Abstract
Effect of the naphthalene 2-nitro-4,8-disulfonic acid preparative reduction conditions on the naphthalene 2-amino-4,8-disulfonic acid yield and current efficiency is studied. In particular, the effects of the electrolyte composition, cathode material, current density, temperature, the parent naphthalene 2-nitro-4,8-disulfonic acid concentration are found out. The optimal conditions for the naphthalene 2-amino-4,8-disulfonic acid preparative electrosynthesis in the ammonia-buffer solutions (pH 7.0–8.4) are determined. The naphthalene 2-amino-4,8-disulfonic acid yield and current efficiency of 97.5–98.5% and 65.0–72.2%, respectively, are obtained on scaled-up laboratory unit and pilot plant with a filter-press-type electrolyzer. Based on the experimental data obtained in the scaled-up laboratory unit and pilot plant, the technology of amino-C-acid electrosynthesis is developed. This technology is presented in the experiment-and-industry regulations for the producing of the amino-C-acid and in the requirements specification for designing of a pilot electrolyzer for the current load of 3 kA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Naphthalene amino-disulfonic acids, in particular, the acidic sodium salt of naphthalene 2-amino-4,8-disulfonic acid (amino-C-acid), naphthalene 1‑amino-3,6,8-trisulfonic acid (Т-acid), isomeric mixture of the naphthalene 1,6- and 1,7-aminosulfonic acids (Cleve-acids) are the most important intermediate products in the dye industry [1, 2]. In the industry, the acidic sodium salt of naphthalene 2‑amino-4,8-disulfonic acid (NADSA) used to be obtained by the reduction of the corresponding naphthalene 2-nitro-4,8-disulfonic acid (NNDSA), previously isolated from nitrosulfonic mass as diammonium salt [3, 4], with cast iron filings. The NADSA yield in the reduction stage is about 100%; in the stage of extraction from the solution, 78–79%. The NADSA production is a complicated multistage process that includes the target sulfonic-acid isomer extraction from reaction masses; it is fraught with a great amount of nonrecycled solid and liquid waste products. In the producing of 1 t of NADSA, 1.7 t of ferric slime is emerged which is transferred to sludge settling tank and cannot be recycled. Moreover, the amino-C-acid yield and quality depend on the cast iron filings activity and preliminary treatment prior to the using in the reduction process.
The bottleneck in the amino-C-acid production is the stage of the corresponding NNDSA diammonium salt reduction. The using of the NNDSA diammonium salt catalytic hydrogenation does not remove all disadvantages inherent in the above-described reduction method. Moreover, the NNDSA catalytic hydrogenation requires using of noble-metal-based catalysts as well as expensive equipment, i.e. autoclaves. Some patents [5, 6] disclose information on the NNDSA reduction as NNDSA magnesium or ammonium salt by using Pd/C or Pt/C catalysts in the presence of sodium formate aqueous solution and iron hydroxide under the pressure of 5–30 bar, temperature 70–120°С, and рН 4–5. The disadvantages of the NNDSA diammonium salt catalytic reduction induced searching for other reduction methods that could compete favorably with traditional ones.
The electrochemical reduction of the NNDSA diammonium salt could be such an alternative method of NADSA production. However, scientific and patent literature contains no information on the NNDSA electrochemical preparative reduction; only data on the isomeric naphthalene 1-nitro-4,8-disulfonic acid and naphthalene 2-nitro-4,8-disulfonic acid polarographic reduction is at hand [7]. Meanwhile, the electrochemical preparative reduction of technical isomeric mixture of 1,6- and 1,7-naphtylnitrosulfonic acids to the corresponding naphthalene aminosulfonic acids (Cleve-acids) in strongly acidic and ammonia-buffer solutions at different cathodic materials was studied [8, 9]. The naphthalene 1-nitro-3,6,8-trisulfonic acid electrochemical reduction in strongly acidic medium was studied more appropriately both under the conditions of preparative and polarographic reduction [10–12]. As the naphthalene nitrosulfonic acid electrochemical reduction to the corresponding naphthalene aminosulfonic acids was carried out in different media judging by the literature publications [8–12] whereas the NNDSA diammonium salt electrochemical reduction has not yet been studied, in this work we developed NADSA preparative electrosynthesis from the corresponding nitro-compound, in order to create electrochemical technology for the amino-C-acids production.
EXPERIMENTAL
Polarization curves at glassy carbon (SU-2000) electrode were recorded using a PU-1 polarograph (Russia) in differential mode. The steady-state electrode we used was end-face of glassy carbon rod, 2 mm in diameter [13].
The cell was a conical glass vessel, with a volume of 20 сm3, provided with a jacket for temperature control. A saturated calomel half-cell was the reference electrode; all potentials are given with respect to this electrode. Another saturated calomel half-cell was the auxiliary electrode.
To obtain reproducible results, the glassy carbon was polished with fine abrasive paper, then mirror-polished with felt impregnated with glycerol–alumina slurry. Upon washing with distilled water, the electrode was exposed to concentrated sulfuric acid and washed carefully again. Right prior to the recording of each current–voltage curve the electrode was washed with water and wiped with filter paper. The electrode was dipped into electrolyte bulbed through with inert gas to remove dissolved oxygen.
The supporting electrolytes were 0.1–1.0 N NaOH solutions and ammonia buffer solutions with рН 7.0–8.4, as well as 0.1–0.4 N ammonium chloride solutions.
The amino-C-acid preparative electrosynthesis in galvanostatic mode was carried out in a glass laboratory electrolyzer with cathodic and anodic compartments separated with МК-40 membrane. The temperature-controlled glass electrolyzer had a ground-in cap provided with nozzles for thermometer, sampling, gas outlet from the cathodic and anodic compartments, and the electrode current collectors.
The cathodes for the electrolysis were made of different construction materials; they were shaped as perforated cylinders with the surface area of 0.30 dm2. Platinum plate served as anode. The electrolyzer operating volume was 0.060–0.070 L. The catholyte temperature was assigned and kept by a U-15 thermostat (Germany). The electrolyte solution was agitated by a ММ-3М magnetic stirrer (Russia).
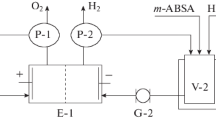
To scale the NADSA electrosynthesis and refine its conditions, we carried out the galvanostatic-mode-electrolysis in a scaled-up laboratory unit and pilot plant at the current load of 20 and 100 А, respectively. Each of the units (Fig. 1) comprised an electrolyzer (E-1), intermediate glass vessels for anolyte (V-1) and catholyte (V-2) provided with jackets, two gear pumps (P-1 and P-2), phase separators (S-1 and S-2), current rectifier, ammeter, voltmeter, and tubings with fixings.
The laboratory electrolyzer (E-1) was a two-compartment filter-press-type apparatus with the cathodic and anodic compartments separated with МК-40 membrane (Russia). The cathode of the N3 nickel perforated plate had working surface area of 1 dm2. The anode was a lead-silver-alloy (~1.0% Ag) plate with the same working surface area. The anolyte was sulfuric acid (10%-solution). The anolyte and catholyte supply and circulation in the corresponding electrode compartments were provided with gear pumps (P-1 and P-2) of a delivery of 150–180 L/h from the temperature-controlled intermediate glass vessels (V‑1 and V-2, respectively). The catholyte volume was 0.25–0.30 L; that of anolyte was 0.15–0.20 L. The gaseous products emerged in the course of electrolysis were separated in the phase separators: hydrogen was separated from the catholyte (S-2); oxygen from the anolyte (S-1).
The current source was a VSА-5А-К rectifier (Russia). The current in the circuit and voltage at the cell and the filter-press electrolyzer were measured by М2018 volt-milliammeters (Russia).
The pilot electrolyzer was a membrane filter-press-type apparatus with two monopolar cells (Fig. 2).
Electrolyzers of this type have the highest capacity among other known industrial electrolyzers [14] which is very important for high-performance production facilities such as the amino-C-acid production is. Each cell comprised a cathode with the working surface area of 4 dm2 and anode with the same working surface area, separated with the МК-40 cation-exchanging membrane. The cathodes were N3 nickel plates; the anodes were manufactured of a lead–silver alloy (~1.0% Ag) as the most strongly corrosion-resistant material in sulfuric acid solutions at the anodic polarization [15]. The possible anode life of the lead–silver alloy (~1.0% Ag) under the conditions of the naphthalene aminosulfonic acid synthesis is 3–4 years. The lead alloying with silver favors the formation of lead dioxide tetragonal modification β-PbO2 during the anodic polarization; its content in the oxide film increased with the increasing of the silver content in the alloy. The lead dioxide tetragonal modification β-PbO2 has smaller crystals as compared with the rhombic modification α-PbO2, and this favors the improving of the film protective characteristics.
The monopolar cells are separated with a polypropylene distributive plug provided with nozzles in its lower and upper parts for the supplying of electrolyte into cathodic compartments of both cells and removal of the electrolysis products therefrom. This goal was advanced by the corresponding openings in the cathodes.
The electrolyte entered to the anodic compartments through the lower openings in the anodes and side gates; the electrolysis products were removed through the openings in the upper parts of the anodes and side gates.
The pressure-tight combining of the electrolyzer elements (the electrodes, rubber inserts, membranes, gates, and the distributive plug) was provided using press plates and tie bolts. Two upper tie bolts and a stud were used in the mounting of the supporting arms onto which the phase separators (for the separating of liquid and gaseous electrolysis products) were placed. The latter were manufactured of polyvinyl chloride. The separators were provided with the corresponding nozzles for the products’ supply and removal according to the flow chart (Fig. 1).
The anolyte and catholyte supply and circulation through the corresponding electrolyzer’s electrode chambers from temperature-controlled intermediate glass vessels (V-1 and V-2) were performed by two gear pumps (P-1 and P-2) with a delivery of 200 L/h. The catholyte volume was 4–6 L; that of anolyte was 2–3 L. The gaseous products emerged in the course of electrolysis were separated in the phase separators: hydrogen was separated from the catholyte (S-2); oxygen from the anolyte (S-1).
The power source of the pilot plant was a VAKG‑18/9-320 rectifying unit (Russia).
The NNDSA diammonium salt as a slurry (the assay percentage about 50–60%) was borrowed from the existing industry: the Berezniki chemical plant.
For the polarographic studies and polarization measurements, the NNDSA diammonium salt was twice crystallized from distilled water with activated carbon. The purified NNDSA diammonium salt was light-creamy crystals with the assay percentage of 99.6–99.8%.
The naphthalene 2-amino-4,8-disulfonic acid (NADSA) was obtained by electrochemical reduction of the corresponding naphthalene nitrodisulfonic acid diammonium salt both in acidic and ammonia-buffer solutions according to the following reaction:

During the preparative electrolysis, we controlled the initial nitro-compound and the reaction product concentrations by using methods of polarography and voltammetry [13].
The concentrations of the intermediate naphthalene nitroso- and hydroxylaminodisulfonic acids were determined in 0.1 М NaOH supporting solution by differential-mode voltammetry [13]; the NADSA concentration in the reaction solution and in the isolated target product, by diazotization [1]. The reaction solution obtained after the NNDSA diammonium salt electrochemical reduction was preliminarily acidified by concentrated sulfuric acid up to its concentration in the solution of 30–40 g/L; then the solution was charged to the temperature-controlled glass apparatus with a gate mixer. Activated carbon was charged to the apparatus at a temperature of 75–78°С and agitation; the solution was treated with the activated carbon for 45–60 min. Then, the naphthalene aminodisulfonic acids was filtered of the activated carbon and transferred to a clean apparatus. The NADSA purified solution was added with sodium chloride at a temperature of 75–78°С and agitation; the sodium chloride concentration was adjusted up to 70–80 g/L. The obtained amino-C-acid suspension was agitated for 1.0–1.5 h at a temperature of 75–78°С. Then, without a break in the solution agitation, the suspension was cooled down to the room temperature. The NADSA evolved from the solution as monohydric sodium salt. The precipitated amino-C-acid was filtered-off by using glass filter, pressed-up carefully and analyzed in order to check the conforming of the obtained product to the technical specifications of the TU 6-14-207-84. The electrochemically obtained amino-C-acid in its appearance, the content of the naphthalene 2-amino-4,8-disulfonic acid monohydric sodium salt in the slurry, the amount of insoluble residue, the diazo-C-acid yield, and the latter’s solubility meets the requirements of the TU 6-14-207-84.
The cathode materials’ corrosion stability was studied in ammonia buffer solution (1.1% NH4Cl + 0.7% NH4OH) with рН 8.0–8.5 at a current density of 10–30 А/dm2, temperature of 50–70°C, and the NNDSA diammonium salt concentration of 0.35 М. A 10%-sulfuric acid was the anolyte.
The studying of the cathode materials’ corrosion stability was carried out in an electrolyzer with the anodic and cathodic compartments separated with a МК-40 cation-exchanging membrane. Samples of different materials sized 40 × 10 × 3 mm were used as cathodes; the anode was platinum plate sized 40 × 10 × 1 mm. The power source was a VAKG-18/9-320 rectifying unit (Russia), the current in the circuit was measured by a М2018 volt-milliammeter (Russia).
The NNDSA diammonium salt solution volume was calculated bearing in mind a 10-h testing of each sample. Prior to experiment, the cathodic samples were degreased in ethanol, washed with distilled water, dried, and weighted accurate to 0.0001 g. After the experiment has been over, the samples were washed with water, dried at 100°С for 1.0–1.5 h, and weighted. The sample corrosion was evaluated by the cathode mass total loss (g/m2), i.e., the sample mass difference prior to and after the experiment. As judged by the electrode mass total loss, the corrosion rate (V, g/(m2 h)) was calculated by the following formula:
where m0 is the initial sample mass, g; m1 is the sample mass after the electrolysis, g; S is the sample surface area, m2; t is the electrolysis duration, h. Then, the corrosion depth index (Vcdi, mm/year) was calculated by the following formula:
where V is the corrosion rate, g/m2 h; γ is the sample specific mass, g/сm3; 8.76 is the conversion factor.
The amount of oxygen evolved in the electrochemical cell in the course of electrolysis was determined by the procedure described in the monograph [14]; in the scaled-up laboratory unit and pilot plant by using a GSB-400 gas meter (Russia).
RESULTS AND DISCUSSION
Because no data on the NNDSA diammonium salt preparative electroreduction is at hand in a scientific literature, the initial stage of the development of the amino-C-acid electrosynthesis conditions required detailed studying of the NNDSA diammonium salt electrochemical behavior. This was carried out by the methods of polarography, voltammetry, and the electrolysis at controlled potential as well as the NNDSA diammonium salt dilute solution electrolysis in a galvanostatic mode. In these preliminary studies we found that the NNDSA diammonium salt dilute solution (0.04 М) reduction is preferable to perform at a nickel cathode in the ammonia-buffer solution (рН 8.3). Here the obtained NADSA yield is 98.0%; the current efficiency is 61.5%. At the same time, the naphthalene 1-nitro-3,6,8-trisulfonic acid preparative reduction to the corresponding naphthalene aminotrisulfonic acid occurs effectively in a strongly acidic medium in a 15–17%-sulfuric acid supporting solution at lead or lead–antimony cathodes [8, 10, 16]. Therefore, it seems advisable studying solutions of different composition for the NNDSA diammonium salt reduction (Table 1).
We can see from Table 1 that the NADSA yield at the lead cathode depends on the electrolyte nature significantly; it comes to 68.9–96.0%. A lower NADSA yield in acidic solutions is explained by the fact that under these conditions, along with the NADSA, the corresponding nitroso- and hydroxylaminodisulfonic derivatives are accumulated in the reaction solutions at great amounts (20.0–24.0%); these derivatives cannot be reduced because of the hydrogen-evolution competitive reaction.
It was reported [1, 16–19] that arylhydroxylamines are reduced by ferrous and cuprous salts at a high rate. In the judgment of the authors of the publications, the ferrous and cuprous salts catalyze the further reduction to the amine thanks to the reversible reactions occurring in the solution bulk: Fe3+ ⇄ Fe2+ and Cu2+ ⇄ Cu+.
In the aromatic amine-compound electrosynthesis, the iron and copper salts are often used as mediator-catalysts [16, 19]. When the NNDSA diammonium salt is reduced at a lead cathode in a 10–15%-sulfuric acid solution added with 0.08% ferrous sulfate, the hydroxylaminodisulfonic acid yield is lowered from 20.0–24.0 to 8.7–11.9%; with the increasing of the ferrous sulfate concentration up to 0.16% it further decreased down to 4.5–6.4%. More increase of the ferrous sulfate concentration in the solution has no effect on the intermediate product yield, its concentration in the reaction solution remains rather large, about 5.6–6.0%.
The largest NADSA yield and current efficiency both at lead and Kh18N10Т-stainless-steel cathodes are achieved in the ammonium chloride and ammonia buffer solutions, in which the post-electrolysis рН is 7.80–8.75. At that, with the increasing of рН, the NADSA yield and current efficiency decreased drastically. Therefore, to optimize the ammonia buffer solution composition we studied the effect of the ammonium hydroxide and ammonium chloride concentration on the NADSA electrosynthesis at an N3-nickel cathode (Tables 2 and 3). As shown earlier [9], at this cathode the naphthalene 1,6- and 1,7-nitrosulfonic acids’ isomeric mixture reduction occurs effectively in the ammonia buffer medium.
We can see in Table 2 that with the increasing of the ammonium hydroxide concentration from 0.35 to 1.40% in the presence of 1.1% NH4Cl, the NADSA yield and current efficiency practically did not change; they are 98.2–98.5% and 72.0–74.2%, respectively. With the increasing of the ammonium hydroxide concentration in the solution of up to 5.0%, the target NADSA yield decreased down to 91.4%. The decrease in the NADSA yield with the increasing of the ammonium hydroxide concentration in catholyte is due to the growth of the solution рН which favors formation of dimeric side compounds [14] which manifests itself through the appearance of resinous materials formed during the electrolysis under such conditions.
As we can see in Table 3, at a change in the ammonium chloride concentration from 0.32 to 1.87%, the ammonium hydroxide concentration being kept constant (0.7%), the NADSA yield and current efficiency remained practically constant; they are 97.6–98.5 and 72.0–73.4%, respectively. It comes from the data presented in Table 3 that it makes the most sense to reduce the NNDSA diammonium salt in the ammonia buffer solutions containing 1.1–1.87% NH4Cl + 0.35–0.7% NH4OH which provides the catholyte рН of 7.40–7.60 in the course of the electrolysis.
It comes from the data presented in Tables 1–3 that the NADSA yield and current efficiency is affected by the cathode material nature in addition to the supporting solution composition. The effect of the cathode material on the NADSA electrosynthesis was studied in the ammonium chloride and the ammonia buffer solutions. The cathode materials were as follows: nickel (N3), titanium (VТ1-0), lead, copper, stainless steel Kh18N10Т, steel St. 3, and stainless steel EP‑467. The results of the study are summarized in Tables 4 and 5 and in Figs. 3 and 4.
Dependence of the cathode potential change in the ammonium chloride 1.1%-solution on the passed charge for different electrode materials: (1) titanium; (2) stainless steel Kh18N10Т; (3) nickel; (4) copper; (5) steel St. 3; (6) lead, and dependence of the hydrogen evolution current efficiency (CE) on the passed charge for different electrode materials: (11) titanium; (31) nickel; (41) copper; (61) lead. NNDSA diammonium salt concentration 0.35 М, current density 10 А/dm2, temperature 70°С.
Dependence of the cathode potential change in the ammonia buffer solution (1.1% NH4Cl + 0.7% NH4OH) with рН 8,3 on the passed charge for different electrode materials: (1) titanium; (2) stainless steel Kh18N10Т; (3) nickel; (4) alloy EP-467; (5) lead; (6) copper; (7) steel St. 3, and dependence of the hydrogen evolution current efficiency (CE) on the passed charge for different electrode materials: (11) titanium; (31) nickel; (51) lead; (61) copper. NNDSA diammonium salt concentration 0.35 М, current density 10 А/dm2, temperature 70°С.
We can see at Tables 4 and 5 that the NADSA yield and current efficiency is affected by the cathode material and electrolyte composition. The highest NADSA yield and current efficiency in ammonium chloride solution was observed at the lead, copper, and nickel cathodes. Somewhat lower NADSA yield (90.0–92.5%) at the titanium- and steel-3-cathodes is due to the incomplete conversion of the initial nitro-compound and intermediate product because of the hydrogen intense evolution (Fig. 3).
In the ammonia buffer medium, as we can see in Table 5, the majority of the studied cathode materials demonstrate a high NADSA yield (94.5–98.5%). An exception to the above is the lead cathode at which the target product yield is somewhat lower of 92.4%. The cathodes made of titanium and EP-467 alloy give a high NADSA yield, yet, a low current efficiency of 55.0 and 42.0%, respectively.
The NNDSA diammonium salt reduction at the titanium cathode both in the ammonium chloride and ammonia buffer solutions is accompanied by a high electrode polarization which manifests itself in the potential shift toward negative values (Figs. 3 and 4).
At the lead electrode in the studied electrolytes, the NNDSA diammonium salt is reduced with a large current efficiency (72.0–76.0%). Evidently, it is caused by the higher overvoltage of a hydrogen evolution at the lead electrode [20]. Indeed, our measurements of the evolved hydrogen volume showed that in the course of the electrolysis at the lead electrode, the hydrogen current efficiency is the lowest: about 25.0% (Figs. 3 and 4). However, at copper and nickel which have a low hydrogen evolution overvoltage [20], the NNDSA reduction in an ammonia buffer solution also show a relatively high current efficiency. The characteristic feature of these metals is a formation of thin spongy sulfide films at their surfaces during the electrolysis. The films are likely to be the result of the NNDSA diammonium salt’s partial desulfurization which produced side products (\({\text{SO}}_{{\text{3}}}^{{{\text{2}} - }},\) H2S, NiS, and CuS). The same products are formed in the desulfurization of sodium allylsulfonate at a nickel cathode in phosphate–borate buffer solutions [14]. During the electrolysis of an ammonia buffer solution (0.55% NH4Cl + 0.7% NH4OH) added with a 6.0 g/L sodium sulfite, the nickel electrode surface is covered with a sulfide film which increased the NADSA electrosynthesis effectiveness. For example, at the current density of 15.0 А/dm2 and the temperature of 70°С, the NADSA yield and current efficiency at a nickel cathode in the ammonia buffer solution (0.55% NH4Cl + 0.7% NH4OH) is 95.0 and 56.6%, respectively; in the presence of a 6.0 g/L sodium sulfite, 97.0 and 70.2%, respectively. At that, we found that the post-electrolysis catholyte added with a sodium sulfite does not practically contain any naphthalene hydroxylaminodisulfonic acid which increased the target product (NADSA) yield as a result of the intermediate product conversion to the amino-compound.
All electrode materials demonstrated the potential gradual shift toward negative values in the course of the electrolysis (Figs. 3 and 4). In the ammonium chloride solution, the passing of charge approaching the theoretically required one for the NNDSA diammonium salt to be reduced to naphthalene hydroxylaminodisulfonic acid (3.0 А h) affected the potential of each of the studied cathodes but insignificantly. Further reduction of a naphthalene hydroxylaminodisulfonic acid into the NADSA resulted in the electrode potential dramatic shift toward the cathodic location; finally, on the passing of a charge required for the initial nitro-compound reduction to NADSA (4.5 А h) the electrode potential takes on the extremely negative value reaching that of a hydrogen evolution. Therefore, the NADSA production in the electrolysis final stage occurs in parallel with the predominant hydrogen evolution, which lowers the target process effectiveness.
In the ammonia buffer solutions, we reached more negative potential values at the studied cathode materials as compared with the ammonium chloride solution. However, the hydrogen evolution current efficiency for nickel and copper cathodes decreased markedly (Fig. 4). This fact must increase the selectivity of the NNDSA diammonium salt electroreduction to NADSA as is the case in the experiment. The relatively high NADSA current efficiency at the copper and nickel electrodes is likely to be due to both decrease of the true current density because of developing copper and nickel spongy sulfides and the cathode surface catalytic activity because of the copper and nickel sulfides’ formation thereon.
For the copper cathode, the increase in the NADSA yield and current efficiency can be caused by the involvement of the mediator-catalysts, the mixed valent copper ions, to the reduction process. The copper ions emerge because of the electrode corrosion during the electrolysis; they mainly affect the stage of naphthalene hydroxylaminodisulfonic acid reduction to NADSA.
The cathode material choice is determined by not only the NNDSA reduction effectiveness, but also by the electrode corrosion resistance under the electrolysis conditions (Table 6). We studied the corrosion resistance of those construction materials, which based cathodes provided the larger NADSA electrosynthesis effectiveness.
We can see from the data of Table 6 that VT1-0 titanium and Kh18N10Т stainless steel demonstrated the highest corrosion resistance. However, the cathodes manufactured of these materials give the NNDSA diammonium salt reduction yield and current efficiency of only 55.0 and 52.1%, respectively, while the nickel cathode provided 72.2%. The corrosion depth index for the nickel cathode increased with the increasing of temperature, current density, and the NNDSA concentration as well as that of ammonium hydroxide. With the increasing of the ammonium hydroxide concentration up to 5.0%, we observed intense corrosive destruction of the nickel electrode for which the corrosion depth index equals 19.0 mm/year. Evidently, for this reason the nickel sample corrosion increases with the increasing of the initial nitro-compound concentration because the NNDSA diammonium salt hydrolysis increased the ammonium hydroxide content in the reaction solution. With the increasing of temperature, the nickel corrosion resistance in the ammonia buffer decreased as a result of a nickel chemical dissolution with the ammoniate formation [21]. Therefore, the nickel cathode can be only used at a current density of 10–15 А/dm2 and a temperature of 50–60°С.
Thus, judging by the corrosion resistance and the selectivity of the NNDSA diammonium salt electroreduction to the corresponding NADSA, we selected the nickel cathode and ammonia buffer solution (1.1% NH4Cl + 0.7% NH4OH) with рН 8.0–8.3 for our further studies.
The current density effect on the NADSA electrosynthesis was studied at a temperature of 50 and 70°С. The results of this study are given at Table 7 and Fig. 5. It comes from the obtained data that with the increasing of the current density both at 50 and 70°С, the NADSA yield and current efficiency decreased while the capacity and energy intensity of the process increased significantly.
Dependence of the electrolyzer capacity (Р) change at a temperature of 50°С (1) and 70°С (2) and the process energy intensity (W) at a temperature of 50°С (11) and 70°С (21) on the current density in the ammonia buffer solution (1.1% NH4Cl + 0.7% NH4OH) with рН 8.3. NNDSA diammonium salt concentration 0.35 М, cathode N3 nickel.
With the increasing of the current density the electrode potential is shifted toward negative values more rapidly that increased the fraction of current which is consumed in the hydrogen evolution side-reaction (Fig. 6). For instance, at a temperature of 50°С with the increasing of the current density from 10 to 40 А/dm2 the NADSA reduction current efficiency decreased from 65.0 to 44.2%; the hydrogen evolution current efficiency increased from 32.0 to 55.0%.
Dependence of the cathode potential change on the passed charge in the ammonia buffer solution (1.1% NH4Cl + 0.7% NH4OH) with рН 8.3 at a different current density, А/dm2: (1) 10; (2) 20; (3) 40, and dependence of the hydrogen evolution current efficiency (CE) on the passed charge at a different current density, А/dm2: (11) 10; (21) 20; (31) 40. NNDSA diammonium salt concentration 0.35 М, temperature 50°С, cathode N3 nickel.
The decrease in the NADSA yield with the increasing of the current density is likely to be connected to the electrode’s high negative potential (–1.82…–1.91 V) observed at current densities of 20–40 А/dm2. At this potential, the occurrence of side-reactions becomes possible, in particular, the NNDSA diammonium salt reductive desulfurization (Fig. 4). Moreover, at a current density of 40 А/dm2 the catholyte is alkalinized significantly in the course of the electrolysis. At that, the catholyte рН increased from 8.3 to 8.8. At the same time, at the current density of 10–30 А/dm2 the catholyte рН increased to only 8.50–8.55. In more alkaline solutions, dimer side-products can be formed [14] which lower the target NADSA yield.
We can see from the data of Table 7 and Fig. 5 that at a temperature of 70°С, the NADSA electrolysis current efficiency and capacity are higher, the energy expenditures lower than at a temperature of 50°С at the same current density. However, with the increasing of temperature and current density the nickel cathode destruction is more intense (Table 6). Therefore, for the NADSA electrosynthesis at a temperature of 50–60°С, the recommended current density is 10 А/dm2 at a temperature of 70°С and 15 А/dm2.
At NNDSA reduction at nickel cathode in the ammonia buffer solution (1.1% NH4Cl + 0.35% NH4OH) with рН 7.5, 0.32 М, we studied changes in the initial, intermediate, and final product concentration in the course of the preparative electrolysis (Fig. 7).
Cyclic differential voltammograms of reaction solution obtained in the NNDSA diammonium salt reduction conducted at a glassy carbon in 0.1 N NaOH solution during electrolysis in the ammonia buffer solution (1.1% NH4Cl + 0.35% NH4OH) with рН 7.5. Start time from the electrolysis: 6 h—1, 11, 12, 13; 7 h—2, 21, 22, 23; 8 h—3, 31, 32, 33; 10 h—4, 41, 42, 43. The D peak is registered at the instrument sensitivity by ten times smaller than that of the А, B and C peaks registration. The electrolysis conditions are as follows: NNDSA diammonium salt concentration 0.32 М, current density10 А/dm2, temperature 60°С, cathode N3 nickel.
At Fig. 7 we give typical cyclic voltammograms of reaction solution sampled at 6, 7, 8, and 10 h after the process start. With the scanning of the cathodic potential, we observed the reduction current peak А at Ер = –0.42 V, that is at the potential more positive than that of the NNDSA reduction peak (the peak B with Ер = –1.0 V). Presumably, the peak A must be ascribed to the reduction of the naphthalene 2-nitroso-4,8-disulfonic acid produced under conditions of the preparative electrolysis to the corresponding naphthalene hydroxylaminodisulfonic acid [22].
Under these conditions, NADSA gives an oxidation current peak D at the voltammogram at Ер = +0.68 V with a height increased in the course of the electrolysis.
The NNDSA diammonium salt preparative reduction in strongly acidic medium (5–15%-sulfuric acid solution) also shows the А peak in the voltammogram; at a mercury dropping electrode the Е1/2 potential is more positive than that at a glassy carbon electrode which equals –0.32 V in 0.1 N NaOH.
In the ammonia buffer solution (1.1% NH4Cl + 0.35% NH4OH) with рН 7.5, the NNDSA diammonium salt is reduced to hydroxylamine derivative which gives an oxidation current peak C at Ер = –0.30 V at an anodic potential scanning from –1.35 V. Such an anodic wave with Ер = –0.28 V was observed [22] in the trisodium salt of the naphthalene 1-nitro-3,6,8-trisulfonic acid’s electroreduction at a gold ring-disc electrode in 0.3 М NaOH + 60% (v/v) СН3СN alkaline solution; it was related to the oxidation of the corresponding naphthalene hydroxylaminotrisulfonic acid to a nitroso-compound. In water–ethanol solutions, N-phenylhydroxylamine also can be oxidized easily [23, 24] giving an anodic oxidation wave at Е1/2 = +0.095…–0.27 V over the supporting solution рН range from 4 to 10. Therefore, the observed voltammetric current peak C at Fig. 7 can undoubtedly be ascribed to the naphthalene hydroxylaminodisulfonic acid oxidation to the corresponding nitroso-compound.
Nitrosobenzene was shown [23–25] to be reversibly reduced polarographically in water–ethanol solutions, with involvement of two electrons, to N-phenylhydroxylamine over the potential range from +0.075 to –0.285 V when the supporting solution рН varied from 4 to 10; nitrobenzene, from –0.415 to –0.74 V over the same рН range. To confirm the suggested nature of the А peak, we took cyclic voltammograms of the NNDSA diammonium salt reduction at a glassy carbon electrode in the supporting ammonia buffer solution (1.5% NH4Cl + NH4OH) with рН 7.5 upon the potential changing in the cathodic direction from +0.2 to –1.3 V and in the anodic direction from –1.3 to +0.2 V (Fig. 8).
We can see at Fig. 8 that the cathodic branches of the cyclic voltammograms demonstrate a single irreversible current peak B of the NNDSA diammonium salt’s four-electron reduction at Ер = –0.83 V to the corresponding hydroxylamine-compound. When the cathodic potential scanning direction is reversed to the anodic one starting from –1.3 and to +0.20 V, the anodic branches of the cyclic voltammograms demonstrated two oxidation current peaks С and S. The value Ер of the anodic peaks S (Fig. 8, curves 11, 21, and 31) practically coincided with the Ер value for the NNDSA diammonium salt reduction (Fig. 8, curves 1, 2, and 3); it does not depend on the nitro-compound concentration. It is about four times lower than the cathodic peak (Fig. 8, curves 11 and 1); its height increased linearly with the increasing of the NNDSA diammonium salt concentration. This data points to the reversible one-electron character of the oxidation process [25]. Therefore, it should come as no surprise that the anodic peaks S represent a reversible process of a radical-anion oxidation to the initial compound [22]. The cathodic peak of the radical-anion formation is likely to be camouflaged by the summary four-electron peak of the hydroxylamine-compound synthesis. However, at Fig. 7 the anodic peak S is not observed; it is likely to be connected with a more complicated composition of the near-cathode solution layer and a higher concentration of the preparative-electrolysis products as compared with the near-cathode solution layer composition in the recording of the cyclic voltammogram of the NNDSA diammonium salt reduction at a glassy carbon electrode.
The anodic peak C (Fig. 8, curves 12, 22, and 32) of the hydroxylamine-compound oxidation is observed over the same potential range as the peak C at Fig. 7; its height increased linearly with the increasing of the NNDSA diammonium salt concentration. Because it is the nitroso-compound that emerged as the hydroxylamine-compound oxidation product [22, 25], it must be reduced during the potential scanning toward a cathodic direction. Indeed, we can see at Fig. 8 that at the potential re-scanning in the cathodic direction, immediately after the reaching of the anodic-scanning final potential (+0.2 В), the voltammogram shows a cathodic peak А (curves 13, 23, and 33) at Ер = –0.3 V which height increased linearly with the increasing of the NNDSA diammonium salt concentration and equals the naphthalene hydroxylaminodisulfonic acid’s oxidation peak height at the same nitro-compound concentration (Fig. 8). Therefore, the А peak at Fig. 8, evidently, is connected with the reduction of the naphthalene nitrosodisulfonic acid produced in the oxidation of the corresponding hydroxylamine derivative. Judging by the data obtained, it is believed that the cathodic peak А (Fig. 7) also reflects the reduction of the naphthalene nitrosodisulfonic acid emerged in the NNDSA diammonium salt preparative electrolysis because the peak observation does not require the potential re-scanning in the cathodic direction.
In the preparative reduction of the naphthylnitrosulfonic acids’ isomeric mixture (nitro-Cleve-acids), we also observed the formation of the corresponding nitroso- and hydroxylamine-compounds in both strongly acidic and ammonia-buffer reaction solutions obtained during the electrolysis that can be registered in the cyclic differential voltammograms recorded at a glassy carbon electrode [9].
In the cyclic voltammograms (Fig. 9, curves 1, 11, 12, and 13) recorded at a glassy carbon electrode in the ammonia buffer solution (1.5% NH4Cl + NH4OH with рН 7.5) with the potential scanning in cathodic direction from +0.2 to –1.6 V and in a reverse (anodic) direction from –1.6 to +0.9 V, no NADSA production has been registered. In all probability, the NADSA which emerged during the recording of a cyclic voltammogram is able to diffuse quantitatively from an electrode surface to the solution bulk during the anodic curve recording prior to the reaching of its oxidation potential. The NADSA formation in a minor amount is observed after a microelectrolysis at a potential approaching that of the supporting electrolyte discharge (–1.6 V) for 30 min (curves 21, 22, and 23; the peak А) which was confirmed by the NADSA spiking into the solution (Fig. 9, curve 3). The microelectrolysis at the potential of –1.6 V allows synthesizing large amounts of the target NADSA and observing its anodic oxidation at the potential of +0.67 V. At the same potential (see Fig. 7) we observed an anodic oxidation of the NADSA obtained in the NNDSA diammonium salt’s preparative reduction.
Cyclic differential voltammogram of NNDSA diammonium salt reduction (1, 11, 12 and 13) and a differential voltammogram of oxidation of the NNDSA diammonium salt reduction products (peaks S, C, and А) formed at a potential of –1.6 V (21, 22 and 23) for 30 min, and a differential voltammogram of NADSA (3) in the ammonia buffer solution (1.5% NH4Cl + NH4OH) with рН 7.5. NNDSA diammonium salt concentration 7.3 × 10–4 М, NADSA concentration 1.5 × 10–4 М, potential scan rate 20 mV/s.
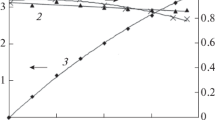
We can see at Fig. 10 that upon the passing of a charge approaching the theoretical value (4.4 А h), the initial NNDSA diammonium salt concentration decreased linearly and reached a high conversion factor (about 80%). At that, the B peak height in the voltammogram decreased (Fig. 7).
Dependence of the concentration changes for NNDSA diammonium salt (1), NADSA (2), 2-hydroxylamino-4,8-disulfonic acid (3), and naphthalene 2‑nitroso-4,8-disulfonic acid (4) as well as the electrode potential (5) and the hydrogen evolution current efficiency (CE) (6) on the passed charge in the ammonia buffer solution (1.1% NH4Cl + 0.35% NH4OH) with рН 7.5. The electrolysis conditions: NNDSA diammonium salt concentration 0.31 М, temperature 60°С, current density 15 А/dm2, cathode N3 nickel.
We can see at Fig. 9 that the reaction intermediate product concentrations first increased, reached maximum, then decreased rapidly upon the electrode potential was reaching –1.1…–1.15 V (the А and C peaks in the voltammogram disappeared by the end of the electrolysis). Herewith, the potential was shifted to the cathodic location abruptly down to –1.45 V. This leads to further reduction of the initial and intermediate products into NADSA (which manifests itself in the significant increase of the latter’s concentration in the solution) as well as the hydrogen evolution. At that, in the ammonia buffer reaction solution a small amount (7–10 g/L) of the naphthalene nitrosodisulfonic acid can be registered during the 0.32–0.40 М of the NNDSA diammonium salt reduction; to the end of electrolysis, it disappeared completely, which, naturally, results in the increase of the NADSA yield. At the same time, in strongly acidic solutions, as was mentioned above, the naphthalene nitroso- and hydroxylaminodisulfonic acids are formed in the reaction solutions up to their 20–24%-concentration. It does not seem possible to reduce them to the corresponding amino-compound without using of a mediator-catalyst, in particular, the ferrous sulfate.
NADSA is produced at the very beginning of the electrolysis; however, its concentration is small, comparable with that of the intermediate nitroso- and hydroxylamino-derivatives. With further continuing of the process, the NADSA concentration in the reaction mixture grows abruptly, as we see at Fig. 10 which is due to the initial and intermediate products’ reduction as a result of the potential shift toward negative values (curve 5). Upon the passing of the charge approaching the theoretical value (4.4 А h), the further NADSA catholyte concentration growth slowed down because of the increase of the current fraction consumed in the hydrogen evolution. Here, the hydrogen evolution current efficiency increased from 12 to 28% (curve 6).
Temperature effects on the NADSA electrosynthesis were studied in the 50–90°С range (Table 8).
We can see at Table 8 that with the increasing of a temperature from 70 to 90°С, the NADSA yield decreased from 98.5 to 92.8%; the current efficiency increased from 65.0 to 75.5%. The increase in the current efficiency can be explained by the increasing solubility of the initial NNDSA diammonium salt as well as the decreasing hydrogen evolution current efficiency, probably, because the cathode potential shifts toward less negative values. However, it is ill-advised conducting the NADSA electrosynthesis at the temperature of 80–90°С, despite the relatively high current efficiency both because of the decrease in the NADSA yield and lowering of the cation-exchanging МК-40 membrane service life as well as the nickel electrode corrosion acceleration (Table 6). Therefore, it makes sense performing the NNDSA diammonium salt reduction at the temperature of 50–60°С.
The NNDSA diammonium salt concentration effect on the NADSA electrosynthesis was studied over the 0.35 to 0.54 М concentration range (Table 9). It comes from the data of Table 9 that the initial nitro-compound concentration does not affect the NADSA yield over the studied concentration range; the yield is 97.0–98.0%. However, with the increasing of the NNDSA diammonium salt concentration from 0.35 to 0.47 М, the NADSA current efficiency grows from 65.0 to 81.8; after the further increase in the concentration up to 0.54 М, the current efficiency decreased down to 78.0%. The increase in the NADSA current efficiency with the increasing of the nitro-compound concentration can be explained by the current redistribution between the hydrogen evolution side-reaction and the target one in favor of the latter that is confirmed by the data concerning the hydrogen evolution current efficiency. At that, the electrode potential becomes less negative.
In the more concentrated NNDSA diammonium salt solutions, the NADSA current efficiency decreased. It is likely to be caused by the decrease in the diammonium salt NNDSA solubility. This hampers the reduction and makes the potential more negative that increased the hydrogen evolution current efficiency.
Judging by the obtained data, we concluded that it is preferable to carry out the NADSA electrosynthesis at the NNDSA concentration ranged within 0.35–0.41 М. At higher nitro-compound concentrations, the current efficiency is larger, yet the nickel electrode is subject to corrosion (Table 6).
Thus, the performed research allowed determining the optimal conditions for the NADSA electrosynthesis in the ammonia buffer solutions with рН 7.0–8.4. They are as follows: the current density 10–15 А/dm2, the temperature 50–70°С, the NNDSA concentration 0.35–0.41 М, the ammonium chloride concentration 1.1–2.2%, the ammonium hydroxide concentration 0.35–0.70%, the cation-exchanging membrane МК-40, the cathode of the N3 nickel or Kh18N10Т stainless steel. These conditions were approbated using the scaled-up laboratory unit and pilot plant; they provided the NADSA yield and current efficiency of 97.5–98.5 and 65.0–72.2%, respectively [26] (Table 10).
We can see from the data of Table 10 that the NADSA yield recalculated per the reduction and isolated from the reaction solution practically does not depend on the scaling of the NNDSA diammonium salt reduction process ; it is 95.0–98.5% and 84.4–89.0%, respectively. Moreover, the amino-C-acid yield recalculated per the isolation from the post-electrolysis reaction solution is significantly larger (see Table 10) than that upon the NNDSA reduction with the cast iron filings (78–79%) [3, 4].
It comes from the data of Table 10 that the NADSA electrosynthesis at a nickel cathode occurs with a larger effectiveness than at other studied metals. Therefore, it is the N3 nickel that is recommended as a cathode material for the experimental-industrial electrolysis.
In the pilot electrolyzer, the NNDSA diammonium salt reduction occurs with a lower current efficiency (44.0–46.0%) than in the simple laboratory one (71.1–72.2%) or in the scaled-up laboratory unit (56.0–60.0%). This lower current efficiency results in the decrease of the plant capacity. For example, the laboratory electrolyzer specific capacity was 1.43 kg/(m2 h) at the current density of 10 А/dm2 and the temperature of 50°С; by contrast, that of the scaled-up laboratory unit and pilot plan was 1.0 and 0.80–0.93 kg/(m2 h), respectively. This current efficiency lowering is caused by the low electrolyte linear velocity in the electrolyzer’s cathodic compartment because of the insufficient delivery rate of the used pumps. Our study of the catholyte linear velocity effect on the NADSA at the Kh18N10Т steel cathode showed the catholyte linear velocity increase from 0.02 to 0.06 m/s to result in the current efficiency increase from 37.0 to 48.0% at the temperature of 60°С and current density of 10 А/dm2.
We can see at Table 10 that upon the temperature increasing up to 70–80°С, the NNDSA diammonium salt reduction process occurs with a higher current efficiency at the same electrolyte linear velocity in the electrolyzer cathodic compartment. However, the increase in the temperature is undesirable from the nickel cathode corrosion standpoint as well as the МК-40 cation-exchange membranes limiting temperature regime operation and the target product quality degradation because of the formation of resinous side-products. Therefore, to increase the NADSA current efficiency, the electrolyte linear velocity in the electrolyzer cathodic compartment must be increased with the use of a more productive pump.
Amino-C-acid produced in the pilot plant conforms with requirements of the TU 6-14-207-84 technical specifications. In addition, the amino-C-acid qualities were tested in the PAO “Khimprom” (Novocheboksarsk, Chuvashia, Russia) for the assay percentage and impurities by using a thin-layer chromatography method. Another test was the synthesis of the active Golden Yellow (2КKh) dye which is most sensitive with respect to the quality of the intermediate used. Additionally, we found that the yield and quality of the dye prepared through the use of the electrochemically produced amino-C-acid well conform with the process regulations and standard sample qualities; the dyeing with the obtained dye conforms with the TU 6-14-53-77 regulatory standard.
CONCLUSIONS
Judging from the obtained results, the following conclusions can be drawn:
(1) The studying of the effects of condition on the NNDSA diammonium salt preparative reduction to NADSA, in particular, the electrolyte composition, the cathode material, the current density, the temperature, and the initial NNDSA diammonium salt concentration allowed establishing the optimal conditions for the NNDSA diammonium salt reduction to NADSA in the ammonia buffer solutions with рН 7.0–8.4. They are as follows: the current density 10–15 А/dm2, the temperature 50–70°С, the NNDSA concentration 0.35–0.41 М, the ammonium chloride concentration 1.1–2.2%, the ammonium hydroxide concentration 0.35–0.70%, the cation-exchanging membrane МК-40, the N3 nickel or Kh18N10Т stainless steel cathode. They provide the NADSA yield and current efficiency of 97.5–98.5 and 65.0–72.2%, respectively which were approbated using the scaled-up laboratory unit and pilot plant with a membrane electrolyzer of a filter-press type.
(2) The ammonia buffer solutions with рН 7.0–8.4 were shown to be more preferable for the NADSA preparative electrosynthesis as compared with acidic ones because they provided not only a higher engineering-and-economic performance of the NNDSA diammonium salt reduction but also are less corrosive, and this simplifies the choice of the electrode and construction materials for the electrolyzer and production facilities for the amino-C-acid production.
(3) By using the experimental data obtained with the scaled-up laboratory unit and pilot plant, an electrochemical technology of the amino-C-acid production is developed. It is shown in the experimental-industrial regulations for the amino-C-acid production and in requirements specification for the designing of experimental-industrial electrolyzer for the producing of amino-C-acid. On its basis, the ММN-3-NS-03 experimental-industrial membrane electrolyzer of a filter-press type at a current load of 3 kА is drafted.
(4) The developed technology for the amino-C-acid electrosynthesis allows increasing the product yield recalculated per the isolating from reaction solution from 79.0 to 84.4–89.0%, cutting back the amount of non-recyclable solid wastes from 1.7 to 0.2 t per 1 t of the product, simplifying the technological process by the excluding stages involving the cast iron filings preparation, filtering, and iron sludge transportation, improving working conditions, providing economic, ecological, and social effect as well as producing the amino-C-acid in conformity with requirements of the ТU 6-14-207-84 technical specifications.
REFERENCES
Vorozhtsov, N.N., The Fundamentals of the Intermediate Products and Dyes Syntheses (in Russian), Moscow: Goskhimizdat, 1955.
The Chemistry of Synthetic Dyes, Venkataraman, K. (Ed.), Academic, 1974.
Efros, L.S. and Gorelik, M.V., Chemistry and Technology of Intermediate Products (in Russian), Leningrad: Khimiya, 1980. 544 p.]
Vigasin, A.A., Amitrova, I.M., Ganiushkina, L.S. et al., USSR Inventor’s Certificate no. 154261, 1963.
Bamfield, P., Quan, P.M., and Smith, T.J., US Patent 4093646, 1978.
Hildreth, J.D., US Patent 4299779, 1981.
Horyna, J. and Jehlicka, V., Polarographie der Mononitroderivate der 1,5- und 1,6-Naphthalindisulfonsauren, Coll., 1959, vol. 24, p. 3353.
Fierz, H.E. and Weissenbuch, P., Ueber die Reduktion von Nitronaphtalinssulfosauren, Helv. Chim. Acta, 1920, vol. 3, p. 305.
Konarev, A.A., Electrochemical synthesis of Cleves-acids, Russ. J. Electrochem., 1998, vol. 34, p. 1160.
Konarev, A.A., Katunin, V.Kh., and Sukhinina, N.G., Method for preparation of naphthalene 1-amino-3,6,8-trisulfonic acid, USSR Inventor’s Certificate no. 1369232, 1987.
Konarev, A.A., Avrutskaya, I.A., and Katunin, V.Kh., Polarographic behavior of a naphthalene 1-nitro-3,6,8-trisulfonic acid, Soviet. Electrochem., 1981, vol. 17, p. 901.
Konarev, A.A. and Avrutskaya, I.A., Features of electrochemical reduction of 1-nitro-3,6,8-trisulfonic acid of naphthalene, Soviet. Electrochem., 1988, vol. 24, p. 1548.
Konarev, A.A., RF Patent 2159424, 2000.
Tomilov, A.P., Mayranovsky, S.G., Fioshin, M.Ya., and Smirnov, V.A., Electrochemistry of Organic Compounds (in Russian), Leningrad: Khimiya, 1968.
Konarev, A.A., Selection of anode material for electrosynthesis of naphthalene aminosulfonic acids, Russ. J. Appl. Chem., 1997, vol. 70, p. 261.
Konarev, A.A., Studies of Intermediates Formed under the Conditions of Preparative Electroreduction of Naphthalene 1-Nitro-3,6,8-Trisulfo Acid, Russ. J. Electrochem., 2012, vol. 48, p. 922.
Organic Electrochemistry. An Introduction and a Guide. Baizer, M. and Lund, H., Eds., New York: Marcel Dekker, 1973.
Mizuch, K.G., Collateral oxidation processes in reduction of aromatic nitrocompounds, The USSR Acad. Sci. J., 1937, vol. 15, no.1, p. 37.
Lukashevich, V.O., Organic Semi-products and Dyes (in Russian), Moscow: Goskhimizdat, 1959.
Antropov, L.I., Theoretical Electrochemistry, Univ. Pacific, 2001.
Akhmetov, N.S., General and Inorganic Chemistry (in Russian), Moscow: Vysshaya Shkola, 1981.
Bakir D., Extended Abstract of Cand. Sci. Dissertation, Moscow Gos. Univ., Moscow, 1989.
Smith, J.W. and Waller, J.G., Polarographic Behavior of Aromatic Nitro Compounds Nitrosobenzene and N‑Phenylhydroxylamine, Trans. Farad. Soc., 1950, vol. 46, p. 290.
Stradins, J., Polarography of Organic Nitrocompounds, Riga: Latvian Akad. Nauk, 1961.
Mayranovsky, S.G., Stradins, J., and Bezugliy, V.D., Polarography in Organic Chemistry (in Russian), Leningad: Khimiya, 1975.
Konarev, A.A., RF Patent 2009125, 1994.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
Rights and permissions
About this article
Cite this article
Konarev, A.A. Electrochemical Synthesis of Amino-C-Acid. Russ J Electrochem 57, 289–305 (2021). https://doi.org/10.1134/S1023193521030058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193521030058