Abstract
A simple, sensitive, and selective analytical method was validated for the determination of mercury, cadmium, lead, arsenic, copper, iron, and zinc in fish muscle using microwave-induced plasma optical emission spectrometry (MIP OES) after acid digestion. The procedure for determining linear range, detection and quantification limits, selectivity, trueness, repeatability, reproducibility, and uncertainty of the method is reported. The results of the validation process demonstrate that the method complies with the provisions of the European Commission guidelines for metals mercury, arsenic, copper, iron, and zinc, but for cadmium and lead, it can only be used as a screening method. The SRM 1946 (Lake Superior Fish Tissue) certified reference material was used to evaluate analytical result quality. The recovery varies between 87 and 99.7%. The HorRat value was equal to or lower than 0.67, and the expanded uncertainty was lower than 37%. The method described can be considered adequate for the simultaneous determination and quantification of the chosen heavy metals in fish matrices. For Cd and Pb, the method could only be employed as a screening tool and not for official control of these contaminants.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals do not disappear from the environment; they change oxidation state and chemical speciation thereby becoming soluble or insoluble in water and consequently remaining in water or sediments. Some heavy metal chemical species and oxidation states are more toxic than others (Mancera Rodríguez and Álvarez León 2006). The most toxic metals include mercury (Hg), lead (Pb), cadmium (Cd), and metalloids like arsenic (As). Meanwhile, copper (Cu), iron (Fe), and zinc (Zn) are essential micronutrients for some living organisms but become toxic in higher concentrations.
Due to the increase in socioeconomic activities of the human population, pollutant presence has increased. This includes heavy metals in water resources that affect food chains and cause organism bioaccumulation and biomagnification (Lozada Zarate et al. 2007; Malik et al. 2010; Jayaprakash et al. 2015).
Due to the fact that fish is consumed by humans, heavy metal maximum levels (MLs) have been established. These are very low, and thus, very sensitive analytical techniques are required to determine concentrations in fish. One of these analytical techniques is microwave-induced plasma optical emission spectrometry (MIP OES) (Herrero Fernández et al. 2014). In this technique, the continuous introduction of the liquid sample along with a system of nebulization forms a spray that is carried by nitrogen to the plasma torch. In the plasma, due to the high temperatures generated, the analytes are atomized and ionized, creating the atomic emission spectra of characteristic lines. The spectra are dispersed by diffraction grating, and the light-sensitive detector is responsible for measuring the intensities of the lines. The information is processed by the computer system through the processing of electronic signals into electrical signals by a phenomenon known as photoelectric effect (Skoog et al. 2013; Laboratorio de Técnicas Instrumentales 2016).
The analyses for this type of matrices are generally performed with flame atomic absorption spectrometry (FAAS), and inductive coupled plasma optical emission spectroscopy (ICP OES) FAAS has been one of the best techniques used for element determination in fish and its derivatives. Comparing the FAAS with the microwave-induced plasma optical emission spectrometry (MIP OES), the MIP OES has higher performance and capacity to monitor multiple elements. For emission spectroscopy systems, several excitation sources such as flames, electrical arc/spark, and plasma instruments are used. Another way to generate the plasma is by induced microwave (MP), in which the plasma is powered by a lower magnetron and waveguide. The use of nitrogen and air as plasma gas in several MP systems has been compared to generation through the ICP source. This has demonstrated that MP performance approaches that of the ICP sources (Li et al. 2013).
The use of this equipment that works with nitrogen plasma is of great interest for many fields of analytical chemistry. This is because the cost of operation is significantly lower, since the running cost can be substantially reduced if this technique is used instead of inductively coupled argon plasma (Li et al. 2013; Karlsson et al. 2015).
Although this equipment has been successfully used to quantify trace metals in animal feed (Amais et al. 2013; Donati et al. 2013; Hettipathirana 2013; Li et al. 2013; Niedzielski et al. 2015; Ozbek and Akman 2015; Zhao et al. 2015; Ozbek et al. 2016; Ozbek and Akman 2016a, b), there is very little scientific information on the functioning and performance of this technology in other matrices such as fish.
Currently, there are national and international standards on the maximum levels allowed in fishing products. Resolution 122 of 2012 issued by the Colombian Ministry of Health and Social Protection sets forth the physical-chemical, microbiological, and chemical pollutant requirements that fish products must comply with. This includes fresh, frozen, deep frozen, precooked, pasteurized, cooked, and canned fish, molluscs, and crustaceans that are for human consumption and are manufactured, processed, prepared, canned, transported, sold, imported, exported, stored, distributed, and commercialized in the national territory. Maximum allowed limits have been determined for mercury, cadmium, and lead of 0.5, 0.05, and 0.3 mg kg−1 wet weight, respectively (Ministerio de Salud y Protección Social de Colombia 2012). Internationally, the European Community has set forth the same maximum contents at wet weight for mercury, cadmium, and lead (European Commission 2011, 2014).
The aim of this work is to implement the MIP OES technique for the determination of Hg, Cd, Pb, As, Fe, Cu, and Zn in fish samples, due to the significance of these elements with respect to human health. The work includes metals necessary for vital functions and nutrition (Fe, Cu, and Zn) as well as those that cause toxicity (Hg, Cd, Pb, As). The methodology was validated using the MP-AES 4100.
Materials and Methods
Reagents and Solution
Metal standard solutions were obtained from Merck (Merck, Darmstadt, Germany) in 1000 mg L−1 solutions. Acids were measured using a Dispensette (Brand, 25 mL) dispenser. Nitric acid, hydrochloric acid, and sulfuric acid were EMSURE products obtained from Merck (Merck, Darmstadt, Germany). Water for the solution preparation was Milli-Q quality (conductivity less than 0.05 μS/cm) (Millipore, Bedford, MA, US). A reductant solution was prepared mixing 5.00 g of sodium borohydride (NaBH4) with 2.50 g of sodium hydroxide (NaOH) in 500 mL of deionized water. For the suppressant solution preparation, 3.00 g of potassium chloride (KCl) and 50 mL of nitric acid (HNO3) were mixed in 1 L of deionized water. The SRM 1946 (Lake Superior Fish Tissue) Certified Reference Material (CRM) was acquired from the National Institute of Standards and Technology (NIST).
Instruments and Equipment
An Agilent 4100 MIP OES Microwave Plasma-Atomic Emission spectrophotometer (Agilent, Santa Clara, CA) with a Multimode Sample Introduction System (MSIS) was used to generate hydrides for Hg and As. For the other metals, a two-way chamber inert OneNeb nebulizer equipped with an Agilent 4107 nitrogen generator (Agilent, Santa Clara, CA) was used. For the weighing process, a Shimadzu AUW120D balance (Shimadzu, Kyoto, Japan) was used. Sample homogenization was performed using a Hobart CC-34 industrial food processor (Hobart Troy, OH). Digestions were performed in a water bath and heating plate (Centricol, Medellin, Colombia). The glassware used was certified and washed four times with a 5% HNO3 solution and rinsed four times with deionized water.
Sample Preparation
Fish frozen at −20 °C were skinned for the analysis and homogenized in a Hobart CC-34 food processor (Hobart Troy, OH). Analytical results are indicated in wet weight.
For Cd, Pb, As, Cu, Fe, and Zn, 5 g of homogenized sample was weighed on an analytical balance directly inside the Erlenmeyer used to conduct the digestion. Ten milliliter of HCl and 5 mL of HNO3 were added along with 1 mL of deionized water, to obtain a smoother reaction. The blank sample was prepared with the same amount of acids added to the samples. The samples were placed on a heating plate at 140 °C for 2 h. After the digestion, they were poured into a 50-mL gauge volumetric flask whose volume was made up with deionized water. For the CRM, half of the amounts were used for the sample, the acids, and the final volume.
Concentrations of the analytes were determined with an emission wavelength of 228.802 nm for Cd, 405.781 nm for Pb, 234.984 nm for As, 324.754 nm for Cu, 371.993 nm for Fe, and 213.857 nm for Zn. The pumping speed was 15 rpm and the nebulizer pressure was 240 kPa for Cd, Pb, Cu, Fe, and Zn, and 120 kPa for As. For the reading of Fe, Cu, and Pb, the suppressant solution was used with a two-way chamber OneNeb nebulizer, in order to suppress the ionization energy of the elements and avoid spectral interferences. The As reading was performed with a multimode sample introduction system and reduction solution, to obtain the metallic element by hydride generation.
For Hg, 1 g of the homogenized sample was weighed on an analytical balance directly inside the Erlenmeyer used to conduct the digestion. Four milliliter of HNO3, 2 mL of H2SO4, and 1 mL of HCl were added. The samples were kept in a water bath at 80 °C for 2 h. To avoid steam release, they were rested for 1 h to cool down. After the digestion, they were poured into a 50-mL gauge volumetric flask whose volume was made up with deionized water.
Hg was determined with a 253.652 nm Hg emission wavelength with a 15 rpm pumping speed and a nebulizer pressure of 240 kPa. The reading was performed with a multimode sample introduction system and the reduction solution, to obtain the metallic element by the cold vapor technique, forming a vapor of elemental mercury.
Analyses were conducted following the Eurachem (Eurachem/CITAC 2012; Eurachem/CITAC 2015) and the European Commission guidelines (European Commission 2011, 2013).
Parameters analyzed for the validation were linearity, detection and quantification limits, selectivity (matrix effect), trueness, repeatability, reproducibility, and uncertainty.
Linearity
In order to provide results directly proportional to the analyte concentration in the sample within a set range, calibration curves were prepared through 1000 mg L−1 pattern solution dilution (Pérez Cuadrado and Pujol Forn 2001).
Calibration curves were prepared with six points and three-point repetitions; 1.00, 2.00, 3.00, 4.00, 5.00, and 6.00 μg Hg L−1 (corresponding to 0.05, 0.10, 0.15, 0.20, 0.25, and 0.30 mg kg−1 Hg in fish); 0.004, 0.007, 0.01, 0.002, 0.03, and 0.05 mg Cd L−1 (corresponding to 0.04, 0.07, 0.10, 0. 20, 0.30, and 0.50 mg kg−1 Cd in fish); 0.03, 0.06, 0.10, 0.15, 0.30, and 0.50 mg Pb L−1 (corresponding to 0.30, 0.60, 1.00, 1.50, 3.00, and 5.00 mg kg−1 Pb in fish); 10, 20, 30, 40, 50, and 60 μg As L−1 (corresponding to 0.10, 0.20, 0.30, 0.40, 0.50, and 0.60 mg kg−1 As in fish); 0.10, 0.30, 0.50, 1.00, 2.00, and 3.00 mg Fe L−1 (corresponding to 1.00, 3.00, 5.00, 10, 20, and 30 mg kg−1 Fe in fish); 0.02, 0.05, 0.10, 0.20, 0.30, and 0.40 mg Cu L−1 (corresponding to 0.20, 0.50, 1.00, 2.00, 3.00, and 4.00 mg kg−1 Cu in fish); and 0.10, 0.30, 0.50, 1.00, 1.50, and 2.00 mg Zn L−1 (corresponding to 1.00, 3.00, 5.00, 10.00, 15.00, and 20.00 mg kg−1 Zn in fish). Blank samples were prepared for each curve in order to adjust the equipment to zero.
Linearity acceptance criteria were as follows: (R 2) > 0.995 determination coefficient, Shapiro-Wilk test p calculated > 0.05, residual normality, Durbin-Watson statistic DW > 1.5 and p calculated > 0.05, residual independence, and Breusch-Pagan test p calculated > 0.05, residual homoscedasticity.
Detection Limit (DL) and Quantification Limit (QL)
Through the quantification limit (QL), the minimum quantities of analytes present in a sample can be determined quantitatively with accuracy and precision, and through the detection limit (DL), the minimum detectable amount of analyte in the sample can be found. Limits were calculated using 10 blank (no detectable amount of analyte) and were determined according to Eqs. (1), (2), and (3) (Morillas 2016)
where, s 0 is the estimated standard deviation of individual results at or near zero concentration, s′0 is the standard deviation used to calculate DL and QL, n is the number of averaged observation replicates, and k Q is the factor that by default has a value of 10 according to IUPAC (Joint Committee for Guides in Metrology (JCGM) 2008a).
Selectivity (Matrix Effect)
Interference presence was evaluated through samples enriched with Hg, Cd, Pb, As, Cu, Fe, and Zn in two concentration levels (low and high) which is as follows: 0.09 and 0.21 mg kg−1 Hg; 0.20 and 0.30 mg kg−1 Cd and As; 0.80 and 2.00 mg kg−1 Pb; 1.00 and 5.00 mg kg−1 Cu; and 3.00 and 10.00 mg kg−1 Fe and Zn.
Student’s t and Snedecor’s F tests were conducted to compare mean homogeneity and pattern solution variance with respect to the fortified sample in each element. For the identification of the atypical values in the fortified samples, the Grubbs test was conducted.
For these tests, the following were the acceptance criteria taken into account: Student’s t p calculated > 0.05, homogeneous means, Snedecor’s F p calculated > 0.05, homogeneous variance, and Grubbs p calculated > 0.05, atypical value inexistence.
Trueness
Three replicates of the SRM 1946 (Lake Superior Fish Tissue) reference material were analyzed to evaluate the analytical result quality. Following the Guidelines for Standard Method Performance Requirements (Eurachem/CITAC 2012; Eurachem/CITAC 2015), and the AOAC Appendix F (AOAC International 2012), recovery percentages corresponding to 80–110% were contrasted as well as the relative standard deviation percentages (% RSD) < 7.3% for Fe and Zn metals and <11% for the rest of the elements. Individual control charts were used to monitor the stability of the analysis system. The external and internal control limits were defined (3SD) as well as the warning internal and external limits (2SD).
Repeatability
Repeatability was measured in terms of precision, in order to determine the variability of independent test results obtained with the same method by the same operator and with the same equipment in a series of analyses on the same sample (Pérez Cuadrado and Pujol Forn 2001). Ten control replicates of fish samples with three concentration levels (low, medium, and high) were analyzed. These concentration levels were 0.05, 0.20, and 0.30 mg kg−1 for mercury; 0.04, 0.2, and 0.05 mg kg−1 for cadmium; 0.3, 1.5, and 5 mg kg−1 for lead; 0.50, 2.00, and 3.00 mg kg−1 for arsenic; 0.10, 0.40, and 0.60 mg kg−1 for copper; 1.00, 10.00, and 30.00 mg kg−1 for iron; and 1.00, 5.00, and 20.00 mg kg−1 for zinc.
The AOAC Appendix F (AOAC International 2012) recovery percentages were between 80 and 110%, and the relative standard deviation percentages (% RSD) for Fe and Zn metals were <7.3% and <11% for the rest of the elements. Predicted Relative Standard Deviation of Reproducibility (PRSDR) was calculated for each concentration level using the Horwitz formula (Eq. (4)) and the HorRatr value (Eq. (5)).
where C is expressed as mass fraction.
where RSDr is the relative standard deviation due to repeatability.
For this parameter, it was taken into account that HorRatr was below the determined limit of <2. The HorRatr was used, meaning the observed relative standard deviation (% RSDr) under repeatability conditions was divided by the RSDr value estimated from the Horwitz equation.
Reproducibility
For this parameter result, the variability of independent tests was studied for the elements with the same method and with the same equipment by different operators at different times (Pérez Cuadrado and Pujol Forn 2001). The procedure was conducted as for repeatability but using the HorRatR value (Eq. (6)).
where RSDR is the relative standard deviation due to reproducibility.
For this parameter, it was taken into account that HorRatR was below the determined limit of <2.
Uncertainty
This parameter was associated with the result of a measurement that characterizes the dispersion of values that may be attributed to the measuring process (Joint Committee for Guides in Metrology (JCGM) 2008b). It is calculated taking into account all contributions during the validation according to the following Eq. (7):
where U is the expanded uncertainty; k is the coverage factor (K = 2); u cal is the relative standard uncertainty regarding the calibration curve; u vol is the relative standard uncertainty regarding the final volume; u m is the relative standard uncertainty regarding sample mass; u treat is the relative standard uncertainty regarding the sample treatment factor; and u tru is the trueness estimation for relative standard uncertainty, using the CRM SRM 1946.
Results and Discussion
Linearity
The results of the acceptance criteria are indicated in Table 1. These, according to the Determination Coefficient (R 2), indicate that the adjusted model explains for every element between 99.88 and 99.96% variability in Y. The correlation coefficients (R) of the elements were equal to 0.999, indicating a positive correlation. The two variables are correlated in the direct sense.
Residual independence was determined through the Durbin-Watson statistic that showed significant values (>0.05), indicating that there was no residual autocorrelation, with a 95% trust level. The Shapiro-Wilk test was conducted to determine residual normality, yielding significant p values for every element. It can be stated that the residuals come from a normal distribution. To determine if residual variance was constant, the Breusch-Pagan test was conducted. Considering that the p value calculated for every element was >0.05, it was concluded that variances were constant (homoscedasticity) in every concentration for every element.
Detection and Quantification Limits
Table 2 shows detection and quantification limits for each element in which the noise signal method was used.
Matrix Effect (Selectivity)
Table 3 shows Student’s t, Snedecor’s F, and Grubbs test results for each element. The analysis did not show differences between the values obtained from standard solutions and enriched samples. This confirms that there is no matrix effect and the result is satisfactory under the criteria, demonstrating mean and variance homogeneity according to Student’s t and Snedecor’s F tests. No atypical values were found for any element, which was confirmed by the Grubbs test.
Trueness
The CRM SRM 1946 results as measured by an MIP OES are described in Table 4. These show that recovery percentages complied with recovery criteria, between 80 and 110%. In addition, the RSD % complies for each element, according to the Guidelines for Standard Method Performance Requirements (Eurachem/CITAC 2012; Eurachem/CITAC 2015). For all analytes, the recovery values are below 100%, which could be attributed to the open vessel digestion system. Although the bias exists, the recoveries obtained are acceptable.
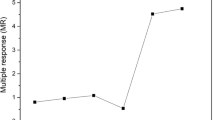
Figure 1 shows the control charts with the CRM values. The values do not exceed control limits corresponding to three times the pattern standard deviation (3 SD).
Repeatability and Reproducibility
Table 5 shows that both RSDr and RSDR values were lower than those calculated by the Horwitz equation, thereby complying with HorRat <2 criteria. This indicates that the method has acceptable repeatability and reproducibility values.
Uncertainty
Table 5 describes the contribution of the sources of uncertainty for each metal and uncertainty (expanded) on the final value. The greatest cause of uncertainty for all metals is the treatment of the sample because of the open system digestions conducted followed by calibration. However, each uncertainty cause complies with the analysis method criteria set forth under 2009/90/CE Standard, <50% (Commission of the European Communities 2009).
Conclusions
The methodology validation was conducted according to the Joint Committee for Guides in Metrology and the European Community standards that developed a method to determine mercury, cadmium, lead, arsenic, iron, and zinc using microwave-induced plasma optical emission spectrometry. This validation took into account linearity with a correlation coefficient of 0.999. The quantification limits were for Hg 0.034 mg kg−1, Pb 0.247 mg kg−1, Cd 0.038 mg kg−1, As 0.076 mg kg−1, Fe 0.448 mg kg−1, Cu 0.406 mg kg−1, and Zn 0.152 mg kg−1. Trueness with recovery percentages was between 87.29 and 99.70, and relative standard deviation was equal to or lower than 7.4%. Repeatability and reproducibility with HorRat were <1 and uncertainty <50%. Each parameter complied with acceptance criteria, indicating that the method is accurate. The described method application field might be extended to determine other metals in similar biological matrices. In this case, it was developed for edible skinless, boneless fish tissue samples, not including other parts of the fish. The method could be used for fish sample screening but not for official control of Cd and Pb. This is because analytically the MIP OES used for validation is not able to decrease the limits of quantification for this type of matrices.
References
Amais RS, Donati GL, Schiavo D, Nóbrega JA (2013) A simple dilute-and-shoot procedure for Si determination in diesel and biodiesel by microwave-induced plasma optical emission spectrometry. Microchem J 106:318–322. doi:10.1016/j.microc.2012.09.001
AOAC International (2012) Appendix F: guidelines for standard method performance requirements. Rockville, USA
Commission of the European Communities (2009) Commission directive 2009/90/EC of 31 July 2009 laying down, pursuant to directive 2000/60/EC of the European Parliament and of the council, technical specifications for chemical analysis and monitoring of water status. Off J Eur Union, Brussels L 201/36-L 201/38
Donati GL, Amais RS, Schiavo D, Nóbrega JA (2013) Determination of Cr, Ni, Pb and V in gasoline and ethanol fuel by microwave plasma optical emission spectrometry. J Anal At Spectrom 28:755. doi:10.1039/c3ja30344f
Eurachem/CITAC (2012) Quantifying uncertainty in analytical measurement, 3rd Ed. United Kingdom
Eurachem/CITAC (2015) Setting and using target uncertainty in chemical measurement, First Ed. United Kingdom
European Commission (2011) Commission regulation (EU) No 420/2011 of 29 April 2011 amending regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union, Brussels L 111/3-L 201/6
European Commission (2014) Commission regulation (EU) No 488/2014 of 12 May 2014 amending regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs. Off J Eur Union, Brussels L 138/75-L 138/79. doi: 10.2903/j.efsa.2011.1975
European Commission (2013) Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed. SANCO/12571/2013
Herrero Fernández Z, Valcárcel Rojas LA, Montero Álvarez A et al (2014) Application of Cold Vapor-Atomic Absorption (CVAAS) spectrophotometry and inductively coupled plasma-atomic emission spectrometry methods for cadmium, mercury and lead analyses of fish samples. Validation of the method of CVAAS. Food Control 1–6
Hettipathirana TD (2013) Determination of boron in high-temperature alloy steel using non-linear inter-element correction and microwave plasma-atomic emission spectrometry. J Anal At Spectrom 28:1242. doi:10.1039/c3ja50103e
Jayaprakash M, Kumar RS, Giridharan L et al (2015) Bioaccumulation of metals in fish species from water and sediments in macrotidal Ennore creek, Chennai, SE coast of India: a metropolitan city effect. Ecotoxicol Environ Saf 120:243–255
Joint Committee for Guides in Metrology (JCGM) (2008a) International vocabulary of metrology—basic and general concepts and associated terms (VIM). Pavillon de Breteuil, Paris
Joint Committee for Guides in Metrology (JCGM) (2008b) Evaluation of measurement data guide to the expression of uncertainty in measurement. Pavillon de Breteuil, Paris
Karlsson S, Sjöberg V, Ogar A (2015) Comparison of MP AES and ICP-MS for analysis of principal and selected trace elements in nitric acid digests of sunflower (Helianthus annuus). Talanta 135:124–132. doi:10.1016/j.talanta.2014.12.015
Laboratorio de Técnicas Instrumentales (2016) Espectroscopía de Plasma. http://laboratoriotecnicasinstrumentales.es/analisis-qumicos/espectroscopa-de-plasma-icp-oes
Li W, Simmons P, Shrader D et al (2013) Microwave plasma-atomic emission spectroscopy as a tool for the determination of copper, iron, manganese and zinc in animal feed and fertilizer. Talanta 112:43–48. doi:10.1016/j.talanta.2013.03.029
Lozada Zarate EJ, Monks S, Pulido Flores G et al (2007) Determinación de metales pesados en Cyprinus carpio en la laguna de Metztitlán, Hidalgo, México. In: IV Foro de Investigadores por la Conservación y II Simposio de Áreas Naturales Protegidas Del Estado De Hidalgo. Universidad Autónoma del Estado de Hidalgo, Pachuca, Hidago, pp 32–39
Malik N, Biswas AK, Qureshi TA et al (2010) Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal. Environ Monit Assess 160:267–276
Mancera Rodríguez NJ, Álvarez León R (2006) Current state of knowledge of the concentration of mercury and other heavy metals in fresh water fish in Colombia. Acta Biológica Colomb 11:3–23
Ministerio de Salud y Protección Social de Colombia (2012) Resolución 000122 de 2012. Ministerio de Salud y Protección Social, Colombia
Morillas P.P. EE y colaboradores (2016) La Adecuación al Uso de los Métodos Analíticos Una Guía de Laboratorio para Validación de Métodos y Temas Relacionados
Niedzielski P, Kozak L, Wachelka M et al (2015) The microwave induced plasma with optical emission spectrometry (MIP-OES) in 23 elements determination in geological samples. Talanta 132:591–599. doi:10.1016/j.talanta.2014.10.009
Ozbek N, Akman S (2016a) Method development for the determination of calcium, copper, magnesium, manganese, iron, potassium, phosphorus and zinc in different types of breads by microwave induced plasma-atomic emission spectrometry. Food Chem 200:245–248. doi:10.1016/j.foodchem.2016.01.043
Ozbek N, Akman S (2016b) Microwave plasma atomic emission spectrometric determination of Ca, K and Mg in various cheese varieties. Food Chem 192:295–298. doi:10.1016/j.foodchem.2015.07.011
Ozbek N, Akman S (2015) Determination of boron in Turkish wines by microwave plasma atomic emission spectrometry. LWT - Food Sci Technol 61:532–535. doi:10.1016/j.lwt.2014.11.047
Ozbek N, Koca M, Akman S (2016) A practical method for the determination of Al, B, Co, Cr, Cu, Fe, Mg, Mn, Pb, and Zn in different types of vinegars by microwave induced plasma optical emission spectrometry. Food Anal Methods 0–4. doi: 10.1007/s12161-016-0421-x
Pérez Cuadrado J, Pujol Forn M (2001) Validación de Métodos Analíticos. Monografías de AEFI, Barcelona, España
Skoog DA, West DM, Holler FJ, Crouch SR (2013) Fundamentals of analytical chemistry. Mary Finch, USA
Zhao Y, Li Z, Ross A et al (2015) Determination of heavy metals in leather and fur by microwave plasma-atomic emission spectrometry. Spectrochim Acta Part B At Spectrosc 112:6–9. doi:10.1016/j.sab.2015.06.017
Acknowledgements
This article was supported by the Diagnosis and Pollution Control Research Group (GDCON), Faculty of Engineering, University Research Center (SIU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the 2015–2016 sustainability fund.
Conflict of Interest
Sara Elisa Gallego Ríos declares that she has no conflict of interest. Gustavo A. Peñuela declares that he has no conflict of interest. Claudia María Ramírez Botero declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Electronic supplementary material
Table 5
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Gallego Ríos, S.E., Peñuela, G.A. & Ramírez Botero, C.M. Method Validation for the Determination of Mercury, Cadmium, Lead, Arsenic, Copper, Iron, and Zinc in Fish Through Microwave-Induced Plasma Optical Emission Spectrometry (MIP OES). Food Anal. Methods 10, 3407–3414 (2017). https://doi.org/10.1007/s12161-017-0908-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0908-0