TN = 230 K) below 140 K.
REFERENCES
A. Urushibara, Y. Moritomo, T. Arima, A. Asamitsu, G. Kido, and Y. Tokura, Phys. Rev. B 51, 14103 (1995).
M. Imada, A. Fujimori, and Y. Tokura, Rev. Mod. Phys. 70, 1039 (1998).
S. Balamurugan, K. Yamaura, A. B. Karki, D. P. Young, M. Arai, and E. Takayama Muromachi, Phys. Rev. B 74, 172406 (2006).
J. S. Srikiran, A. Das, P. L. Paulose, and S. K. Paranjpe, Appl. Phys. A 74, S814 (2002).
N. Gayathri, A. K. Raychaudhuri, and S. K. Tiwary, Phys. Rev. B 56, 1345 (1997).
G. V. Bazuev, A. V. Korolyov, M. A. Melkozyorova, and T. I. Chupakhina, J. Magn. Magn. Mater. 322, 494 (2010).
A. Sundaresan, J. L. Tholence, A. Maignan, C. Martin, M. Hervieu, B. Raveau, and E. Suard, Eur. Phys. J. B 14, 431 (2000).
W. J. Lu, B. C. Zhao, R. Ang, W. H. Song, J. J. Du, and Y. P. Sun, Phys. Lett. 346, 321 (2005).
P. Mandal, A. Hassen, and A. Loidl, Phys. Rev. B 69, 224418 (2004).
H. Gu, H. Chen, L. Gao, Y. Zheng, X. Zhu, and L. Guo, Electrochem. Acta 54, 3532 (2009).
S. Balamurugan, K. Yamaura, M. Arai, and E. Taka-yama Muromachi, Phys. Rev. B 76, 014414 (2007).
A. Maignan, B. Raveau, S. Hebert, V. Pralong, V. Caignaert, and D. Pelloquin, J. Phys.: Condens. Matter 18, 4305 (2006).
P. D. Battle, T. C. Gibb, and C. W. Jones, J. Solid State Chem. 74, 60 (1988).
H. Wu. K. Zhu, G. Xu, and H. Wang, Phys. B (Amsterdam, Neth.) 407, 770 (2012).
Z. Zhang, C. J. Howard, D. Kennedy, M. Matsuda, and M. Miyake, J. Phys.: Condens. Matter 21, 124218 (2009).
T. I. Chupakhina and G. V. Bazuev, Inorg. Mater. 47, 1361 (2011).
S. N. Shamin, V. V. Mesilov, M. S. Udintseva, A. V. Ko-rolev, T. I. Chupakhina, G. V. Bazuev, and V. R. Galakhov, Curr. Appl. Phys. 16, 1597 (2016).
G. V. Bazuev and A. V. Korolyev, J. Magn. Magn. Mater. 320, 2262 (2008).
G. V. Bazuev, Russ. Chem. Rev. 75, 749 (2006).
A. Maignan, S. Hebert, N. Nguyen, V. Pralong, D. Pelloquin, and V. Caignaert, J. Magn. Magn. Mater. 303, 197 (2006).
R. D. Shannon, Acta Crystallogr., A 32, 75 (1976).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Bondareva
Rights and permissions
About this article
Cite this article
Bazuev, G.V., Chupakhina, T.I. & Korolev, A.V. Effect of Cobalt on the Crystal Structure and Magnetism of Electron-Doped Sr0.8Ce0.2MnO3 Oxide. Phys. Solid State 60, 2491–2497 (2018). https://doi.org/10.1134/S1063783418120089
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063783418120089