Abstract
The crystal structure and magnetic state of Sr0.8Y0.2CoO2.65 layered perovskite have been studied using neutron diffraction and synchrotron radiation diffraction and measuring the magnetization. It is shown that the crystal structure in the temperature range of 90–375 K can be described within the framework of the monoclinic sp. gr. A2/m with a cell 4√2ap × 2√2ap × 4ap. The unit-cell parameter a is doubled (sp. gr. A2/m) below TN ≈ 375 K. The basic magnetic structure can be described as G-type antiferromagnetic ordering with cobalt ion magnetic moments of 2.7 µB and 1.7 µB in the anion-deficient CoO4+γ and stoichiometric CoO6 layers, respectively. Based on the magnetization measurements, the ferromagnetic component of cobalt ion magnetic moment amounts to 0.27 µB at 8 K. In both layers, the Co3+ ions are predominantly in the mixed low/high-spin state. The ferromagnetic component is assumed to be due to the orbital ordering in the CoO5 pyramids at TN and the ferromagnetic exchange coupling between CoO5 pyramids in anion-deficient layers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rare earth cobaltites with a perovskite structure are of great interest both from the viewpoint of technological applications and for basic research in the field of physics of magnetic phenomena [1, 2]. The state of the basic compound LaCoO3 at low temperatures (Т ≤ 30 K) is very close to diamagnetic, whereas at temperatures above 30 K a partial spin crossover occurs from the low-spin state to the high-spin paramagnetic state [3, 4]. Doping of LaCoO3 with Sr2+ up to 18% results in an occurrence of long-range ferromagnetic order [5]. At further replacement, there is an almost linear increase in the Curie temperature TC from 180 to ∼305 K and in the magnetization up to Мs = 2.5 µB for the stoichiometric compound SrCo4+O3, which can be obtained only under high pressure [6]. A deviation from the stoichiometric oxygen content in cobaltites gives rise to antiferromagnetic ordering, for which the Néel point may be much higher than room temperature [7]. It was shown in [8, 9] that light doping with yttrium leads to stabilization of Sr1 – хYхCoO3 – δ with a perovskite-type layered structure due to the large deviation from oxygen content. The CoO6 stoichiometric layers alternate with anion-deficient CoO4 + γ layers [9, 10]. The basic antiferromagnetic ordering of the G-type occurs at a point much higher than room temperature and is accompanied by the occurrence of a small ferromagnetic component of cobalt ion magnetic moment.
A great number of scenarios of the ferromagnetic component occurrence was proposed: orbital ordering in the CoO6 layers stoichiometric with respect to oxygen [11], uncompensated ferrimagnetic moment in anion-deficient layers [12], zigzag-like chains in stoichiometric CoO6 layers [12], spin “bags” in these layers [13], and non-collinear magnetic structure [14]. The following questions remain open: whether the magnetic ordering is accompanied by a structural transition and what is its role in the occurrence of ferromagnetic component? To answer them, we performed a complex study of the crystal structure and magnetic and elastic properties of Sr0.8Y0.2CoO2.65 and some compounds with a close yttrium content.
EXPERIMENTAL
A polycrystalline Sr0.8Y0.2CoO2.65 sample was obtained by conventional ceramic technology in air. The starting reagents Y2O3, Co3O4, and SrCO3 of high purity were taken in the stoichiometric ratio and carefully mixed in a Retsch PM-100 planetary ball mill with a speed of 250 rpm for 30 min. Before weighing, the Y2O3 oxide was preliminary annealed at a temperature of 1000°C to eliminate moisture. The sample synthesis involved two stages. The first was firing at 1000°C. The final synthesis was performed at 1185°С for 8 h. Then the sample was cooled to 300°C for 12 h. The oxygen content was determined with an error of ±0.03 by the mass loss upon sample decomposition to simple oxides and metallic cobalt, as well as from the neutron diffraction data. An X-ray diffraction analysis (T = 95–420 K) was performed on the synchrotron radiation source at the Research Center of Paul Scherrer institute (Willingen, Switzerland). Neutron diffraction studies in the range of 10–400 K were carried out on a D2B high-resolution diffractometer at the Institut Laue–Langevin (Grenoble, France). The parameters of crystal and magnetic structures were refined by the Rietveld technique using the FullProf software package [15]. Young’s modulus was measured by the method of resonance oscillations in the frequency range of 1–10 kHz. The magnetic and magnetotransport properties were investigated using a Cryogenic Ltd. setup in a magnetic field up to 14 T in the temperature range of 5–315 K. The conductivity was measured by the four-contact method; indium contacts were deposited by ultrasound.
RESULTS AND DISCUSSION
The parameters of Sr0.8Y0.2CoO2.65 crystal structure were refined (using synchrotron radiation diffraction) in the space groups I4/mmm, Cmma, and A2/m. We could index the X-ray diffraction reflections only in the monoclinic sp. gr. A2/m with the super-cell 2√2ap × 2√2ap × 4ap (400 K) and 4√2ap × 2√2ap × 4ap (350 K), where ap is the primitive-cell parameter. Figure 1 shows the low-angle parts of diffraction patterns at 400 and 350 K. Changes in the diffraction peaks indicate that a phase transformation with unit-cell doubling along the a axis occurs at a temperature lying between 400 and 350 K. The unit-cell parameters are a = 21.704 Å, b = 10.667 Å, c = 15.343 Å, and γ = 90.61° at 10 K; a = 21.723 Å, b = 10.651 Å, c = 15.358 Å, and γ = 90.57° at 300 Κ; and a = 10.829 Å, b = 10.821 Å, c = 15.378 Å, and γ = 90.17° at 400 K.
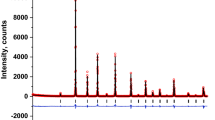
Figure 2 shows the low-angle parts of neutron diffraction patterns measured at 10 and 400 K. No magnetic contribution to neutron scattering was observed at 400 K. A number of additional peaks appeared with a decrease in temperature; a part of them can be identified in the sp. gr. I4/mmm. These peaks are indicated by arrows and designated as AF. Very weak reflections, which can be indexed in sp. gr. Cmma or A2/m, are indicated by asterisks. The crystal and magnetic structures were calculated within the framework of the most simple tetragonal group I4/mmm (2ар × 2ар × 4ар), since the peaks that are not indexed in I4/mmm are very weak. According to the Rietveld refinement, the magnetic structure of Sr0.8Y0.2CoO2.65 is G-type antiferromagnetic, with magnetic moments of 1.7 µB in the stoichiometric CoO6 layers and 2.7 µB in the anion-deficient ones (Table 1). The inset in Fig. 2 shows the temperature dependence of the intensity of reflection 112, from which we can see that the Néel point is about 375 K. Judging from the fact that the intensity of the reflections indicated by asterisks is greatly affected by temperature, the magnetic cell is much larger than that considered in Table 1. However, the intensity of these reflections is very low. Thus, the presented approximation describes the magnetic structure rather well. According to the refined oxygen content, the cobalt ions are in the trivalent state, and the chemical formula is Sr0.8Y0.2CoO2.65.
(Color online) Neutron diffraction patterns of Sr0.8Y0.2CoO2.65, recorded at (a) 10 and (b) 400 K. Bars indicate the Bragg reflections corresponding to the sp. gr. I4/mmm. Asterisks indicate the peaks that cannot be identified within the sp. gr. I4/mmm. The inset shows the temperature dependence of the intensity of magnetic contribution to the peak 112.
It can be seen in Fig. 3 that the Young’s modulus minimum corresponds to the Néel point. This circumstance suggests that the crystal structural transformation coincides with the magnetic ordering.
It is difficult to estimate correctly the spontaneous magnetization from the field dependence of magnetization at 8 K (Fig. 4, inset) because of the absence of magnetization saturation in the fields up to 14 T. However, we can conclude that spontaneous magnetization is not lower than 0.27 µB. Generally, the temperature dependence of the magnetization has a standard shape; however an inflection is clearly seen at 280 K. Note that the compound with х = 0.2 has a highest spontaneous magnetization in the series Sr1–хYхCoO3–δ. The temperature dependence of resistivity for the Sr0.8Y0.2CoO2.65 composition exhibits semiconductor behavior; at 5 K, the resistivity amounts to 104 Ω cm. The magnetoresistance is small and amounts to ∼2% at 5 K in a field of 14 T.
To explain the magnetic properties, one must know the spin state of cobalt ions. Let us assume that Co3+ ions in both layers are in the mixed low/high-spin state. This assumption was based on the following facts.
The magnetic structure is G-type antiferromagnetic; i.e., the magnetic moments of the nearest neighbors are directed oppositely in both layers; the Néel point is high and amounts to TN ≈ 400 K. For the Co3+ ions in the intermediate spin state, the ferromagnetic interaction results in a low Curie temperature TС. For example, TС ≈ 90 K in ferromagnetic epitaxial films LaCoO3 [16], whereas TN amounts to 540 K for SrCoO2.5, where cobalt ions are in the high-spin state [7]. High resistivity at 5 K (104 Ω cm) and low magnetoresistance indicate a good stability of the semiconductor antiferromagnetic state. The intermediate spin state is characterized by low resistivity and ferromagnetic exchange coupling.
In the CoO6 octahedra, the Co3+ ion (high-spin state) is isotropic. However, if CoO5 pyramids are connected by bases, a dedicated axis appears. It is believed that such linking of pyramids in the YBaCo\(_{2}^{{3 + }}\)O5.5-type layered perovskites leads to a non-collinear magnetic structure and appearance of a ferromagnetic component of cobalt ion magnetic moment, which has the same value (0.25 µB) in compounds of both classes [17, 18]. The non-collinear magnetic structure is stabilized due to the ferromagnetic bonds between cobalt ions in CoO5 pyramids. A small substitution of iron for cobalt ions in Sr0.78Y0.22CoO2.65 destroys the orbital ordering and, simultaneously, the ferromagnetic component [9].
CONCLUSIONS
It was shown that the crystal structure of layered cobaltite Sr0.8Y0.2CoO2.65 can be described within the monoclinic sp. gr. A2/m with a cell 2√2ap × 2√2ap × 4ap at temperatures above TN = 375 K and a cell 4√2ap × 2√2ap × 4ap at temperatures below 375 K. All Co3+ ions are in the mixed low/high-spin state. At the temperature TN, the unit cell increases due to the orbital ordering in the CoO5 pyramids articulated by vertices in the anion-deficient layers. The main magnetic structure is G-type antiferromagnetic, with a minor ferromagnetic component of the cobalt ion magnetic moment of about 0.27 µB due to the presence of ferromagnetic exchange coupling and orbital ordering in the anion-deficient layers. The stability of the non-collinear magnetic structure is also due to the high magnetic anisotropy. The magnetic moments of the Со ions in CoO4 + γ and CoO6 layers are 2.7 µB and 1.7 µB, respectively.
REFERENCES
N. B. Ivanova, S. G. Ovchinnikov, M. M. Korshunov, et al., Usp. Fiz. Nauk 179, 837 (2009).
B. Raveau and M. M. Seikh, Cobalt Oxides: From Crystal Chemistry to Physics (Wiley-VCH Verlag GmbH and Co. KGaA, Weinheim, 2012).
A. Podlesnyak, S. Streule, J. Mesot, et al., Phys. Rev. Lett. 97, 247208 (2006).
M. W. Haverkort, Z. Hu, J. C. Cezar, et al., Phys. Rev. Lett. 97, 176405 (2006).
J. Wu and C. Leighton, Phys. Rev. B 67, 174408 (2003).
Y. Long, Y. Kaneko, Sh. Ishiwata, et al., J. Phys.: Condens. Matter 23, 245601 (2011).
A. Muñoz, C. de la Calle, J. A. Alonso, et al., Phys. Rev. B 78, 054404 (2008).
M. James, D. Cassidy, K. F. Wilson, et al., Solid State Sci. 6, 655 (2004).
I. O. Troyanchuk, D. V. Karpinskii, V. M. Dobryanskii, et al., J. Exp. Theor. Phys. 108, 428 (2009).
D. V. Sheptyakov, V. Yu. Pomjakushin, O. A. Drozhzhin, et al., Phys. Rev. B 80, 024409 (2009).
H. Nakao, T. Murata, D. Bizen, et al., J. Phys. Soc. Jpn. 80, 023711 (2011).
D. D. Khalyavin, L. C. Chapon, E. Suard, et al., Phys. Rev. B 83, 140403 (2011).
J. L. Bettis, H. Xiang, and M.-H. Whangbo, Chem. Mater. 24, 3117 (2012).
I. O. Troyanchuk, M. V. Bushinskii, V. M. Dobryanskii, et al., Pis’ma Zh. Eksp. Teor. Fiz. JETP Lett. 94, 849 (2011).
T. Roisnel and J. Rodríquez-Carvajal, Mater. Sci. Forum. 378–381, 118 (2001).
K. Gupta and P. Mahadevan, Phys. Rev. B 79, 020406 (2009).
M. Itoh, Y. Nawata, T. Kiyama, et al., Physica B: Condens. Matter 329–333, 751 (2003).
J. Padilla-Pantoja, C. Frontera, O. Castaño, et al., Phys. Rev. B 81, 132405 (2010).
Funding
This study was supported by the Belarusian Republican Foundation for Fundamental Research, project no. F18R-159.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by A. Zolot’ko
Rights and permissions
About this article
Cite this article
Troyanchuk, I.O., Bushinsky, M.V., Tereshko, N.V. et al. Crystal Structure and Ferromagnetic Component in Layered Perovskite Sr0.8Y0.2CoO2.65. Crystallogr. Rep. 64, 975–978 (2019). https://doi.org/10.1134/S1063774519060245
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774519060245