Abstract
The effect of matrix elements (Na, In, Bi, Zn, Cu) and nitric and hydrochloric acids on the analytical signals of impurity elements in nitrogen microwave-plasma atomic emission spectrometry (MP AES) was studied. In the range of acid concentrations 0.1–0.5 M (for rare-earth elements, 0.1–0.9 M), the analytical signals of the elements did not change. The presence of a matrix element in a concentration of 1 wt % led to a significant change in the signal for most elements, which must be taken into account in selecting a calibration strategy. In MP AES, it is necessary to fully comply with the conformity of samples and calibration solutions regarding the type and concentration of acid and matrix elements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Atomic emission spectrometry is a widespread multielement method. It is known that, for all variants of spectral excitation, the method is subjected to matrix effects of very different nature [1, 2]. A spectrometer for atomic emission spectrometry with microwave plasma, recently appeared on the market of analytical equipment, has several advantages (simplicity, low cost due to the use of nitrogen, etc.) that make it attractive for routine analysis [3]. The equipment for MP AES, commercially available in recent years, is used to analyze samples of different nature [4–10]; however, studies of matrix effects in atmospheric-pressure nitrogen plasma are few [11, 12]. It is known that, in analyzing samples with high concentrations of matrix elements by inductively coupled plasma (ICP)–AES, a sample dilution ratio is selected at which the matrix effect is minimal, or calibration samples must contain the matrix element [13–15]. Tasks of this kind often arise in the analysis of functional materials, various compounds and alloys, and geological samples.
The aim of this work was to determine the analytical capabilities of MP AES for analyzing samples containing matrix elements (Na, In, Bi, Zn, Cu) and study the effect of the acid used on the magnitudes of analytical signals of impurity elements.
EXPERIMENTAL
Equipment and reagents. The measurements were performed using a 4100 MP–AES spectrometer (Agilent Technologies, Australia) equipped with a conventional magnetron with a frequency of 2450 MHz, a Czerny–Turner monochromator, and a CCD detector thermostated using a Peltier device. To obtain nitrogen, a 4107 nitrogen generator (Agilent Technologies, China) was used. Plasma power was 1 kW; plasma was observed in an axial view in the “zero” position. An inert OneNeb nebulizer (Agilent, Spain) and a Cyclonic Spray Chamber, Double Pass chamber (Agilent, United States) were used to inject the samples. Plasma stabilization time was 15 s; the number of sample injections was 3; the number of pixels for processing analytical signals was 1. The most intense analytical lines of impurity elements free of spectral superpositions were selected (Table 1).
To prepare solutions of matrix elements, we used metals Bi, In, Cu, Zn (all of high-purity grade), NaCl (high-purity grade), HNO3 (high-purity grade), HCl (high-purity grade), and deionized water with a resistivity of ≥18 MΩ/cm (Ultrapure Water System Direct Q-3, Millipore). Impurities were introduced using solutions of multielement reference samples (multielement standards, MESs): MES 1 (50 mg/L of Al, Ca, Cd, Fe, Mg, Mn, Na, Cr, Zn, K, and P and 10 mg/L of Li), MES 2 (50 mg/L of B, V, Bi, Ga, In, Co, Cu, Ni, Si, and Ti), MES 3 (50 mg/L of As, Sn, Rb, Pb, Se, Sb, and Te; 20 mg/L of Ba and Sr; 10 mg/L of Be, Ag, and Au; and 5 mg/L of Hg), MES 4 (50 mg/L of W, Hf, Mo, Nb, Re, Ta, and Zr), and MES 5 (50 mg/L of Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu) (Skat, Russia).
To study the effect of acid, we prepared solutions containing 2 mg/L of impurity elements (for the elements contained in the initial mixtures in concentrations below 50 mg/L, the final concentration was proportionally lower); the concentration of HNO3 or HCl was increased to 1.2–1.4 M. In experiments with matrix elements, the concentration of impurities was also 2 mg/L, and the concentration of the matrix element was 0–1 wt % for In, Bi, Zn, and Cu and 0–0.4 wt % for Na.
Processing of spectral data. We calculated the difference in the intensities of the impurity elements with the matrix and the “pure” matrix of the same concentration to take into account the background and possible control experiment. The matrix effect was calculated as the ratio of the intensity of the line with the acid (or matrix) to the intensity without the matrix. The error in measuring intensity was, on average, 2%, and for some elements (Se, Te, etc.), it was up to 5%. Therefore, the error in the ratio of intensities (matrix effect) was a maximum of 7.5%. This range is represented by dashed lines in the graphs. A change in the value within ±7.5% of the initial value was considered insignificant.
RESULTS AND DISCUSSION
Effect of solution acidity. Because samples are, mainly, solid and require transition into a solution, we studied the effect of nitric acid, hydrochloric acid, and their mixtures on the change in the analytical signals of elements. It is known that the acidity of a solution can affect the intensity of the analytical signal in a low-pressure helium MP [16]; for nitrogen plasma, signal suppression was observed with an increase in the concentration of nitric acid to 10 wt % (in a chamber developed by Okamoto [12]). For most elements, the analytical signal changed insignificantly with a change in acid concentration, while for alkali metals, alkaline earth elements, Fe, Si, Bi, Ni, and V, it decreased significantly (Fig. 1). It was also found that for B, Cd, Fe, Mn, Ga, Si, and Ti, the direction of the change in the analytical signal in the presence of hydrochloric and nitric acids is different. For most rare earth elements (REEs), the magnitude of the analytical signal changed slightly with increasing acid concentration. The signals significantly increased for Tb, Tm, Yb, and Lu in the presence of hydrochloric acid, significantly decreased for Sc, Y, Gd, and Er in the case of both acids, and increased for Eu, Sm, and Pr only in the presence of hydrochloric acid. An increase in the intensity of the analytical signal in a hydrochloric acid solution of alkali chlorides was also observed for Cd, Fe, and Mn in helium MP [16, 17] and for Fe in argon MP [18]. The reason for this phenomenon is in the higher volatility of chlorides compared with oxides [17]. The acidity range, in which there was no significant change in the analytical signal, was 0.1–0.5 M for most elements and 0.1–0.9 M for REEs. Thus, in MP AES, the task of matching the type of acid and its concentration in samples and reference samples remains relevant regardless of the presence or nature of the matrix element.
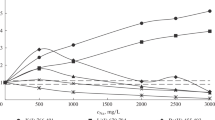
Effect of matrix element.Figure 2 shows the change in the matrix effect with increasing the concentration of the matrix element. For matrix elements of sodium and indium, the impurity elements exhibited two types of dependence: an increase in the analytical signal with a subsequent decrease or a steady decrease (Zn, Cd, B, W, Be, Cu, and Si). The list of impurities for which these types of changes are characteristic coincided for both matrix elements. For all studied lines, the change in intensity was significant. In the presence of bismuth and zinc, the matrix effect was much weaker. The change in intensity in the presence of bismuth for most elements lied in the range of 20% (with the exception of Ba, Sr, and Cu) and within 10% in the case of zinc. In the presence of copper, only a few lines demonstrated the absence of a matrix effect; the intensity of most lines monotonously and significantly increased.
Given the significant change in the analytical signal of impurity elements, it was necessary to add a matrix element to the reference solutions for calibration. The significant effect of sodium on most analytical lines also made it challenging to analyze mineral waters with different sodium concentrations by MP AES. It was necessary to verify the accuracy of the analysis results for the samples containing copper at a concentration of more than 200 mg/L.
Selection of the concentration of the matrix element. In analyzing real samples, it was essential to select a dilution ratio that ensured the minimum limits of detection. To identify the optimal concentration of the matrix element, we used the method proposed in [19]. We constructed the dependences of the analytical signal intensity taking into account the dilution of the sample (for example, in the analysis of metal, 0.1 wt % of the base element corresponds to the dilution of the sample by 1000 times; 1 wt % of the base element corresponds to dilution, respectively, by 100 times, etc.) on the concentration of the matrix element. The value along the ordinate axis of unity corresponds to the maximum analytical signal, taking into account the dilution (R) of the metal sample. Some examples are shown in Fig. 3. It is seen that for bismuth, the maximum signals, taking into account the dilution of the sample, are observed at the maximum bismuth concentration in the solution. Similar results were obtained for zinc and copper. In the presence of indium, two maxima are observed: at 0.2–0.3 wt % (B, Si, Ti, and Nb) and 1 wt %. Thus, a quantitative analysis requires two series of solutions with different matrix concentrations for two groups of elements. Similar results were obtained for sodium.
In determining impurities in bismuth, zinc, and copper by MP AES, the limits of detection for impurities are n × 10–6 wt % for Li and Sr; n × 10–5 wt % for Al, B, Ba, Be, Cr, Cu, Fe, K, Mn, Ni, Ti, V, and Zr (n = 1–9); n × 10–4 wt % for Ca, Cd, Co, Ga, In, Mg, Mo, Na, Pb, Rb, Re, and W; n × 10–3 wt % for As, Hf, Nb, Sb, Si, Sn, Ta, Te, and REEs, which is one or two orders of magnitude higher than those for ICP–AES [19]. In metallic indium, the following limits of detection for impurities were found: n × 10–6 wt % for Li and Sr; n × 10–5 wt % for Al, B, Ba, Be, Cr, Cu, Fe, K, Mn, Ni, Ti, V, and Zr; n × 10–4 wt % for Ca, Cd, Co, Ga, In, Mg, Mo, Na, Pb, Rb, Re, and W; n × 10–3 wt % for Hf, Nb, Sb, Si, Sn, Ta, and REEs; n × 10–2 wt % for As and Te. The method of MP AES can be used to analyze metals with purity less than 98–99% and alloys. In general, the response to a change in the composition of the sample is more pronounced in MP AES than in ICP–AES.
CONCLUSIONS
Thus, when acid concentration (HNO3 or HCl) is higher than 0.5 M (for REEs, higher than 0.9 M), the MP AES analytical signal changes for Na, Rb, Ca, Ba, Co, Cd, Ga, Mn, Ni, Se, Sr Ti, V, Er, Y, and Tm. In preparing reference solutions, it is necessary to observe the same conditions not only in terms of acidity but also in the nature of the acid used. The presence of matrix elements in a concentration of up to 1 wt % in the solution significantly changes the analytical signals for impurities, which must be taken into account in calibration.
REFERENCES
Jankowski, K.J. and Reszke, E., Microwave Induced Plasma Analytical Spectrometry, RSC Analytical Spectroscopy Monographs, no. 12, London: R. Soc. Chem., 2011.
Li, W., Simmons, P., Shrader, D., Herrman, T.J., and Dai, S.Y., Talanta, 2013, vol. 112, p. 43.
Hammer, M., Spectrochim. Acta, Part B, 2008, vol. 63, p. 456.
Balogh, Z., Harangi, S., Gyulai, I., Braun, M., Hubay, K., Tóthmérész, B., and Simon, E., Environ. Sci. Pollut. Res., 2017, vol. 24, p. 4851.
Karlsson, S., Sjöberg, V., and Ogar, A., Talanta, 2015, vol. 135, p. 124.
Simon, E., Kis, O., Jakab, T., Kolozsvari, I., Málnás, K., Harangi, S., Baranyai, E., Miskolczi, M., Tóthmérész, B., and Dévai, G., Ecotoxicol. Environ. Saf., 2017, vol. 136, p. 55.
Zhao, Y., Li, Z., Ross, A., Huang, Z., Chang, W., Ou-Yang, K., Chen, Y., and Wu, C., Spectrochim. Acta, Part B, 2015, vol. 112, p. 6.
Ozbek, N. and Akman, S., Food Chem., 2016, vol. 192, p. 295.
Gallego Ríos, S.E., Peñuela, G.A., and Ramirez Botero, C.M., Food Anal. Methods, 2017, vol. 10, p. 3407.
Ozbek, N. and Akman, S., LWT—Food Sci. Technol., 2015, vol. 61, p. 532.
Burman, J.-O. and Bostrom, K., Anal. Chem., 1979, vol. 51, p. 516.
Zhang, Z. and Wagatsuma, K., Spectrochim. Acta, Part B, 2002, vol. 57, p. 1247.
Gras, L., Hernandis, V., and Mora, J., J. Anal. At. Spectrom., 2002, vol. 17, p. 142.
Donati, G.L., Amais, R.S., and Williams, C.B., J. Anal. At. Spectrom., 2017, vol. 32, p. 1283.
Dubuisson, C., Poussel, E., and Mermet, J.M., J. Anal. At. Spectrom., 1998, vol. 13, p. 1265.
Kawaguchi, H. and Vallee, B.L., Anal. Chem., 1975, vol. 47, p. 1029.
Atsuya, I., Kawaguchi, H., Vellion, C., and Vallee, B.L., Anal. Chem., 1977, vol. 49, p. 1489.
Jin, Q., Zhang, H., Wang, Y., Yuan, X., and Yang, W., J. Anal. At. Spectrom., 1994, vol. 9, p. 851.
Medvedev, N.S., Shaverina, A.V., Tsygankova, A.R., and Saprykin, A.I., Spectrochim. Acta, Part B, 2018, vol. 142, p. 23.
Funding
This work was supported by the Program of the Federal Scientific Research Institute of State Academies of Sciences for 2013–2020 (project no. V.45.1.4.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Polyakova, E.V., Nomerotskaya, Y.N. & Saprykin, A.I. Effect of Matrix Element and Acid on Analytical Signals in Nitrogen Microwave-Plasma Atomic Emission Spectrometry. J Anal Chem 75, 474–478 (2020). https://doi.org/10.1134/S1061934820040115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934820040115