Abstract
Microwave plasma atomic emission spectrometry was used to determine 36 impurity elements in the zinc metal by a calibration method without adding the matrix element. The values of the nebulizer gas flow pressure are selected that ensure the maximum analytical signals of impurity elements. The limits of detection for most elements are 10–5–10–4 wt %; the error of the results of analysis is 10%. The accuracy is confirmed by the standard addition method and the analysis of standard reference samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Conventional methods for determining the impurity composition are inductively coupled plasma atomic emission spectrometry (ICP AES), inductively coupled plasma mass spectrometry, and electrothermal atomization atomic absorption spectrometry [1–3]. In 2011, Agilent Technologies launched the Agilent 4100 atomic emission spectrometer with excitation of spectra in a microwave plasma, in which nitrogen is used as the plasma-forming gas. The design of the device is similar to that of ICP spectrometers, and the cost of its operation is much lower, as the necessary nitrogen enters the generator from the air. The temperature of microwave nitrogen plasma is 4500–5500 K, which is lower than the temperature of argon inductively coupled plasma. Therefore, many elements show only atomic lines as the most intense, except for alkali and alkaline-earth elements and those elements, the intense atomic lines of which overlap with the spectrum of the nitrogen plasma, while the emission spectra of the elements are simpler than those obtained in the ICP [4]. It was demonstrated that atomic emission spectrometry with spectrum excitation in microwave plasma (microwave plasma atomic emission spectrometry, MP AES) is suitable for the analysis of geological and technogenic samples after acid decomposition [5–7], biological samples [8–11], food [12–14], drinks [15, 16], natural waters [17], and leather and textile materials [10, 18]. However, there is practically no information on the MP AES analysis of objects with an inorganic matrix, for example, alloys or functional materials [19–21]. It is known that the matrix effects on the AES results can be significant [4, 22]. Although, in general, the limits of detection in MP AES are somewhat higher than those in ICP AES [4, 23], it is interesting to evaluate the possibilities of the method for determining the impurity composition in the presence of a matrix element.
The matrix element, being present in a significant amount as compared with the impurity elements, can change the parameters of the plasma, which affects the intensity of the analytical signals. For correcting the matrix effect on the results in AES analysis, various methods are used: dilution (selection of the concentration of the matrix element in the sample), use of calibration curves with the addition of the main element, or use of the internal standard [24–26].
The purpose of this work is to study the possibility of obtaining the correct analysis results and estimate the limits of detection for impurity elements by MP AES in the presence of a matrix element with a high ionization potential, which does not form additional compounds in the plasma. Zinc having Eioniz = 9.39 eV satisfies these requirements. As an object of analysis, we select a zinc alloy used for hot galvanizing of metals and metal products in order to prevent corrosion; the zinc concentration in the alloy is 92–99%. Technologically important impurities in the alloy are Pb, Bi, Fe, Cu, Mn, Ni, Sn, Al, Si, etc.
EXPERIMENTAL
Reagents and equipment. We used nitric acid and deionized water with a specific resistance of ≥18 MΩ/cm purified in an Ultrapure Water System Direct Q-3 (Millipore) to transfer zinc samples into a solution. Calibration curves were plotted using solutions of multielement reference samples (multielement standards, MESs): MES 1 (50 mg/L of Al, Ca, Cd, Fe, Mg, Mn, Na, Cr, Zn, K, and P and 10 mg/L of Li), MES 2 (50 mg/L of B, V, Bi, Ga, In, Co, Cu, Ni, Si, and Ti), MES 3 (50 mg/L of As, Sn, Rb, Pb, Se, Sb, and Te; 20 mg/L of Ba and Sr; 10 mg/L of Be, Ag, and Au; and 5 mg/L of Hg), and MES 4 (50 mg/L of W, Hf, Mo, Nb, Re, Ta, and Zr) (Skat, Russia). The reference samples were diluted with 1% (vol) nitric acid.
The measurements were performed using a 4100 MP AES spectrometer (Agilent Technologies) equipped with a conventional magnetron with a frequency of 2450 MHz, a Czerny–Turner monochromator, and a CCD detector thermostated at 0°C using a Peltier device. To obtain nitrogen, a 4107 Nitrogen Generator (Agilent Technologies) generator was used. The plasma power was 1 kW; the plasma was observed in an axial direction in the “zero” position. An inert OneNeb nebulizer (Agilent) and a Cyclonic Spray Chamber, Double Pass chamber (Agilent) were used to inject the samples. The plasma stabilization time was 15 s; the number of sample injections was 3; the number of pixels for processing analytical signals was 1.
Spectra were processed using the Agilent MP Expert software (ver. 1.5.2.7948). In the “auto” spectral peak processing mode, the software simulates the peak shape based on the spectra of a “blank” sample and calibration solutions; in “off-peak” modes, background signal metering points are manually selected by the user.
Dissolution of samples. A portion of zinc weighing 250 mg was placed in a polypropylene test tube; 1.5 mL of nitric acid (1 : 1) was added dropwise. After cooling to room temperature, the resulting solution was diluted and analyzed. A blank solution was prepared similarly.
It is known that microwave plasma is very sensitive to the salt composition of the injected solution. The manufacturer does not recommend spraying solutions with a total salt concentration above 2%. In this regard, we additionally diluted the sample 20-fold; that is, the concentration of the matrix did not exceed 1%. To determine the elements with the levels of 0.01–0.1 wt % in the sample, we used dilution by 50 and 200 times. The concentration of components in the solution was determined from calibration curves plotted using multielement reference samples without adding the matrix element.
One of the parameters affecting the magnitude of the analytical signal is the nebulizer gas flow pressure, which mainly determines the residence time of the sample solution aerosol in the plasma. The nebulizer pressure was varied in the working range from 80 to 240 kPa with an interval of 40 kPa to obtain the maximum analytical signal. For this purpose, solutions of multielement reference samples with a concentration of 10 mg/L were sprayed into the plasma. The intensity of the analytical signals obtained at various pressures was normalized to the intensity at a minimum gas pressure of 80 kPa.
RESULTS AND DISCUSSION
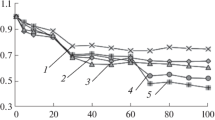
Optimization of nebulizer gas flow pressure. The analytes can be divided into two groups by the nature of the change in the analytical signal with increasing pressure of the nebulizer flow: (1) the relative intensity increases with increasing gas flow pressure (Al, Ba, Ca, Cr, Fe, Li, Mg, Mn, Na, K, Ga, Rb, Mo, Sn, Re, Pb, and Sr; Figs. 1a‒1d) and (2) the dependence reaches a plateau or has a maximum at a medium pressure (As, B, Bi, Cd, Cu, Co, Ni, Sb, Si, Ta, Te, Ti, Hf, Nb, and Zn; Figs. 1d‒1h). The selected values of pressure and the energy characteristics of the lines are given in Table 1. The nebulizer flow pressure affects the intensity ratio of most elements significantly; the change can amount several times. The first group includes elements with atomic lines with low excitation energy, as well as ionic lines with a sum of ionization and excitation energies from 5.96 to 7.93 eV (except for Mg II 279.553 with Eex 12.07 eV). It is known [27] that an increase in the flow rate leads to a change in the plasma temperature by several hundred degrees in the “zero” observation position; in addition, the electron density increases by two or three times. The presence of ionic lines, the intensity of which does not decrease at the maximum pressure of the nitrogen flow, indicates the effective evaporation of the increased number of aerosol droplets. The excitation energies of the atomic lines of the second group of elements intersect with those of the first group. Apparently, the nature of the element plays a role. The atomic lines of nonmetals, as well as metals forming refractory compounds, are in the range of 4.5–6.6 eV. Ionic lines with a sum of energies greater than 9 eV belong to elements forming refractory compounds, such as Zr, Hf, Ti, Nb, and Be. Taking into account the plasma temperature (approximately 4500 K [27]), it can be assumed that for the elements of the second group, the decisive factors are the lack of plasma energy for the conversion of elements into atomic form; the increased electron density, which decreases the intensity of ionic lines; and the local decrease in temperature with an increasing amount of solvent, accompanying the increase in the nebulizer flow pressure. We note for comparison that in the ICP, the medium value of the nebulizer flow rate is optimal for all elements, while the plasma power has a crucial role [28], especially for atomic lines with Eex > 5 eV and Eioniz > 10 eV.
Change in the magnitude of the analytical signal with increasing nitrogen flow pressure: (a) (1) Sn 317.505, (2) Na 588.995, (3) Al 393.152, and (4) Re 346.046; (b) (1) Ga 417.204, (2) Mo 379.825, (3) Fe 371.993, and (4) Sr 407.771; (c) (1) Ba 455.403, (2) Li 670.784, (3) Cr 425.433, and (4) Mg 285.213; (d) (1) Rb 780.027, (2) Sb 231.147, (3) K 766.491, and (4) As 193.695; (e) (1) Bi 306.772, (2) Cd 228.802, (3) Hf 339.980, (4) Be 234.861, and (5) B 249.772; (f), (1) Ti 334.941, (2) Nb 309.418, (3) Te 214.281, and (4) Si 251.611; (g) (1) W 400.875, (2) V 309.311, (3) Zr 343.823, and (4) Ta 268.473; and (h) (1) Co 345.351, (2) Ni 352.454, (3) Cu 324.754, and (4) Ca 393.366.
Calibration dependences, signal processing, account of background signal. In contrast to ICP, the calibration dependences for most elements (the dependence of intensity on concentration) are nonlinear and are approximated by second-order curves, covering ranges of two orders of magnitude. Therefore, to plot the calibration dependence, it is necessary to obtain 4–6 points (Table 2).
We aimed at accounting for the modified background signal in the presence of the matrix element properly and considered various options provided by the software: “auto,” “left–right,” “left of the peak,” and “right of the peak.” The results of the standard addition experiment served as the selection criterion, in which a solution of a multielement reference sample was added to a solution of a zinc sample diluted 20–50 times. Several lines were considered for each element; the most intense of those and free from overlays of the matrix element and the main impurities—Pb, Fe, Cu, Bi, Ni, and Sn—was selected (Table 2). For half of the selected lines, the background signal is almost unchanged and is satisfactorily taken into account in the “auto” mode. More significant changes in the background signal are well handled by selecting accounting limits on both sides of the peak (“left–right” mode). Only the lines of Be at 313.042 nm, Re at 346.046 nm, Sb at 217.581 nm, and Sn at 303.412 nm require one-way accounting of the background signal. The results of the spiking experiment for the selected lines and options for accounting for the background signal are listed in Table 3. The recovery (the found-to-added ratio, taking into account the concentration in the sample solution) is 0.90–1.08 for most elements, and 0.79–1.26 for As, B, Ga, Si, and Ti.
Verification of accuracy. To verify the accuracy of measurements, a standard sample of zinc composition with impurity concentrations of n × 10–3% was additionally analyzed (Table 4). The results agree well with the certified values, which indicates the absence of a significant systematic error. The analysis error does not exceed 10% for two parallel determinations. The limits of detection for impurities in zinc, calculated by the 3s criterion, were n × 10–6–10–3 wt %.
CONCLUSIONS
It is shown that nebulizer flow pressure significantly affects the magnitudes of the analytical signals of the elements in MP AES. The maximum intensities of the analytical signals are obtained at the maximum pressure or in the middle of the working pressure range, depending on the sum of excitation and ionization energies and the nature of the element. A possibility of determining 36 elements, being impurities in the zinc metal, at a level of 10–6–10–3 wt % is demonstrated. A wide range of determined impurities makes it possible to calculate the concentration of the main element from the difference between 100% and the total concentration of impurities in one analysis. The method can be recommended for determining the composition of zinc-based alloys and technical zinc as an alternative to ICP AES or direct current arc atomic emission spectrometry [29].
REFERENCES
Inorganic Chemical Biology: Principles, Techniques and Applications, Gasser, G., Ed., Chichester: Wiley, 2014.
Handbook of Mineral Elements in Food, de la Guardia, M. and Garrigues, S., Eds., Chichester: Wiley, 2015.
Handbook of Trace Analysis. Fundamentals and Applications, Baranowska, I., Ed., Springer, 2016.
Jankowski, K.J. and Reszke, E., Microwave induced plasma analytical spectrometry, RSC Analytical Spectroscopy Monographs, Cambridge: The Royal Soc. Chem., 2011, no. 12.
Helmeczi, E., Wang, Y., and Brindle, I.D., Talanta, 2016, vol. 160, p. 521.
Zhdanov, P.A., Seregin, I.F., Bol’shov, M.A., Volkov, A.I., and Seregin, A.N., Inorg. Mater., 2016, vol. 52, p. 1431.
Balogh, Z., Harangi, S., Gyulai, I., Braun, M., Hubay, K., Tóthmérész, B., and Simon, E., Environ. Sci. Pollut. Res., 2017, vol. 24, p. 4851.
Karlsson, S., Sjöberg, V., and Ogar, A., Talanta, 2015, vol. 135, p. 124.
Simon, E., Kis, O., Jakab, T., Kolozsvári, I., Málnás, K., Harangi, S., Baranyai, E., Miskolczi, M., Tóthmérész, B., and Dévai, G., Ecotoxicol. Environ. Saf., 2017, vol. 136, p. 55.
Zhao, Y., Li, Z., Ross, A., Huang, Z., Chang, W., Ou-Yang, K., Chen, Y., and Wu, C., Spectrochim. Acta, Part B, 2015, vol. 112, p. 6.
Harangi, S., Baranyai, E., Fehér, M., Tóth, C.N., Herman, P., Stündl, L., Fábián, I., Tóthmérész, B., and Simon, E., Biol. Trace Elem. Res., 2017, vol. 177, p. 187.
Ozbek, N. and Akman, S., Food Chem., 2016, vol. 192, p. 295.
Ozbek, N. and Akman, S., Food Chem., 2016, vol. 200, p. 245.
Gallego Ríos, S.E., Peñuela, G.A., and Ramírez Botero, C.M., Food Anal. Methods, 2017, vol. 10, p. 3407.
Ozbek, N. and Akman, S., LWT—Food Sci. Technol., 2015, vol. 61, p. 532.
Ozbek, N., Koca, M., and Akman, S., Food Anal. Methods, 2016, vol. 9, p. 2246.
Eken, M.D. and Akman, B., Fresenius Environ. Bull, 2017, vol. 26, p. 4845.
Sungur, Ş. and Gülmez, F., J. Spectrosc., 2015, vol. 2015, 640271. www.hindawi.com/journals/jspec/2015/640271/. Accessed September 7, 2017.
Hettipathirana, T.D., J. Anal. At. Spectrom., 2013, vol. 28, p. 1242.
Feng, X.-J., Metall. Anal., 2014, vol. 34, p. 58.
Amorin, A., Direct determination of Al, B, Co, Cr, Mo, Ti, V and Zr in HF acid-digested nickel alloy using the Agilent 4210 Microwave Plasma-Atomic Emission Spectrometer, Agilent Technologies, 2016, publ. no. 5991-7283EN. www.agilent.com/cs/library/applications/5991-7283EN.pdf. Accessed September 7, 2017.
Li, W., Simmons, P., Shrader, D., Herrman, T.J., and Dai, S.Y., Talanta, 2013, vol. 112, p. 43.
Bashilov, A. and Rogova, O., Analitika, 2013, no. 5, p. 48. http://www.j-analytics.ru/files/article_pdf/3/ article_3881_102.pdf. Accessed September 7, 2017.
Todolí, J.L., Gras, L., Hernandis, V., and Mora, J., J. Anal. At. Spectrom., 2002, vol. 17, p. 142.
Donati, G.L., Amais, R.S., and Williams, C.B., J. Anal. At. Spectrom., 2017, vol. 32, p. 1283.
Dubuisson, C., Poussel, E., and Mermet, J.M., J. Anal. At. Spectrom., 1998, vol. 13, p. 1265.
Goncalves, D.A., McSweeney, T., and Donati, G.L., J. Anal. At. Spectrom., 2016, vol. 31, p. 1097.
Tsygankova, A.R., Makashova, G.V., and Shelpakova, I.R., Metody Ob’ekty Khim. Anal., 2012, vol. 7, no. 3, p. 138. http://www.moca.net.ua/12/3/pdf/ 07032012_138-142.pdf. Accessed September 7, 2017.
GOST (State Standard) 17261-2008: Zinc. Methods of Atomic-Emission Spectral Analysis, Moscow: Standartinform, 2016.
Funding
This work was supported by the Federal Agency for Scientific Organizations of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Polyakova, E.V., Nomerotskaya, Y.N. & Saprykin, A.I. Determination of Zinc Impurity Composition by Microwave Plasma Atomic Emission Spectrometry. J Anal Chem 74, 693–700 (2019). https://doi.org/10.1134/S1061934819070074
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934819070074