The effect of the oxygen content δ in layered NdBaCo2O5 + δ cobaltite, where 0.37 ≤ δ ≤ 0.65, on the metal‒insulator transition, as well as on the magnetic and spin states of Co3+, is studied for the first time. An increase in δ reduces the metal‒insulator transition temperature TMI, the antiferromagnetic ordering temperature TN, and the Curie temperature TC by about 100–150 K. For all values of δ, the metal‒insulator transition occurs when the spin state of Co3+ ions changes from the HS/LS state in the metallic phase to the IS/LS state in the semiconducting phase, whereas with an increase in δ, the spin state of Co3+ ions changes from the IS/LS to HS/LS state. At δ ~ 0.65, a heavily doped semiconductor–bad metal transition occurs without any change in the spin state of Co3+ ions. The ferromagnetic behavior of NdBaCo2O5 + δ in the antiferromagnetic phase below TN is interpreted in terms of the metamagnetic model as the effect of the size of the rare earth Nd3+ ion on the antiferromagnetic state in layered cobaltites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Broad interest in ordered layered RBaCo2O5 + δ cobalt oxides, where R stands for a R3+ rare earth ion and δ is the oxygen content, is due to their unusual magnetic and transport properties [1, 2]. They have the layered perovskite-type crystal structure, consisting of layers located along the c axis, in which ROδ and BaO layers alternate with CoO2 layers. RBaCo2O5 + δ compounds with δ ≈ 0.5, where R = Eu, Gd, Tb, etc., from the middle of the rare earth row, are studied in the most detail [1–7]. RCo2O5.5 contains only Co3+ ions, which are located in the crystal lattice within an equal number of CoO6 octahedra and square CoO5 pyramids [1]. They exhibit a number of successive metal‒insulator (MI), paramagnetic (PM), ferromagnetic (FM), and antiferromagnetic (AFM) phase transitions [1–7].
The main problem concerns the nature and driving forces of the metal‒insulator transition in these materials. In contrast to manganites, the MI transition in cobaltites is not related to magnetic ordering. The properties of RBaCo2O5 + δ compounds, as well as of LaCoO3, are unusual mainly because cobalt ions can be in three different states: low-spin (LS), intermediate-spin (IS), and high-spin (HS) states. The energy differences between the spin states are mostly small [8] and can be easily overcome with a change in the temperature, forming unusual sequences of structural and other phase transitions, including the MI transition. The structural and magnetic data [4] indicate that, in GdBaCo2O5.5, the transition from the insulating to the metallic phase is related to the excitation of electrons from the LS state to the HS state corresponding to the eg band formed by Co3+ ions in octahedra without any changes in the IS state of Co3+ in pyramids. Although this model contradicts the structural data, it is widely accepted. Many researchers adhere to this model of the metal‒insulator transition in other RBaCo2O5.50 cobaltites as well. Refinement of the paramagnetic contribution of R3+ ions shows [9] that the transition can occur when the spin states of Co3+ ions in octahedra and pyramids change, in agreement with the structural data [4].
The size of the rare earth ion affects the crystal field at the Co ions and, therefore, it can change their spin state and the magnetic state of RBaCo2O5.5 [10]. The largest rare earth ions are Pr3+ and Nd3+ [11]. The neutron and synchrotron X-ray powder diffraction data [12] and muon spectroscopy data [13] show that, although the phase transition temperatures in NdB-aCo2O5.5 are similar to those of known cobaltites, their microscopic magnetic nature is quite different. In particular, the FM state is retained in NdBaCo2O5 + δ with δ ≈ 0.5 [14] and PrBaCo2O5.50 [15] below TN ∼ 230–250 K, while other cobaltites remain in the AFM state [1–7]. The metamagnetic state and ferromagnetic behavior of NdBaCo2O5 + δ with δ ≈ 0.5 at low temperatures is explained in [14] within the Landau model of metamagnetism [16] by the large size of rare earth ions and by the spin-state ordering (SSO) of Co3+ ions below T ∼ TSSO [12, 17–19].
The oxygen content δ in RBaCo2O5+δ, which can be varied in a wide range 0 ≤ δ ≤ 1, plays an important role [1]. It controls not only the average valency of Co ions (which can vary from 3.5+ for δ = 1 to 2.5+ for δ = 0) but also their oxygen (pyramidal or octahedral) environment and therefore has a strong effect on the spin state of Co ions. As a result, the magnetic and transport characteristics of these compounds are largely determined by the oxygen content [1–7].
Much less is known about the properties of R-BaCo2O5 + δ compounds with higher oxygen content δ > 0.5. The properties of electron- (δ < 0.5) and hole-doped (δ > 0.5) GdBaCo2O5 + δ single crystals are asymmetric. With an increase in the density of charge carriers in electron-doped compounds, the electrical resistivity increases and the magnetization decreases, and in the hole-doped ones, the electrical resistance decreases and the magnetization increases [3]. There are several studies of RBaCo2O5+δ compounds with a fixed composition, where R = Nd, Pr and δ ~ 0.7 [20–22]. Near the metal–insulator transition, the resistivity of NdBaCo2O5 + δ at δ ≈ 0.7 has an activation character [20]. The magnetic properties and paramagnet‒ferromagnet phase diagram of PrBaCo2O5+δ, where 0.35 ≤ δ ≤ 0.8, are unusual and differ from the properties of known layered cobaltites [15]. The metal‒insulator transition in PrBaCo2O5 + δ, where 0.5 ≤ δ ≤ 0.7, is explained by a change in the spin states of Co3+ ions [19]. It is of interest to compare the properties of -PrBaCo2O5 + δ [15, 19] and of the related NdBaCo2O5 + δ compound at different oxygen contents.
In this work, we present the results of our studies on the effect of the oxygen content on the PM‒FM (TC), FM‒AFM (TN), and metal‒insulator transitions and on their relation to the change in the Co3+ spin states in NdBaCo2O5 + δ polycrystals, where 0.37 ≤ δ ≤ 0.65. The paramagnetic contribution of the Nd3+ rare earth ions is taken into account, in contrast to other well-known works [15, 19, 21, 22]. It is found that the spin state of Co3+ ions in the metallic phase of NdBaCo2O5 + δ cobaltite is independent of δ, whereas the effective magnetic moment μeff/Co below the MI transition temperature increases with δ, approaching the value corresponding to the spin state of the metallic phase in NdBaCo2O5 + δ.
RESULTS
NdBaCo2O5 + δ polycrystals were synthesized by the solid-state reaction technique using Nd2O3, BaCO3, and Co3O4 as initial components. The preparation process includes stepwise annealing in air at T = 900–1125°C and slow cooling down to room temperature [1]. The absolute oxygen content was determined by the method of sample reduction in hydrogen. The initial samples had the oxygen content δ = 0.65 ± 0.02. The required oxygen content δ was achieved by additional annealing of the original sample at T = 350–800°C followed by quenching and was determined from the change in the sample weight [3], under the assumption that δ = 0.65. The weight of the samples was chosen such that the accuracy of determining δ was no worse than 0.01. According to the X‑ray powder diffraction data, all samples were single-phase. At room temperature, the samples with δ = 0.48‒0.65 have an orthorhombic structure and are described by the space group Pmmm (no. 47) with the ap × 2ap × 2ap unit cell, where ap is the pseudocubic perovskite lattice constant. The sample with δ = 0.37 also has the orthorhombic structure (no. 47) with the ap × ap × 2ap unit cell. The unit cell volume decreases with an increase in δ. The structural parameters of the samples agree with the published data [23]. The resistivity was measured by the four-probe method. The magnetic measurements were performed using the MPMS-5XL (Quantum Design) facility at the Shared Research Center, Mikheev Institute of Metal Physics, Ural Branch, Russian Academy of Sciences.
In Fig. 1, we show the temperature dependence of the magnetization of six NdBaCo2O5+δ samples with δ ranging from 0.37 to 0.65. At δ ≠ 0.5, in addition to Co3+ ions, Co2+ or Co4+ ions with the content |δ − 0.5| appear. The samples were cooled at zero magnetic field from 300 K to 10 K and measured in the magnetic field H = 1 kOe at temperatures up to 400 K. The form of the magnetization curves M(T) turns out to be nearly the same for all known layered RBaCo2O5.5 cobaltites. The magnetization increases sharply below TC ~ 280 K. Within a narrow temperature range, the sample appears to be in the FM state, where M(T) has the maximum at Tmax = TN ~ 250 K, below which it decreases gradually, suggesting the transition of the sample to the AFM state [1–7].
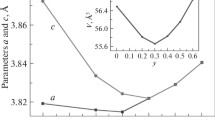
(Color online) Temperature dependence of the magnetization of NdBaCo2O5 + δ, (0.37 ≤ δ ≤ 0.65) at H = 1 kOe. For clarity of images, the values of M(T) for δ = 0.37–0.53 below 175 K are not shown. Their magnetization decreases monotonically with temperature. The inset demonstrates the dependence of the Curie temperature TC and of the AFM ordering temperature TN on the oxygen content. The PM contribution from Nd3+ ions is subtracted.
The presented temperature dependences of the magnetization M(T) of NdBaCo2O5+δ differ from those characteristic of known layered RBaCo2O5.5 cobaltite, in which the transition temperatures to FM (TC) and AFM (TN) are nearly independent of the choice of R [1–7]. As the oxygen content increases, the magnetization peak Mmax(δ) at TN changes nonmonotonically, exhibiting a minimum at δ = 0.60; then it increases, and the TN values become strongly lower (Fig. 1). The Curie temperature TC(δ), determined from the maximum value of dM/dT (see inset of Fig. 1), decreases with increasing oxygen content from 260 to 120 K and changes slightly up to δ = 0.53, and TC(δ) exhibits the maximum change at δ > 0.53‒ 0.60. The spontaneous magnetization Ms determined from M(H) up to 50 kOe at T = const appears about 15–20 K higher than the value TC determined from the maximum of dM/dT; i.e., the magnetic order apparently arises at TC(δ) ≈ 280–140 K. The formation of Ms at T ~ 140 K and δ = 0.65 agrees with the data reported in [20]. The transition temperature to the AFM state, determined from the temperature of the magnetization peak Tmax(δ) ≈ TN, is approximately 20 K lower than TC(δ), and the TC(δ) dependence has a similar form (see the inset of Fig. 1).
The main difference between NdBaCo2O5+δ and known cobaltites, except for PrBaCo2O5 + δ [15] and LaBaCo2O5.50 [24], is that the magnetization below TN(δ) exhibits the ferromagnetic behavior and remains finite at nonzero magnetic field. The data on M(T, H = 1 kOe) below 175 K for δ = 0.37–0.53 are not shown in Fig. 1, but the magnetization also remains finite, as for δ = 0.60–0.65. In PrBaCo2O5 + δ (0.37 ≤ δ ≤ 0.80), the FM interactions are also present at all temperatures below TC and even in the AFM phase [15].
The ferromagnetic behavior of NdBaCo2O5.48 below TN was explained by the metamagnetic state of this compound [14]. Below T ~ 20 K, NdBaCo2O5.48 at zero magnetic field is in the AFM state, and in a low magnetic field of 10–20 kOe, it transforms to the metamagnetic state, i.e., to the mixed FM + AFM state. Above T ~ 20 K, the sample involves a mixture of exchange-coupled ferromagnetic and antiferromagnetic phases. This situation is confirmed by the discovery of an exchange bias in NdBaCo2O5.48 [14].
Layered cobaltites are AFM materials with weakly coupled spin sublattices [3] and are metamagnets even at high temperatures [7]. In RBaCo2O5.50, where R = Gd, Tb, at T ∼ TN ∼ 250 K, the applied magnetic field reduces the AFM/FM transition temperature by about 1 K at Hcr ∼ 10 kOe, and at T = 0, the field Hcr ∼ 200–300 kOe is required to generate this transition [3, 7, 14].
At TN ~ 275 K and at zero magnetic field, Co3+ ions in a similar NdBaCo2O5.47 compound are ordered, forming a G-type AFM structure [12]. In the temperature range TN ∼ 275 K > T > TSSO ∼ 230 K, Co3+ ions are located in two positions with the pyramidal and octahedral oxygen environments. Below TSSO ∼ 230 K, the AFM spin-state ordered (SSO) phase arises in NdBaCo2O5.47, in which Co3+ ions are in four different states: in two different octahedra and pyramids. Following the Landau model of metamagnetism [16], it was assumed that the FM bond in Co layers in the layered compounds in the SSO state remains strong, whereas the AFM bond between Co layers separated by NdOδ layers is weakened because of a large size of Nd3+ ions. At not a high magnetic field, the transition between the AFM and FM states occurs.
We assume that such a model is also applicable at δ ≈ 0.37–0.65. Note that the effect of the oxygen content δ on TC and TN in RBaCo2O5 + δ with R = Gd [3], Pr [15], and Nd (see the inset of Fig. 1) is nearly the same: the values of TC(δ) and TN(δ) vary only slightly at δ ≈ 0.35–0.5 and decrease strongly (by ~100 K) at δ = 0.5–0.7. For the composition with δ = 0.7, the FM order in GdBaCo2O5 + δ also arises at temperatures T < 150 K, and an abrupt transition from the FM to AFM state occurs at T < 100 K [3], in contrast to NdBaCo2O5 + δ and PrBaCo2O5 + δ [15]. In L-aBaCo2O5.50, where La is the largest nonmagnetic rare earth ion [11], the FM behavior below TN is also explained by the effect of the size of the La3+ ion [24]. RBaCo2O5 + δ compounds with δ = 1 and R = Pr and La are ferromagnets with TC = 210 and 179 K, respectively [25, 26]. The formation of the ferromagnetic state below TN in RBaCo2O5 + δ with R = La, Pr [24–26], and Nd having a large ionic radius and its absence in the GdBaCo2O5 + δ compound [3] with a smaller ionic radius suggest that the ferromagnetic state of NdBaCo2O5 + δ below TN is determined by the large size of Nd3+ ions.
The exchange bias detected in NdBaCo2O5 + δ at δ = 0.37–0.53 and T = 77 K indicates the phase separation in this compound into exchange-coupled FM and AFM phases, which is characteristic of the metamagnetic state, and thus supports this assumption.
RBaCo2O5.50 compounds with the largest sizes of R3+ = La, Pr, and Nd ions exhibit the FM behavior at all temperatures below TC, even in the AFM phase [24–26], while compounds with smaller sizes of R3+ ions demonstrate the AFM behavior [1–7]. These results confirm the effect of the ionic size of R on the FM state in layered cobaltites.
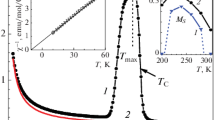
In Fig. 2, we show the temperature dependence of the resistivity ρ(T) of NdBaCo2O5 + δ at 0.37 ≥ δ ≥ 0.65 in the temperature range 100–400 K. For comparison, we present the ρ(T) data for δ = 0.70 [1]. The temperature dependence of the resistivity ρ(T) exhibits a semiconducting behavior: ρ(T) decreases monotonically with increasing temperature and oxygen content. After a sharp decrease in ρ(T) above TMI indicated in Fig. 2 by arrows, the sample passes to a state with the resistivity only slightly dependent on the temperature. In fact, the transition from the quasimetallic to the semiconductor state rather than the metal‒insulator transition occurs [1, 2]. The derivative dρ/dT remains negative above TMI, suggesting the semiconducting behavior of ρ(T), possibly related to the polycrystalline structure of the sample.
In a narrow temperature range (∼100–150K below TMI), the resistivity of NdBaCo2O5 + δ with 0.48 ≥ δ ≥ 0.65 can be described by the activation-type relationship [20]
With an increase in δ, the activation energy ΔE ch-aracterizing the resistivity changes from ΔE ∼ (50 ± 10) meV to ΔE ≈ 30 meV for δ = 0.65 (0.70). The pre-exponential factor also decreases from ρ0 ≈ 3 × 10‒3 Ω cm, which is characteristic of a disordered medium, to the value ρ0 ≈ 2×10–4 Ω cm, which is larger than that typical of semiconductors. The positive magnetoresistance MR = [ρ(H) − ρ(H = 0)]/ρ(H = 0) up to 2.5% at H = 10 kOe characteristic of semiconductors is observed in NdBaCo2O5.65 in the temperature range TC < T < TMI ~ 250 K.
It is interesting to note that TMI(δ), TN(δ), and TC(δ) (see insets of Figs. 1 and 2), differing in magnitude by about 100 K, at different oxygen contents, have approximately the same form: they change slightly at δ ≤ 0.53 and decrease steeply at δ > 0.60. The changes in ρ(T) decrease with an increase in δ. From 100 K to TMI, the resistivity changes by almost three orders of magnitude at δ = 0.37, whereas at δ = 0.65, it changes by less than one order of magnitude. At δ = 0.65, a heavily doped semiconductor–bad metal transition occurs with almost no change in the spin state. Qualitatively, the behavior of ρ(δ, T) agrees with changes in the effective magnetic moment μeff(δ, T) (see below).
Magnetic methods are among the main ones for determining the spin states of Co3+ ions in cobaltites. The spin state of Co3+ ions is determined from the measurements of the paramagnetic susceptibility described by the Curie–Weiss law χ(T) ∼ μ2eff/(T − θPM) above and below TMI [1–7]. For these purposes, the magnetic methods turn out to be rather complicated because it is difficult to distinguish the contribution of Co3+ ions in RBaCo2O5+δ and the PM contribution from rare earth ions R3+. The discrepancies between the spin states of Co3+ ions reported in different publications are probably due to the fact that the latter contribution is ignored or is taken into account incorrectly (see [9]). It is usually assumed that this contribution coincides with that of free R3+ ions and is determined using the expression for the paramagnetic susceptibility χ = μ2eff/3k(T − θPM), where μeff is the effective magnetic moment of the R3+ ion, k is the Boltzmann constant, and θPM = 0 is the Weiss paramagnetic temperature [3, 6, 7, 27]. The values of μeff and θPM are determined on the basis of the saturation of magnetization at a high magnetic field at low temperatures [9, 28], which for rare earth ions R3+ is described by the Brillouin function [29]
where \({{B}_{S}}(x)\) is the Brillouin function, NA is the Avogadro number, x = gμBJH/(k\((T - {{\theta }_{{{\text{PM}}}}})\)), g is the Landé g-factor, μB is the Bohr magneton, J is the total magnetic moment of an R3+ ion, and H is the applied magnetic field. In this work, we assume that the РМ contribution from Nd3+ ions in NdBaCo2O5 + δ is independent of δ and is described by Eq. (2) at \({{\theta }_{{{\text{PM}}}}} = - 18{\kern 1pt} \) K, as in NdBaCo2O5.48 [14].
In Figs. 3a and 3b, the symbols denote the temperature dependence of the inverse paramagnetic susceptibility χ–1(T) of NdBaCo2O5 + δ (δ = 0.37–0.65, H = 10 kOe), determined by the subtraction of the PM contribution from the Nd3+ ion. Circles are the measured values of \(\chi _{{\exp }}^{{ - 1}}\)(T) at δ = 0.48 and 0.63. All samples were cooled at H = 0 from 300 to 10 K, and the magnetization was measured up to 400 K at H = 1, 10, and 50 kOe. The solid lines show the χ–1(T) data for δ = 0.53 at H = 50 kOe and for δ = 0.65 at H = 1 kOe, which hardly differ from the χ–1(T) values at H = 10 kOe. The same data were obtained for other values of δ. These results prove the applicability of Eq. (2) for determining the PM contribution from Nd3+ ions.
(Color online) Temperature dependence of the inverse paramagnetic susceptibility χ–1(T) and of the differential magnetic moment \(\mu _{{{\text{eff}}}}^{{{\text{dif}}}}\)/Co of NdBaCo2O5 + δ (0.37 ≤ δ ≤ 0.65). Symbols denote the data at H = 10 kOe and lines correspond to H = 1 kOe or 50 kOe (see the main text). The paramagnetic contribution from Nd3+ ions is subtracted. The arrows indicate the metal‒insulator transition temperatures.
The temperature dependences χ–1(T) for δ = 0.37–0.60 are nearly the same and are similar to the observed dependences χ–1(T) in known layered cobaltites with δ ≈ 0.5. In these samples, it is almost impossible to separate a linear segment in the χ–1(T) plots. In fact, this means that the behavior of χ–1(T) cannot be described at a constant value of μeff(T), and the transition is accompanied by the changes in μeff(T) with temperature. On the other hand, for δ = 0.63 and 0.65 above and below TMI, a linear behavior of χ–1(T) is observed. To reveal the features of the metal‒insulator transition, the differential values \(\mu _{{{\text{eff}}}}^{{{\text{dif}}}}\)/Co(T) were determined. The values of χ–1(T) were measured with an interval of ΔT = 5 K, and at each step, the differential values \(\mu _{{{\text{eff}}}}^{{{\text{dif}}}}\)/Co3+(T) were determined taking into account the contribution of the specific content of Co2+ and/or Co+4 ions for all values of δ (Figs. 3c and 3d).
In a certain temperature range below 400 K, \(\mu _{{{\text{eff}}}}^{{{\text{dif}}}}\)/Co at δ = 0.37–0.60 remains constant (Figs. 3c and 3d). The temperature at which the slope of the χ‒1(T) plot changes sharply (which corresponds to a sharp decrease in \(\mu _{{{\text{eff}}}}^{{{\text{dif}}}}\)/Co) is accepted as the metal‒insulator transition temperature TST related to the spin-state transition. This temperature, TST, is approximately 10–15 K higher than the TMI temperature at δ = 0.37–0.53 (see inset of Fig. 2). These discrepancies are explained by the contribution of intergranular resistance to the resistivity of polycrystals. At δ = 0.60–0.65, neither ρ(T) shown in Fig. 2 nor χ–1(T) plotted in Figs. 3a and 3b demonstrates any pronounced transition boundary. According to the intuitive definition of this boundary, TST and TMI coincide with each other. Below 400 K, we observe a decrease in χ–1(T) proportional to the temperature at δ = 0.63(0.65) above and below TMI. The slopes of the χ‒1(T) curve above and below TMI are slightly different (see the solid lines plotted through the symbols corresponding to δ = 0.63 and 0.65 in Fig. 3b). Their behavior indicates that a small change in the spin state of Co3+ ions occurs near TMI.
In the metallic phase (δ = 0.37–0.65) at T > TMI (Figs. 3c and 3d), \(\mu _{{{\text{eff}}}}^{{{\text{dif}}}}\)/Co3+ = (3.43 ± 0.02)μB is independent of the oxygen content and corresponds to the HS/LS state of Co3+ ions in the ratio of 1 : 1. The significant deviation of \(\mu _{{{\text{eff}}}}^{{{\text{dif}}}}\)/Co from this state at δ = 0.37 and its slight deviation at δ = 0.60–0.65 are explained by the different contributions from Co2+ and Co4+ ions. Co2+ ions are always in the HS (S = 3/2) state because of a weaker crystal field than that for Co3+ ions, whereas Co4+ ions are always in the LS (S = 1/2) state because of a stronger crystal field [30]. With an increase in the content of Co2+ or Co4+ ions, the deviation of \(\mu _{{{\text{eff}}}}^{{{\text{dif}}}}\)/Co3+ from the HS/LS state increases, which is in reasonable agreement with the calculations of the contribution from Co2+ and Co4+ ions.
In the semiconductor phase at TC < T < TMI and δ = 0.37–0.53 (Fig. 3c), the compounds are in the IS/LS state, and most of the Co3+ ions are in the LS state. Then, an abrupt transition to the HS/LS state occurs in a narrow temperature range. At δ = 0.60–0.63 (Fig. 3d), the \(\mu _{{{\text{eff}}}}^{{{\text{dif}}}}\)/Co3+ values exceed those corresponding to the IS/LS state of Co3+ ions in a ratio of 1 : 1; i.e., most of the Co3+ ions are in the IS state.
Next, in Figs. 3a and 3b, we identify the fragments with a nearly linear temperature dependence on χ‒1(T) curves, find the values of \(\mu _{{{\text{eff}}}}^{{{\text{dif}}}}\)/Co3+ from the Curie–Weiss law (lines 1 and 2 in Fig. 4), and determine the Weiss paramagnetic temperature θPM (lines 3 and 4 in Fig. 4) above and below TMI depending on the oxygen content δ (Fig. 4). In the metallic phase (line 1 in Fig. 4) at T > TMI, μeff/Co3+ = (3.43 ± 0.02)μB does not depend on the oxygen content δ = 0.37–0.65 and corresponds to the HS/LS state of Co3+ ions in the ratio of 1 : 1. The significant deviation of μeff/Co from this state at δ = 0.37 and its slight deviation at δ = 0.65 are explained above by different contributions from Co2+ and Co4+ ions. In the paramagnetic phase at TC < T < TMI, μeff/Co (line 2 in Fig. 4) at δ = 0.37–0.53 is constant; as δ increases further to 0.65, μeff/Co increases to the μeff/Co value at T > TMI. With an increase in the Co4+/Co3+ ratio to 15/85 (δ = 0.65), the semiconductor–bad metal transition (Fig. 2) occurs without a change in the spin state. The results on μeff/Co agree with the \(\mu _{{{\text{eff}}}}^{{{\text{dif}}}}\)/Co data shown in Fig. 3.
Since the Weiss paramagnetic temperature θPM is related to the characteristics of the exchange interaction [29], determining θPM below and above TMI, one can obtain information about the exchange interaction depending on the oxygen content. In the semiconducting phase at TC < T < TMI, as δ increases, the values of θPM decrease from about 260 to 100 K at δ = 0.65 (line 3 in Fig. 4). In the metallic phase, θPM increases from θPM ≈ −100 K to positive values (line 4 in Fig. 4). At δ = 0.65, θPM is about 110 K, coinciding with θPM at T < TMI. The change in the sign of θPM at δ ≈ 0.55–0.6 means that the exchange interaction changes from AFM + FM to FM exchange and the FM exchange becomes stronger with an increase in δ.
As the oxygen content increases, both the magnetization Mmax(δ) at TN (Fig. 1) and the spontaneous magnetization Ms (according to our preliminary data) first decrease to a minimum at δ = 0.60 and then increase. The spontaneous magnetization of N-dBaCo2O5+δ first decreases from Ms ≈ 0.40μB at δ = 0.48 to a minimum of Ms ≈ 0.2μB at δ = 0.60 and then increases to 0.85μB at δ = 0.65. The nonmonotonic behavior of Mmax(δ) and Ms(δ) suggests the existence of competing FM and AFM interactions in these compounds. The FM exchange can be caused by the Co3+‒O‒Co4+ double exchange mechanism [30] or, according to the empirical Goodenough–Kanamori rule, by the presence of Co3+‒O‒Co4+ FM superexchange interactions [31] as well as of the Co3+‒O‒Co3+ AFM superexchange [32].
The temperature and field dependences of the magnetization of PrBaCo2O5+δ, where 0.35 ≤ δ ≤ 0.8 [15], and of NdBaCo2O5 + δ, where 0.37 ≤ δ ≤ 0.65 (Fig. 1), are similar; TC and TN, depending on δ, also decrease by about 100 K, and most sharply at δ > 0.6 [15]. The magnetic behavior of GdBaCo2O5 + δ also changes noticeably when the oxygen content is δ > 0.55. The authors of [3] suggest that such a change is a manifestation of a change in the positions of spins of Co3+ and Co4+ ions. One can suppose that the features appearing at δ ~ 0.6 are also inherent in other layered cobaltites; they are due to a change in the exchange interaction from AFM + FM to FM behavior with an increase in the oxygen content.
The values of TC(δ) and TN(δ) for NdBaCo2O5 + δ differ by about 20 K and have similar δ dependences (see inset of Fig. 1). The result seems to be natural, since the FM state smoothly transforms to the AFM state within a narrow temperature range. A slight change in TC(δ) and TN(δ) for NdBaCo2O5 + δ at δ up to 0.53 and its strong decrease above δ = 0.6, as well as the nonmonotonic behavior of the magnetization Mmax(δ) (Fig. 1), can be qualitatively explained, in our opinion, by a change in the nature of the exchange interactions. At δ < 0.53, AFM + FM interactions are present; therefore, Mmax and TN decrease, but slightly. The dominant role of FM interactions at δ > 0.6 leads to an increase in the magnetization Mmax(δ), to a decrease in TC(δ) and TN(δ) by about 100–150 K with an increase in the Co3+‒O‒Co4+ FM exchange, and, accordingly, to a weakening of the Co3+‒O‒Co3+ AFM exchange with an increase in the contribution of the magnetization related to the Co4+ ions.
However, an alternative explanation for the behavior of TC(δ) is also known [33]. It is usually assumed that a transition to the AFM state occurs at T = TN, although the behavior of the magnetization M(T) is not characteristic of an antiferromagnet: the magnetization remains nonzero well below TN. The behaviors of the magnetization M(T, H = 1 kOe) of Nd-BaCo2O5.48 near TC (Fig. 1) and the spontaneous magnetization Ms are also not characteristic of a pure ferromagnet. The spontaneous magnetization of NdBaCo2O5 + δ at δ ≈ 0.5 arises at T ~ 300 K, which is 15–20 K higher than TC determined from dM/dT (see inset of Fig. 3 [14]). According to our preliminary data, a similar behavior of TC and Ms is characteristic of compounds with other values of δ (Fig. 1). Numerical calculations allowed Wu [33] to assume that a noncollinear AFM state arises at TN, and no transition to the FM state occurs at T = TC, whereas a smooth transition from the PM state to the canted, noncollinear AFM state occurs, and a transition to another collinear AFM state occurs below TN. According to the muon spectroscopy data, another AFM structure arises in NdBaCo2O5.50 at approximately 100 K below TN = 265(5) K [13].
CONCLUSIONS
Polycrystals of layered NdBaCo2O5 + δ cobaltites with different oxygen content 0.37 ≤ δ ≤ 0.65 have been synthesized by the solid-state reaction technique. The metal‒insulator transition in NdBaCo2O5 + δ occurs when the spin state of Co3+ ions changes from HS/LS in the metallic phase to the IS/LS state in the semiconducting phase, as well as in the related RBaCo2O5.5 compound, where R = Gd and Tb [9, 28]. With an increase in δ, the spin states of Co3+ ions in the semiconducting phase of NdBaCo2O5 + δ approach those characteristic of the HS/LS state. The observed deviations of the spin states from the HS/LS state are in reasonable agreement with the possible effects introduced by Co2+ and/or Co4+ ions.
The ferromagnetic behavior of NdBaCo2O5 + δ below TN in the antiferromagnetic phase is explained by the large size of Nd3+ ions.
We argue that the decrease in TN, TC, TMI, and TST by about 100–150 K, the nonmonotonic behavior of the magnetization Mmax(T = TN), and its increase at δ > 0.53–0.6 are caused by a change in the exchange interactions between Co3+ and Co4+ ions from AFM + FM to FM exchange with an increase in δ.
REFERENCES
A. Maignan, C. Martin, D. Pelloquin, N. Nguyen, and B. Raveau, J. Solid State Chem. 142, 247 (1999).
C. Martin, A. Maignan, D. Pelloquin, N. Nguyen, and B. Raveau, Appl. Phys. Lett. 71, 1421 (1997).
A. A. Taskin, A. N. Lavrov, and Y. Ando, Phys. Rev. B 71, 134414 (2005).
C. Frontera, J. L. García-Muñoz, C. Ritter, D. Martín y Marero, and A. Caneiro, Phys. Rev. B 65, 180405(R) (2002).
Y. Moritomo, T. Akimoto, M. Takeo, A. Machida, E. Nishibori, M. Takata, M. Sakata, K. Ohoyama, and A. Nakamura, Phys. Rev. B 61, 13325(R) (2000).
Z. X. Zhou and P. Schlottmann, Phys. Rev. B 71, 174401 (2005).
M. Baran, V. I. Gatalskaya, R. Szymczak, S. V. Shiryaev, S. N. Barilo, K. Piotrowski, G. L. Bychkov, and H. Szymczak, J. Phys.: Condens. Matter 15, 8853 (2003).
N. B. Ivanova, S. G. Ovchinnikov, M. M. Korshunov, I. M. Eremin, and N. V. Kazak, Phys. Usp. 52, 789 (2009).
N. I. Solin, S. V. Naumov, and V. A. Kazantsev, J. Exp. Theor. Phys. 130, 690 (2020).
C. Frontera, J. L. García-Muñoz, A. E. Carillo, M. A. G. Aranda, I. Margiolaki, and A. Caneiro, Phys. Rev. B 74, 054406 (2006).
R. D. Shannon, Acta Crystallogr., A 32, 751 (1976).
F. Fauth, E. Suard, V. Caignaert, and I. Mirebeau, Phys. Rev. B 66, 184421 (2002).
A. Jarry, H. Luetkens, Y. G. Pashkevich, P. Lemmens, H.-H. Klaus, M. Stingaciu, E. Pomjakushina, and K. Conder, Phys. B (Amsterdam, Neth.) 404, 765 (2009).
N. I. Solin and S. V. Naumov, JETP Lett. 114, 150 (2021).
S. Ganorkar, K. R. Priolkar, P. R. Sarode, and A. Banerjee, J. Appl. Phys. 110, 053923 (2011).
L. Landau, Phys. Zs. Sowjet. 4, 675 (1933).
D. D. Khalyavin, O. Prokhnenko, N. Stüßer, V. Sikolenko, V. Efimov, A. N. Salak, A. A. Yaremchenko, and V. V. Kharton, Phys. Rev. B 77, 174417 (2008).
D. Chernyshov, V. Dmitriev, E. Pomjakushina, K. Conder, M. Stingaciu, V. Pomjakushin, and A. Podlesnyak, Phys. Rev. B 78, 024105 (2008).
P. Miao, X. Lin, S. Lee, Y. Ishikawa, S. Torii, M. Yonemura, T. Ueno, N. Inami, K. Ono, Y. Wang, and T. Kamiyama, Phys. Rev. B 95, 125123 (2017).
L. S. Lobanovskii, I. O. Troyanchuk, H. Szymczak, and O. Prokhnenko, J. Exp. Theor. Phys. 103, 740 (2006).
S. Vlakhov, N. Kozlova, L. S. Lobanovskii, R. Wawryk, and K. A. Nenkov, Phys. Rev. B 84, 184440 (2011).
C. Frontera, J. L. García-Muñoz, A. E. Carrillo, C. Ritter, D. M. y Marero, and A. Caneiro, Phys. Rev. B 70, 184428 (2004).
J. C. Burley, J. F. Mitchell, S. Short, D. Miller, and Y. Tang, J. Solid State Chem. 170, 339 (2003).
E.-L. Rautama, V. Caignaert, Ph. Boullay, A. K. Kundu, V. Pralong, M. Karppinen, C. Ritter, and B. Raveau, Chem. Mater. 21, 102 (2009).
Md. M. Seikh, V. Pralong, O. I. Lebedev, V. Caignaert, and B. Raveau, J. Appl. Phys. 114, 013902 (2013).
E.-L. Rautama, V. Caignaert, P. Boullay, A. K. Kundu, V. Pralong, M. Karppinen, and B. Raveau, Chem. Mater. 21, 102 (2009).
S. Kolesnik, B. Dabrowski, O. Chmaissem, S. Avci, J. P. Hodges, M. Avdeev, and K. Swierczek, Phys. Rev. B 86, 064434 (2012).
N. I. Solin, S. V. Naumov, and S. V. Telegin, JETP Lett. 107, 203 (2018).
S. V. Vonsovskii, Magnetism (Nauka, Moscow, 1971; Wiley, New York, 1971), Chap. 9.
C. Zener, Phys. Rev. 81, 440 (1951).
J. Goodenough, Magnetism and the Chemical Bond (Wiley Intersci., New York, 1963).
P. W. Anderson, Phys. Rev. 115, 2 (1959).
H. Wu, J. Phys.: Condens. Matter 15, 503 (2003).
ACKNOWLEDGMENTS
We are grateful to A.V. Telegin for fruitful discussions and to A.V. Korolev for his assistance with the magnetic measurements.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (state assignment no. AAAA-A18-118020290104-2, project Spin) and partially by the Russian Foundation for Basic Research (project no. 20-02-00461).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by K. Kugel
Rights and permissions
About this article
Cite this article
Solin, N.I., Naumov, S.V. Effect of the Oxygen Content on the Metal‒Insulator Transition and on the Spin State of Co3+ Ions in the Layered NdBaCo2O5 + δ Cobaltite (0.37 ≤ δ ≤ 0.65). Jetp Lett. 115, 531–538 (2022). https://doi.org/10.1134/S0021364022100472
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0021364022100472