Abstracts
The results and prospects of the in situ (in the cell volume) and ex situ (outside the cell) use of aqueous solutions of hydrogen peroxide electrogenerated from oxygen in gas-diffusion electrodes (GDE) of carbon black are discussed. It is shown that using GDE based on technological A-437E carbon (acetylene black) and mesostructured carbon CMK-3 allows the Н2О2 solution with the concentration higher than 3 M to be obtained. It is found that electrosynthesized hydrogen peroxide may be used in situ with the high efficiency both in the indirect electrosynthesis of important organic and inorganic target products and in the destruction of organic and inorganic pollutants present in waste waters of different origin. Under the ex situ conditions, it is possible to synthesize the more concentrated solutions of Н2О2, organic peroxoacids, and inorganic peroxosolvates and also to carry out mineralization of exometabolites in autonomous life-support systems. These results may be helpful in selecting the most appropriate versions of using hydrogen peroxide solutions electrogenerated from oxygen for solving particular problems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The concept of the development of modern chemical technologies corresponding to the principles of Green Chemistry and steady development focuses attention on the wide use of aqueous solutions of hydrogen peroxide as the environmentally clean oxidizer [1]. Their application region is extraordinarily wide: the aqueous Н2О2 solutions prepared by commercial technologies are used in the pulp and paper industry, in the production of detergents and disinfectants, in hydrometallurgy plants, for cleaning waste waters, in liquid-phase oxidation of exometabollites in closed life-support systems, and in many other life activity fields which require the use of clean oxidizers [2].
In the recent 20 years, attention was focused on indirect electrolytic oxidation of organic and inorganic substrates by active oxygen forms (AOF) (О3, Н2О2, \({\text{H}}{{{\text{O}}}^{● }}\), \({\text{HO}}_{2}^{ - }\), \({\text{HO}}_{2}^{● }\), RООН, etc.), which can be generated at the anodic oxidation of water and hydrogen peroxide on Pt electrodes, boron-doped diamond electrodes (BDDE), and electrodes based on dioxides of lead, tin, manganese, etc., and also by the cathodic reduction of oxygen to hydrogen peroxide on carbographite materials [3–8]. It is assumed that based on these solutions, one can carry out highly efficient advanced oxidation processes (OAPs) [8]. However, the wide use of hydrogen peroxide is limited by its relatively high cost when it is produced by traditional technologies [9, 10]. In connection with this, for many years, attempts were undertaken to find the cheaper and easier methods of production of its aqueous solutions with the required concentration.
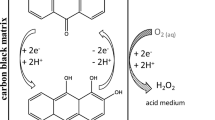
It is well known [2, 9–12], that hydrogen peroxide can be synthesized relatively easily by electroreduction of oxygen on carbographite electrodes in alkaline solutions (the Berl process [11])
and by the following reaction in acidic solutions:
It deserves mention that this method is wasteless and allows producing Н2О2 in the site of its consumption in the form of dilute aqueous solutions and using them latter as a commercial product without extracting Н2О2, which considerably reduces the cost [2, 10, 12]. Such aqueous solutions of hydrogen peroxide can be easily concentrated because the water vapor pressure is higher than that of Н2О2 by one order of magnitude (17 against 1.36 mm Hg, 20°С) [13], which allows solutions with the required Н2О2 concentration to be prepared with the high benefit-cost ratio to be used ex situ, i.e., outside the electrolyzer volume.
It is well known that in homogeneous reactions, hydrogen peroxide can interact with substrates and oxygen-containing intermediates \({\text{OH}}_{2}^{ - }\), \({\text{HO}}_{2}^{● }\), \({\text{H}}{{{\text{O}}}^{● }}\); the intrinsic activity of the latter differs by 5–6 orders of magnitude [14]. It is generally accepted that the most active of the mentioned radicals is \({\text{H}}{{{\text{O}}}^{● }}\) (Е0 \({\text{H}}{{{\text{O}}}^{● }}\)/Н2О = 2.8 V) and the least active is \({\text{HO}}_{2}^{● }\) (Е0 \({\text{HO}}_{2}^{● }\)/Н2О = 1.65 V) [6]. Hence, the use of Н2О2 allows carrying out the oxidation processes of different depth under in situ conditions with the use of the conjugated reactions of the heterogeneous oxygen oxidation to hydrogen peroxide and the homogeneous oxidation of organic and inorganic substrates in the electrolyte volume [15–17]. This was repeatedly discussed in the Russian and foreign literature, for example, in [2–6, 15, 17–32].
This study is aimed at the analysis of experimental results obtained by the research group of the Institute of Chemistry and Technology, Siberian Branch, Russian Academy of Sciences in the course of a long-term complex investigation of the electrosynthesis of aqueous solutions of Н2О2 from О2 in carbon-black gas-diffusion electrodes (GDE) and also to the discussion of prospects of practical application of this approach under in situ and ex situ conditions.
ELECTROSYNTHESIS OF Н2О2 FROM О2
The earlier results on the electrosynthesis of hydrogen peroxide from oxygen in GDE based on carbographite materials produced in Russia (acetylene black A437-E, furnace blacks P268E, P324-E, P701, P602, P805-E, P399-ET) in solutions of different pH are summarized in [12, 17]. The tests of GDE fabricated based on A437-E with the apparent surface area of 64 cm2 in an electrolyzer of the filter-press type, in electrolyte containing K2SO4 (0.5 М) + H2SO4 (0.2 М) + TBAB (0.005 М) produced the solution of Н2О2 with the concentration 3.0 М in 9 h electrolysis with the current efficiency (CE) approaching 80.0%. The similar results were obtained for the GDE of the analogous composition with the apparent surface of 900 cm2. These results suggest that the considerable increase (by a factor of 14) in the apparent surface of these GDE had virtually no effect on the electrosynthesis efficiency. Moreover, it was shown that acetylene black (A437-E) is the most efficient electrocatalyst among the considered carbon blacks of this type.
In the recent years, we carried on our studies on selecting and testing new types of technological carbon produced by the company Omsktekhuglerod (Russia) as the promising electrocatalysts for electrosynthesis of Н2О2 from О2 in GDE [33, 34]. It was found that in GDE based on carbon materials N220, С140, and СН85, the oxygen reduction in alkaline and acidic electrolytes involves the stage of formation of hydroxide ions and hydrogen peroxide with the relatively high selectivity (γ = 0.8). Thus, in GDE based on technological carbon N220, the hydrogen peroxide solutions with the 0.56 М concentration were obtained in 1 h with the 87% current efficiency. These results demonstrated the good prospects of new types of carbographites as the electrocatalysts in electrosynthesis of Н2О2 from О2. Especially good results were obtained for mesostructured carbon CMK-3. Mesostructured mesoporous carbon materials are the new promising materials for various applications in electrochemistry. The main advantage of mesostructured carbon materials prepared by the template synthesis, as compared with other carbons, is the high surface area, the ordered mesoporous structure, the high conductivity, and relatively low cost [35]. The authors of [36, 37] showed the data on mesostructured carbon СМК-3 (Carbon Mesostructured by KAIST) as the electrocatalysts for electrosynthesis of Н2О2 from О2 in a gas-diffusion electrode in alkaline and acidic solutions. Figure 1 shows the results of preparative electrosynthesis of Н2О2. It is evident that the kinetics of Н2О2 accumulation obeys the parabolic law and the current efficiency and selectivity of this process exceed 80% and 0.8, respectively. The 7.5 h electrolysis at the current density of 1500 A/m2 produced hydrogen peroxide with 3 M concentration in the solution containing 0.5 М K2SO4 : 0.1 М H2SO4 (3 : 1). The shown characteristics of the efficiency of synthesizing aqueous Н2О2 solutions considerably exceed the results present the world literature [38], which points to the good prospects of using this material in gas-diffusion electrodes for many applications important for practice.
In situ PROCESSES
INDIRECT ELECTROSYNTHESIS
Oxidation of Formaldehyde to Formic Acid
In [39] we studied the oxidation of formaldehyde by \({\text{HO}}_{2}^{ - }\) generated in situ from О2 in a carbon-black GDE in 1 M NaOH in cells with cathodic and anodic compartments separated by a cation-exchange MF‑4SK-100 membrane at 20°С. It was shown that the formaldehyde oxidation proceeds only to formic acid in quantitative yields according to the equation
The presence of formic acid in the formaldehyde solution volume had virtually no effect on the electrode polarization and generation of \({\text{HO}}_{2}^{ - }\) ions, and the increase in the current from 250 to 2000 A/m2 did not affect the CE of \({\text{HO}}_{2}^{ - }\).
Oxidation of Maleic Acid to Malic Acid
In [40] we studied the kinetics and selectivity of maleic acid oxidation in a cell with a carbon-black gas-diffusion cathode, a platinum anode, and a cation-exchange membrane in 1 М Na2SO4 at the current density of 500 and 1000 A/m2, with the initial substrate concentration of 0.215 М at 12–30°С. The analysis of samples by IR spectroscopy showed that the products represented a mixture of the malic-acid salt as the main product, the unreacted maleic-acid salt, and a small amount of epoxide. It was found that the increase in current density and temperature had virtually no effect on the oxidation rate of the substrate so that its conversion after 12 h electrolysis was by 56–58%. It was shown that electrolysis in a membrane-free cell led to mineralization, where the final products of maleic acid oxidation were only СО2 and Н2О, and the following mechanism was proposed:
Indirect Electrosynthesis of Adipinic Acid from Cyclohexanol
Adipinic acid is used widely, and attempts are still undertaken to develop more environmentally friendly methods of its synthesis [41]. In [42, 43], the authors studied the possibility of synthesizing adipinic acid by the redox-mediator oxidation of cyclohexanol and cyclohexanone on an oxide-hydroxide nickel electrode (OHNE), i.e., Ni(O)OH anode, and also proposed the mechanism of oxidation of cyclohexanol on the Ni(O)OH electrode in aqueous NaOH solutions. It was shown that the oxidation proceeds via the limiting stage of cyclohexanone formation with its subsequent oxidation to adipinic acid
In [44], we studied the effect of several factors (current density, substrate concentration, the charge passed) on the efficiency of this process when it was carried out on a porous Ni(O)OH electrode in the presence of AOF. It was shown that the current efficiency of adipinic acid reached its maximum at the current density of 220 A/m2 with the 50.0% current yield and 50.0% substance yield by the reaction scheme involving hydrogen peroxide. The selectivity of adipinic acid formation also increased with the increase in the current density and reached 91.0% at 250 A/m2 in the presence of Н2О2 in electrolyte solution; however, the current efficiency with respect to the target product decreased to 42%. The side products were glutaric and succinic acids.
Analysis of the results of electron paramagnetic resonance (EPR) has shown that the selectivity of adipinic acid formation can reach 89% at the electrolysis with the optimal current density (220 A/m2) and in the presence of AOF sources, i.e., the appearance of additional amounts of AOF increases the selectivity of adipinic acid formation. In this case, two oxidation paths are realized simultaneously ‑ heterogeneous electrocatalytic and homogeneous chemical. The increase in the amount of passed electricity above its theoretical value (Q/Qtheor) to 3 leads to a decrease in the selectivity of synthesis of adipinic acid due to the formation of side products (glutaric and succinic acids). Based on these results, it can be concluded that for the redox-mediator mechanism of cyclohexanol oxidation on the OHNE anode containing higher oxides of nickel, which is accompanied by the in situ generation of AOF from Н2О2 and О2, the optimization allows reaching the record-breaking selectivity of 89% and obtaining the target product in a single stage.
Indirect Electrocatalytic Oxidation of Aliphatic Alcohols to Carboxylic Acids
Aliphatic carboxylic acids, their salts, and esters are widely used in pharmaceutical, vitamin, cosmetic, and food industries [45]. It is well known that the chemical methods of production of carboxylic acids through oxidation of aliphatic alcohols have several drawbacks associated with the use of relatively expensive and toxious chemical oxidants such as potassium permanganate, chromium compounds, etc. [45, 46]. For this reason, the quest is carried out for alternative methods of synthesis including those electrochemical. As was shown earlier, for the oxidation of alcohols, the OHNE containing specially formed higher oxides of nickel is the most suitable anodic material which can efficiently convert saturated aliphatic alcohols to carboxylic acids in alkaline medium [4, 43, 46].
In [47–50], we studied the indirect oxidation of butyl, hexyl, nonyl, and decyl alcohols to butyric, caproic, pelargonic, and capric acids by active forms of chemically bound oxygen generated in situ from О2, Н2О, and Н2О2 on the following anodes: OHNE, lead-dioxide electrode, and BDDE. The following three schemes of electrolysis were realized: (1) oxidation on the anode where the process can proceed under both homogeneous and heterogeneous conditions; (2) oxidation on an anode in the presence of Н2О2; (3) paired electrolysis, i.e., oxidation on the anode with the parallel generation of \({\text{HO}}_{2}^{ - }\) on a graphite cathode blown round with oxygen.
It is shown that in the paired electrosyntheses, the oxidation of alcohols proceeds with the high current efficiency and produces the corresponding caboxylic acid according to the pseudo-first order mechanism and also that the optimal current density is 50–100 A/m2. According to the data of UV spectroscopy and the results on the chemical oxygen demand (COD), in the interval of 150–500 A/m2, the oxidation proceeds with subsequent mineralization to СО2 and Н2О.
Preparative experiments on accumulation and extraction of target products (acids) with the use of the scheme of paired electrolysis showed the following results. The synthesis on OHNE in 1.2 M NaOH produced butyric, caproic, and capric acids with the CE of 168, 148, and 64%, respectively. On BDDE in 0.1 M H2SO4, pelargonic acid was synthesized with 98% CE. In [50], the results of approximate calculations of the charge consumed per t of target product are shown.
Thus, it was found that the highest efficiency in oxidation of alcohols to the target carboxylic acids is achieved under the conditions of paired electrolysis where active oxygen is formed in parallel with the main process, which provides an additional channel for the homogeneous oxidation of substrates.
Indirect Oxidation of Acetic Acid to Peracetic Acidе
Attention paid to peracetic acid is associated with the wide spectrum of its applications as a strong oxidant [51]. Peracetic acid is an ideal disinfectant in medicine and food industry [52]. However, its wide use is prevented by its high cost which is partly associated with its limited production. Peracetic acid is produced in the reaction of concentrated hydrogen peroxide with glacial acetic acid in the presence of an acidic catalyst
The attention paid to the in situ synthesis of peracetic acid is associated with the fact that it is safer to use dilute solutions of hydrogen peroxide and acetic acid.
The authors of [53, 54] were the first to demonstrate the possibility of synthesizing peracetic acid with the use of H2O2 generated in situ on a gas-diffusion cathode at the reduction of О2 and a catalyst based on Nafion and Pt. However, the obtained concentrations of peracetic acid were low: 0.0021–0.0062 М for the H2O2 concentration of 0.147 М. We studied [55] how the electrolyte composition, the current density, and the nature of catalysts affect the efficiency of indirect electrosynthesis at the higher concentrations of peracetic acid in the presence of hydrogen peroxide generated in situ in a GDE based on technological carbon 437-E and a platinum anode. It was found that the addition of sulfuric acid to the solution as the acidic catalyst increased the concentration of peracetic acid to 0.0092 М. The increase of the acetic acid concentration to 3.5 М had a negative effect on the GDE operation—its electrochemical activity substantially decreased. The addition of ammonium molybdate as the Н2О2-activating catalyst had a positive effect on the total efficiency. As the charge passed increased with the increase in the current density to 1500 A/m2, the produced concentrations of peracetic acid also increased to 0.021 М and the H2O2 concentration increased to 1.46 М. It was shown that the ex situ aging of solutions after the electrolysis (10 and 25°С) increased the yield of CH3CO3H to its equilibrium concentration equal to 0.056 M. This suggests that the kinetics of the chemical stage is slow. Moreover, when the concentrations of CH3CO3H and H2O2 obtained in electrolysis were higher, the concentration of peracetic acid formed in the “maturing” stage was also higher. The use of two types of catalysts, namely, sulfuric acid and ammonium molybdate increased the rate of peracetic acid formation and their simultaneous use allowed solutions with the concentration of 0.021 M to be obtained, i.e., one order of magnitude higher as compared with [51, 53, 54]. We obtained the close results [56] at the hydrogen peroxide electrogeneration from oxygen in aqueous solutions of K2SO4 + H2SO4 in the presence of formic, acetic, and butyric acids. These higher results were associated with the higher productivity of the gas-diffusion cathode as regards Н2О2 generation at the current density of 1500 against 27 A/m2 used in [51, 53, 54].
DECOMPOSITION OF ORGANIC AND INORGANIC POLLUTANTS
It is known [2] that the consumption of electric energy in disinfection of organic toxicants in waste waters is proportional both to their concentration and to the number of electrons required for their destruction to СО2. As a rule, the so deep oxidation of organic molecules requires from 20 to 150 electrons. According to patent [57], the electric energy necessary for electrosynthesizing Н2О2 from О2 is 4.5 kW h per 1 kg of 100% Н2О2. Thus, the synthesis of one Н2О2 molecule from О2 required two electrons, the mineralization of organic pollutants requires from 10 to 75 peroxide molecules. Hence, the use of the method of indirect oxidation at the low concentrations (before sending them to biological cleaning) of organic substrates-“tails” contained in waste water by electrochemically generated Н2О2 is economically expedient. As an example of indirect destructive decomposition of organic and inorganic substrates, we studied the processes of oxidation of pollutants encountered most widely in technological and waste waters of various origin.
Mineralization of Formaldehyde and Formic Acid
В [58, 59], the oxidation of formaldehyde was studied in the presence of Fe(II) salts in acidic solutions (electro-Fenton process) where hydroxide radicals can be formed by the reaction
It was shown that the oxidation products are НСООН, СО2, and Н2О. The optimal conditions are realized at pH 3–4 and 60°С. The increase in the current density from 250 to 2000 A/m2 did not affect the degree of conversion of formaldehyde, the latter was close to 100% but increased the conversion rate and decreased the time of electrolysis 8-fold. The kinetics of this process was limited by the Н2О2 generation rate. The final oxidation products were СО2 and Н2О.
In [59] the destructive oxidation of formic acid by intermediates \(^{● }{\text{OH}}\) and \({\text{HO}}_{2}^{● }\) generated in situ from Н2О and Н2О2 in a diaphragmless cell with a platinum anode and a GDE cathode in electrolytes of different pH is described. It was found that as the current density increased to 1000 A/m2, the conversion of НСООН increased and then remained unchanged; in acidic electrolytes the conversion degree was higher than in alkaline solutions being equal to ~99.0% at рН 3.2. The oxidation products were, as expected, СО2 and Н2О.
Destructive Oxidation of Phenol
It is well known that phenol is one of the most abundant and difficult-to-oxidize environmental toxicants. Its content can vary widely (0.106–2.12 mM) and it can be oxidized by hydrogen peroxide to СО2 and Н2О by the reaction
We studied [60–62] the indirect destructive oxidation of phenol in electrochemical cells with and without cation-exchange membrane, with a carbon-black gas-diffusion cathode, platinum and lead-dioxide anodes, in alkaline, neutral, and acidic media, at current densities of 250–1500 A/m2 and 20°С. It was found [60] that in the alkaline medium in a three-chamber cell with cation-exchange membrane MF-4SK-100, the oxidation products were organic acids and ketones. The intermediate products with the conjugated double bonds were accumulated when the content of oxidant was insufficient. At these current densities, the efficiency of this process substantially depended on the concentration of sodium hydroxide. The higher efficiency of oxidation (78%) was achieved in 0.1 M NaOH in 3 h electrolysis at the current density of 500 A/m2.
In [61], we studied the dependences of the rate and efficiency of destructive oxidation of phenol by hydrogen peroxide generated electrochemically from oxygen in a gas-diffusion electrode in alkaline medium in an electrolyzer without cation-exchange membrane in 0.05, 0.1, and 0.5 M NaOH solutions at the current density of 250, 500, 1000, 1500 A/m2. Smooth platinum was used as the anode. In the electrolyzer containing no cation-exchange membrane, \({\text{HO}}_{2}^{ - }\) was transferred to the anode to be oxidized there to perhydroxyl radical \({\text{HO}}_{2}^{● }\). Moreover, the oxidation of ОН– on the anode produced \(^{● }{\text{OH}}\) radicals. This is why in an electrolyzer without cation-exchange membrane the presence of a platinum anode allowed one to expect the deeper oxidation of phenol up to its mineralization. The found values of characteristic times of destruction at different current densities showed that the optimal conditions were realized in 0.1 M solution at the current densities of 1000 and 1500 A/m2. The efficiency of phenol oxidation decreased with the increase in its initial concentration—at the initial concentration of 0.265, 0.53, 1.06, and 2.12 mM, the efficiency was 86, 85, 70, and 60%, respectively. IR and UV spectroscopic studies have shown that the phenol oxidation proceeds by two pathways: the direct oxidation on the anode to form resinous compounds and its homogeneous oxidation by active intermediates (\(^{● }{\text{OH}}\), \({\text{HO}}_{2}^{● }\)) in the near-anode layer. After 2–3 h electrolysis, the electron spectra contained no absorption bands of phenol and intermediates (carboxyl acids with the bands at 3350 and 1725 cm–1, β-diketones in the enol form with the bands at 1592 and 2900, quinones and α-diketones), which pointed to the virtually complete mineralization of phenol at the current density of 1000 and 1500 A/m2 and for its initial concentration of 0.265–0.53 mM.
In [62], we studied the indirect oxidation of phenol in acidic (рН 2–3) and neutral (рН 6–7) media in a diaphragmless electrolyzer with a gas-diffusion cathode, platinum and lead-dioxide anodes at the current density of 500 and 1000 A/m2. The UV spectroscopic data for solutions during their electrolysis at the same charge passed pointed to the different depth of phenol oxidation in acidic and neutral electrolytes. Thus, in acidic electrolytes, the phenol oxidation proceeded to quinone and hydroquinone (as the intermediate products) which was followed by opening of the aromatic ring to form iron complexes with carboxyl ligands and their further decomposition to СО2 and Н2О. In neutral solution, the absorption bands typical of diketones and carboxyl acids were observed. It deserves mention that the similar results were obtained in several studies by foreign authors under similar conditions [30, 63–66] but with a GDE less efficient in electrogeneration of hydrogen peroxide.
Thus, we can state that the process of phenol oxidation with 96–98% mineralization to СО2 and Н2О can proceed in alkaline electrolyte (0.1 М NaOH) without cation-exchange membrane with involvement of \(^{● }{\text{OH}}\), \({\text{HO}}_{2}^{● }\) radicals and also in acidic electrolytes where НО• is the oxidant, whereas in the neutral medium the oxidation proceeds with involvement of less active radical ions \({\text{HO}}_{2}^{● }\), which allows conversing the poorly oxidizable phenol to simple mono- and dicarboxylic acids which can be utilized by microorganisms in the course of their further biochemical cleaning.
Destructive Oxidation of N-Methyl-p-Aminophenol (Metol)
Aromatic amines are widely used in industry, e.g., as the precursors and intermediate products in the production of dyes, drugs and are also typical contaminants of the hydrosphere [67].
In [68–70], we studied the indirect electrochemical oxidation of N-methyl-p-aminophenol by active oxygen forms generated in situ from О2, Н2О, and Н2О2. The oxidation of N-methyl-p-aminophenol was carried out in a diapragmless electrolyzer with a gas-diffusion electrodes of carbon-black and graphite and anodes of Pt and PbO2 in alkaline and acidic electrolytes. It was shown that the efficiency of oxidation increased with the increase in the current density; moreover, the process was more efficient in alkaline electrolyte as compared with acid electrolyte. In alkaline electrolyte at 700 A/m2, the efficiency of mineralization was 98–99%, whereas in acidic electrolyte at 200 A/m2, the efficiency was 30%. The indirect oxidation of metol by hydrogen peroxide generally proceeded with mineralization to СО2 and Н2О by 98% in 0.1 М NaOH, whereas in 0.2 М NaOH the efficiency of mineralization decreased to 65%.
The results of UV spectroscopic studies showed that the oxidation of metol at different pH proceeded by different pathways of successive oxidation: at pH 2–3, the intermediates were largely of the carboxylate type, whereas at pH 9–10 the metol hydroxylation products were formed and successively transformed to aliphatic acids and the latter were oxidized to СО2 and water. This scheme of metol oxidation was described in the literature [71, 72]. Summarizing, it should be noted that in the alkaline medium, the metol oxidation with mineralization to СО2 and Н2О in diaphragmless cells can proceed with 97–99% efficiency on all anodic materials studied.
Destructive Oxidation of ß-Naphthol
Waste waters of pulp and paper industry contain considerable amounts of naphthol and its content is strictly controlled. The maximum permissible concentration (MPC) of naphthol is 1.39 mM [73]. It is known that the process of destructive oxidation of naphthol is described by the following rough equation [74]:
It follows from the above equation that in the ideal case, the complete mineralization of a β-naphthol molecule requires 46 electrons, i.e., this process is comparatively energy consuming. In connection with this, the more economic version of naphthol neutralization is the interruption of this process at the stage of formation of biologically utilizable aliphatic carboxyl compounds.
In [75, 76], we studied the indirect electrocatalytic oxidation of β-naphthol by active forms of oxygen generated in situ from О2, Н2О, and Н2О2 in an diaphragmless electrolyzer with carbon-black gas-diffusion and graphite cathodes and anodes of Pt and Pb/PbO2 in alkaline and acidic electrolytes of 0.1 М NaOH and 0.5 М H2SO4. In the cell containing gas-diffusion cathode and Pt anode, we observed the cymbate growth of the ß-naphthol oxidation rate with the increase in the current density (i.e., with the increase in the oxidant generation rate). To assess the efficiency of electrochemical-cell operation in the process of β‑naphthol destructive oxidation, we determined the efficiency coefficient of the cell (η) as in [77], which allowed comparing the efficiency of different cells and choosing the optimal conditions for electrolysis. For example, for a cell with a gas-diffusion electrode and the naphthol concentration of 1.387 mM, the oxidation is expedient to carry out at the current density of 500 A/m2. In this case, η = 16%, while for i = 1500 A/m2, η = 7.5%. For substrate concentration of 6.94 mM and i = 900–1000 A/m2, η = 29%. Similar results were obtained in [78]. This suggests that the cell with gas-diffusion cathode best operates in the region of low current densities. For a cell with graphite cathode and alkaline electrolyte blown through with oxygen, at the current of 0.50 A and the initial naphthol concentrations of 2.081 and 3.47 mM, it is better to use a platinum anode (η = 18%), rather than a lead anode (η = 6.5%). For acidic electrolytes, one can use both the lead anode and the platinum anode (η = 15.6%) at the similar conditions of electrolysis. The value of η is higher when the substrate concentration increases, which is probably determined by the rate of diffusion of dissolved substances to the reaction zone. The results of COD determination have shown that the efficiency of β-naphthol mineralization decreases with the increase in its initial concentration in solution. Thus, at the initial concentration of β-naphthol of 1.389 mM, the efficiency was 95%, and at 6.94 mM, it was 75% for the current density of 900 A/m2.
Based on the results of UV spectroscopy, the following scheme of the sequential oxidation of naphthol was proposed: β-naphthol → hydroxylated derivatives of naphthol → quinones → ketones → organic acids → mineralization products (СО2 and Н2О). These results suggest that the efficiency of β-naphthol oxidation in a cell with GDE depends on the current density and the substrate concentration. In a cell with a graphite cathode and oxygen bubbling through solutions, Pt is the best anodic material in acid electrolytes and Pb/PbO2 is the best choice in alkaline electrolytes. The efficiency of oxidation depends on the substrate concentration.
Destructive Oxidation of Benzene
In [69], we studied the indirect destructive oxidation of benzene by intermediates generated in situ from О2, Н2О, and Н2О2 in the two- and three-chamber electrochemical cells containing a carbon-black gas-diffusion cathode capable of generating Н2О2 from О2 and anodes of Pt and lead dioxide in aqueous solutions of different pH. The scheme with Н2О2 generated in situ from О2 and the scheme with the addition of hydrogen peroxide into electrolyte were realized. It was found that benzene hydroxylation to phenol with the use of a gas-diffusion cathode in a two-chamber cell was inefficient due to passivation of the carbon black cathode by adsorbed benzene. The benzene oxidation in the three-chamber cell with a filtering diaphragm that prevented immediate contact of benzene with the GDE proceeded to its mineralization to СО2 and Н2О, the degree of oxidation varied from 94.8% (Pb/PbO2, pH 2) to 63.5% (Pt anode, рН 2.8, \(c_{{{\text{Fe}}}}^{{2 + }}\) = 7.1 × 10–6 М (electro-Fenton version)). The main intermediates were phenol, hydroquinone, resorcinol, p-benzoquinone. The authors of [79] observed the similar intermediates of benzene oxidation on a rotating BDD disk anode. The lower efficiency of oxidation for the scheme with electro-Fenton in a cell containing a platinum anode was probably associated with the use of difficult-to-oxidize oxalate complexes with Fe3+ the presence of which was confirmed by UV spectroscopic data. The final products of benzene oxidation were СО2 and Н2О in all these schemes of destruction. The comparison of our results with literature data [79] made it possible to conclude that depending on the anode materials, the efficient indirect oxidation of benzene can proceed to phenol and further via opening of the benzene ring up to complete mineralization both in the presence of Н2О2 and with anodes of metal oxides capable of in situ generation of НО• radicals at the Н2О oxidation by the rough reaction
Destructive Oxidation of Aniline
Aniline is a highly toxic and carcinogenic substance. Its content in water is strictly regulated. In [80], we studied the indirect oxidation of aniline on Pb/PbO2 and BDDE in sulfuric acid electrolyte in the presence of AOF generated by various schemes in a diaphragmless electrolyzer at current density of 250, 500, and 1000 A/m2 at 20°С. The initial content of aniline in solutions was 0.537, 1.074, 2.147 mM. It was found that the process of indirect aniline oxidation on the Pb/PbO2 electrode at рН 2–3 was the most efficient when we used the scheme “anodic oxidation + Fenton reagent.” As the current density increased, the efficiency of this process became almost independent of the method of AOF generation. On BDDE in 0.1 М H2SO4, the efficiency of aniline oxidation was 90.0% for the oxidation scheme “anodic oxidation + Н2О2.” The UV spectroscopic studies showed that the indirect oxidation of aniline proceeded via the intermediate p-benzoquinole; moreover, in the presence of hydrogen peroxide the oxidation rate of all intermediates approximately doubled. Based on the results obtained, we can conclude that the process of indirect aniline oxidation can be considered as a method alternative (to the extraction and adsorption methods) for its degradation in waste waters of industrial plants on electrodes of Pb/PbO2 and BDD.
Destructive Oxidation of Thiocyanates
The main source of aqueous solutions of thiocyanates (rhodanides) are the hydrometallurgical processes of gold and silver extraction from sulfide-containing ores and concentrates. Before being discharged or reused, the waste and circulating waters containing thiocyanates should be subjected to deep cleaning from these highly toxic components, which is carried out with the use of chlorine-containing chemicals and the latter results in rapid corrosion of the equipment and complicate the environmental situation.
The destructive oxidation of thiocyanates by hydrogen peroxide proceeds by the rough reaction
and allows achieving a considerable environmental effect because decreases the mineralization of waste waters and lowers down the costs for disinfection by regeneration of cyanide-based chemicals.
In [81, 82], we studied the kinetics and the efficiency of thiocyanate oxidation in alkaline medium with the use of a Pt anode and a gas-diffusion cathode that generated hydrogen peroxide from oxygen, in an electrochemical cell with and without cation-exchange membrane. It was found that when the thiocyanate oxidation was carried out in alkaline medium in a cell with a membrane and the current increased from 0.25 to 0.50 A, the oxidation rate increased approximately 3-fold. The efficiency of destruction of thiocyanates with the initial concentration c0 = 18.36 mM was 65.0 and 80.0% for the current of 0.25 and 0.50 A, respectively, at the equal charge passed (1 A h). The oxidation products of thiocyanates were mainly \({\text{CO}}_{3}^{{2 - }}\), \({\text{SO}}_{4}^{{2 - }}\), \({\text{NH}}_{4}^{ + }\), and also a small amount of CNO−. When the process was carried out in a membraneless cell, in addition to the SCN− oxidation by hydrogen peroxide, its direct oxidation on the Pt anode was possible. Moreover, on the anode, the oxidation of the produced \({\text{HO}}_{2}^{ - }\) was possible as well and the oxidation of water or hydroxyl ions to \({\text{HO}}_{2}^{● }\) and НО•. The appearance in the system of an additional oxidizing agent in the form of \({\text{HO}}_{2}^{● }\) increased both the rate of thiocyanate oxidation and its efficiency. For instance, the efficiency of 30 min electrolysis at a current of 0.50 A was 96% when hydrogen peroxide was formed in the gas-diffusion electrode, whereas at the anodic oxidation only, the efficiency was 62%. It was found that in the acidic medium, the oxidation of thiocyanates was more efficient in a membraneless cell as compared with the classical Fenton process in a cell with membrane. The thiocyanate conversion in 30 min electrolysis was 71 and 52%, respectively.
When the process is carried out in a membraneless cell, it should be taken into account that the direct oxidation of SCN− in acidic medium proceeds to СN− ions which can either be oxidized further or hydrolyzed to form toxic hydrogen cyanide.
It should be noted that at the high initial concentration of thiocyanates, the process can be carried out in a membraneless cell with the aim of regeneration of cyanides to be reused in the leaching process [81]. However, at low concentrations, this method is economically unattractive and, without control over evolving HCN‑environmentally unsafe. In the latter case, our method of thiocyanate oxidation in alkaline medium is the most advisable.
Ex situ PROCESSES
INDIRECT ELECTROSYNTHESIS OF PEROXOSOLVATES—THE PRODUCTS OF MOLECULAR ADDITION
It is well known that Н2О2 can form molecular compounds with a large number of substances, which are highly stable, can be extracted in the solid state (peroxosolvates, peroxohydrates), and stored for a long time [83]. In [84], we studied the electrosynthesis of Н2О2 from oxygen in a gas-diffusion electrode based of technological carbon А437-E in solutions of salts capable of forming molecular compounds with hydrogen peroxide and also the preparation of concentrated solutions of these compounds and their extraction in the solid state. As the electrolytes we took KF, sodium and potassium salts of phosphoric acid with different composition.
After the electrolysis, some of Н2О2 solutions were concentrated. For this purpose, the solutions were kept in air in open polyethylene vessels at room temperature for 10–15 days under the conditions preventing the access of solar light and artificial illumination. As mentioned above, under these conditions, the Н2О2 concentration increases [13]. The concentration of solutions containing KF and KCl was carried out before the extraction of solid compounds and their drying. Concentration of phosphate solutions produced a thick gel-like mass. It was found that when the substances capable of forming molecular compounds with Н2О2 were present at the electrolysis, the latter produced the higher concentrations of hydrogen peroxide with the high current efficiency. The best results were obtained in solutions (рН 7–8) of KF which can form stable compounds with Н2О2: the 7.5 h electrolysis at the current density of 1500 A/m2 produced hydrogen peroxide solution with the concentration of 3.6 М with 92% current efficiency. The close results were obtained in solutions of Na2HPO4. The lowest current efficiency and Н2О2 concentration were obtained in solutions of potassium phosphates. The resulting Н2О2 solutions were subjected to concentration; for instance, KF solutions were concentrated to 20 М and higher in 20 days and then stored in closed vessels in order to prevent evaporation of water and Н2О2. The lowest loss of H2О2 was observed when concentrating KF solutions. For instance, the concentration of solutions containing KF with the addition of H3PO4 up to рН 5–6 produced the solution with hydrogen peroxide concentration of 26.9 M; and the solid target product contained ~50.0 wt % Н2О2 in the molar ratio 1 KF per 1.7 Н2О2. It was shown that during the mentioned period, the Н2О2 concentration remained virtually unchanged, which pointed to the high stability of synthesized solutions and solid peroxosolvates. Based on these results, it was concluded that aqueous solutions of salts capable of forming compounds with Н2О2 electrogenerated under in situ conditions may also find their application in the processes of ex situ concentration of Н2О2 solutions up to 26.9 М and also for producing the target product in the solid state, e.g., KF ⋅ 1.7 Н2О2 with the Н2О2 content of ~50.0 wt % (see Table 2 in [84]).
ELECTROGENERATION OF Н2О2 IN AQUEOUS SOLUTIONS OF MINERALIZED LIQUID AND SOLID DOMESTIC WASTES
One of important problems in the development of autonomous life support systems (ALSS) is the processing of domestic wastes on the basis of biological mass-exchange processes. This problem is solved most efficiently by the liquid-phase oxidation of exometabolites in the presence of 30% Н2О2 under alternating current [85]. At the same time, a substantial drawback of this method is the necessity of storing considerable volumes of hydrogen peroxide in a closed system which is unsafe and also substantially limits the lifetime of the system as a whole. In connection with this, to increase the safety and lifetime of ALSS, it is necessary to produce Н2О2 solutions in situ from substances present in closed space and concentrating them under ex situ conditions. The electrolysis of Н2О2 from О2 and Н2О in GDE satisfies these conditions.
In [86], we studied the process of Н2О2 electrosynthesis in aqueous solutions (рН 1–9) of mineralized liquid and solid solutions of domestic wastes. As the catholyte, we used the solutions of mineralized exometabolites (MEM) prepared in the Institute of Biophysics, Siberian Branch of the Russian Academy of Sciences (Krasnoyarsk, Russia). The anolyte represented 1 M H2SO4 solution. It was shown that the generation of hydrogen peroxide proceeds at the potentials more positive than the potential of hydrogen evolution under these conditions, which increases the safety and efficiency of its production. It is evident that the rate of Н2О2 accumulation in electrolyte is determined by the difference of the rate of its synthesis in GDE and the rate of its chemical decomposition in electrolyte [12]. In MEM solutions, its higher concentrations and current efficiencies can be reached as compared with the 0.5 М КОН solutions; moreover, the changes in pH have no effect on the efficiency of the target product. The high selectivity of this process in all syntheses demonstrated that no processes of Н2О2 dissolution and or its further reduction to water take place in the electrode volume. This suggests that the presence in MEM of the large number of various compounds does not affect the characteristics of Н2О2 synthesis and even favors its concentration. This phenomenon was observed at electrosynthesis of Н2О2 in alkaline aqueous suspension of brown coal containing a large number of various organic and inorganic compounds [87, 88]. For the current density of 2000 A/m2, the value of γ was 0.97–0.95 in this solution.
The electrosynthesis of Н2О2 in GDE with the working surface of 64 cm2 at the current density of 1000 A/m2 produced the target product in the concentration reaching 1.6 М at 50% CE. In this case, the lower CE values were associated with the increase in the electrolysis temperature to 40°С and decomposition of peroxide. To test the stability of synthesized Н2О2 solutions, the latter were kept ex situ in air in closed polyethylene vessels for 10 days. These experiments showed that the solutions were stable and the target product remained virtually unaffected. When the solutions were stored in open vessels for 5 days, the Н2О2 concentration increased from 2.4 to 11.3 М; the next 5 days of storage increased its concentration to 19 М. The losses of Н2О2 in the process were less than 20%. These experiments showed that hydrogen peroxide can be concentrated in MEM solutions and that the electrolytic method is promising for its synthesis based on biological mass-exchange processes, which is of principal importance for mineralization of exometabolites in ALSS.
These results demonstrate the principal possibility of a considerable extension of the ex situ application field of hydrogen peroxide solutions electrogenerated in GDE from oxygen, for instance, for their concentration, indirect electrosynthesis of organic peroxoacids, liquid and solid inorganic products with the high content of active oxygen, and mineralization of exometabolites in ALSS.
CONCLUSIONS
Based on the totality of results known from the literature and obtained in our experimental studies, we can state that hydrogen peroxide solutions electrogenerated from oxygen and water can be used in situ with the high efficiency in the traditional fields of bleaching of cellulose semi-finished products, indirect electrosynthesis and target organic and inorganic products, and also in destruction and mineralization of organic and inorganic pollutants in technological waste waters of different origin. Under ex situ conditions, such solutions can be concentrated to considerable concentrations (26.9 М and higher) and used in the production of organic peroxoacids, inorganic peroxosolvates, and in mineralization of exometabolites in ALSS. It deserves mention that at present, of these two directions of the use of Н2О2 solutions electrogenerated from О2, the in situ direction is better developed as compared with the ex situ direction but we believe that the latter direction is also highly promising.
Furthermore, the highly efficient generation of hydrogen peroxide solutions from oxygen in the concentration above 3 M in carbon-black gas-diffusion electrodes based on acetylene black А437-E or mesostructured carbon CMK-3 in cells-electrolyzers of different design and its use in preparation of active oxygen forms with different oxidation activity allows the oxidation processes of different depth to be carried out, while the use of different modes of electrolysis (paired electrolysis) makes it possible to carry out this process with the high efficiency. It deserves mention that the production of such solutions can be organized in the sites where they are used, for instance, in inaccessible sufficiently electrified territories from technological oxygen and also from air oxygen with electrolysis characteristics lower by ~25–30%. These results can be used in selecting the most reasonable version of the use of hydrogen peroxide solutions electrogenerated from oxygen in solving concrete problems.
REFERENCES
Anastas, P.T. and Warner, J.C., Green Chemistry: Theory and Practice. London: Oxford University, 1998.
Pletcher, D., Indirect oxidations using electrogenerated hydrogen peroxide, Acta Chem. Scand., 1999, vol. 53, p. 745.
Kornienko, V.L., Indirect oxidation of organics by hydrogen peroxide electrochemically generated in situ from oxygen, Khim. Interesakh Ustoich. Razvit., 2002, vol. 10, p. 391.
Ogibin, Yu.N., Elinson, M.N., and Nikishin, G.I., Mediator oxidation systems in organic electrosynthesis, Russ. Chem. Rev., 2009, vol. 78, p. 89.
Brillas, E., Bastida, R.M., and Llosa, E., Electrochemical destruction of aniline and 4-chloroaniline for wastewater treatment using a carbon-PTFE O2-fed cathode, J. Electrochem. Soc., 1995, vol. 142, p. 1733.
Brillas, E., Sires, I., and Oturam, M.A., Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry, Chem. Rev., 2009, vol. 109, p. 6570.
Giomo, M., Boso, A., Sandona, G., Boye, B., and Farnia, G., A small-scale pilot plant using an oxygen-reducing gas-diffusion electrode for hydrogen peroxide electrosynthesis, Electrochim. Acta, 2008, vol. 54, p. 808.
Noyori, R., Pursuing practical elegance in chemical synthesis, Chem. Comm., 2005, p. 1807.
Schumb, W.C., Satterfield, C.N., and Wentworth, R.L., Hydrogen peroxide, New York: Chapman and Hall, 1958.
Chemistry and Technology of Hydrogen Peroxide, Seryshev, G.A., Ed., London: Chemistry, 1984.
Berl, B.E., A new cathodic process for the production H2O2, Trans. Electrochem. Soc., 1939, vol. 76, p. 359.
Kornienko, V.L., Kolyagin, G.A., and Saltykov, Yu.V., Electrosynthesis H2O2 from O2 at carbon electrodes in alkali medium, Russ J. Appl. Chem. 1999. vol. 72. p. 353.
Titova, V.K., Nikol’skaya, V.P., Buyanov, V.V., and Suprun, I.P., Methods for concentration of hydrogen peroxide to obtain it in anhydrous form, Russ. J. Appl. Chem., 2002, vol. 75, p. 1903.
Vysotskaya, N.A., Reactivity of HO•, O•-, \({\text{HO}}_{2}^{● }\) radicals and oxygen atoms in aqueous solutions of aromatic compounds, Usp. Khim., 1973, vol. 42, p. 1843.
Kornienko, V.L., Chaenko, N.V., and Kornienko, G.V., Indirect electrochemical oxidation of organics by active oxygen forms, inElektrokhmiya organicheskikh soedinenii v nachale XXI veka (Electrochemistry of Organic Compounds in the Beginning of XXI Century), Gul’tyay, V.P., Krivenko, A.G., and Tomilov, A.P., Eds., Moscow: Sputnic+, 2008, p. 147.
Nagiev, T.M., Conjugate reactions of oxidation by hydrogen peroxide), Usp. Khim., 1985, vol. 54, p. 1654.
Kornienko, V.L., Kolyagin, G.A., and Saltykov, Yu.V., Elektrosintez v gidrofobizirivannykh elekrodakh (Electrosynthesis in Hydrobized Electrodes), Tomilov, A.P., Ed., Novosibirsk: Sib. Otd. Nauk, 2011, p. 109.
Brillas, E., Mur, E., and Casado, I., Iron(II) catalysis of the mineralization of aniline using a carbon-PTFE–O2 fed cathode, J. Electrochem. Soc., 1996, vol. 143, p. L.49.
Brillas, E., Sauleda, R., and Casado, I., Peroxi-coagulation of aniline in acidic medium using an oxygen diffusion cathode, J. Electrochem. Soc., 1997, vol. 144, p. 2374.
Moreira, F.C., Boaventura, R.A.R., Brillas, E., and Vilar, V.J.P., Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters, Appl. Catal. B, 2017, vol. 202, p. 217.
Kahoush, M., Behary, N., Cayla, A., and Nierstrasz, V., Bio-Fenton and bio-electro-Fenton as sustainable methods for degrading organic pollutants in wastewater, Process Biochem., 2018, vol. 64, p. 237.
Chen, Z., Dong, H., Yu, H., Yu, H., Zhao, M., and Zhang, X., Performance and mechanism of in situ electro-catalytic flue gas desulfurization via carbon black-based gas diffusion electrodes doped with MWCNTs, Electrocatal., 2017, vol. 8, p. 103.
Vijapur, S. H., Hall, T.D., Snyder, S.T., Inman, M., Taylor, E.J., and Skinn, B.T., Electrochemical peroxide generation, ECS Trans., 2017, vol. 77, p. 947.
Fukuzumi, S. and Yamada, Y., Hydrogen peroxide used as a solar fuel in one-compartment fuel cells, ChemElectroChem., 2016, vol. 3, p. 1978.
Gravotto, G., Di Carlo, S., Ondruschka, B., Tumiatti, V., and Roggero, C.M., Decontamination of soil containing POPs by the combined action of solid Fenton-like reagents and microwaves, Chemosphere, 2007, vol. 69, p. 1326.
Mousset, E., Trellu, C., Oturan, N., Rodrigo, M.A., and Oturan, M. A., Soil remediation by electro-Fenton process: in electro Fenton process, HEC, 2017, vol. 61, p. 399.
Kornienko, V.L., Kolyagin, G.A., Kornienko, G.V., Chaenko, N.V., Kosheleva, A.M., Kenova, T.A., and Vasil’eva, I.S., Use of aqueous hydrogen peroxide solutions prepared by cathodic reduction of oxygen for indirect oxidation of chemical substances in situ: achievements and prospects, Russ. J. Appl. Chem., 2014, vol. 87, p. 3.
Garcia-Rodriguez, O., Lee, Y.Y., Olvera-Vargas, H., Deng, F., Wang, Z., and Lefebvre, O., Mineralization of wastewater by electro-Fenton with on enhanced graphene-based gas diffusion cathode, Electrochim. Acta, 2018, vol. 276, p. 12.
Zhang, Z., Meng, H., Wang, Y., Shi, L., Wang, X., and Cha, S., Fabrication of graphene graphite-based gas diffusion electrode for improving H2O2 generation in electro-Fenton process, Electrochim. Acta, 2018, vol. 260, p. 112.
Luo, H., Li, C., Wu, C., Zheng, W., and Dong, X., Electrochemical degradation of phenol by in situ electro-generated and electro-activated hydrogen peroxide using an improved gas diffusion cathode, Electrochim. Acta, 2015, vol. 186, p. 486.
Kharlamova, T.A. and Aliev, Z.M., Use of electrolysis under pressure for destructive of phenol and azodyes, Russ. J. Electrochem., 2016, vol. 52, p. 251.
Perazzolo, V., Durante, C., and Gennaro, A., Nitrogen and sulfur doped mesoporous carbon cathodes for water treatment, J. Electroanal. Chem. 2016, vol. 782, p. 264.
Kornienko, G.V., Kolyagin, G.A., Kornienko, V.L., and Parfenov, V.A., Graphitized carbon materials for electrosynthesis of H2O2 from O2 in gas-diffusion electrodes, Russ. J. Electrochem., 2016, vol. 52, p. 983.
Kornienko, V.L., Kolyagin, G.A., Kornienko, G.V., Parfenov, V.A., and Petin, A.A., Electrosynthesis of H2O2 from O2 in gas diffusion electrodes based on soot CH600, Russ. J. Electrochem., 2017, vol. 53, p. 1329.
Solyanikova, A.S., Chayka, M.Yu., Boryak, A.V., Kravchenko, T.A., Glotov, A.V., Ponomarenko, I.V., and Kirik S.D., Composite electrodes of electrochemical capacitors based on carbon materials with different structure, Russ. J. Electrochem., 2014, vol. 50, p. 419.
Kolyagin, G.A., Kornienko, G.V., Kornienko, V.L., and Ponomarenko, I.V., Electrochemical reduction of oxygen to hydrogen peroxide in gas-diffusion electrode based on mesoporous carbon, Russ. J. Appl. Chem., 2017, vol. 90, p. 1143.
Kornienko, V.L., Kolyagin, G.A., Kornienko, G.V., Parfenov, V.A., and Ponomarenko, I.V., Electrosynthesis of H2O2 from O2 in gas diffusion electrodes based on mesostructured carbon CMK-3, Russ. J. Electrochem., 2018, vol. 54, p. 192.
Luo, H., Li, C., Wu, C., and Dong, X., In situ electrosynthesis of hydrogen peroxide with an improved gas diffusion cathode rolling by carbon black and PTFE, RSC Advances, 2015, vol. 5, p. 6527.
Vasiľeva, I.S., Kolyagin, G.A., and Kornienko, V.L., Highly selective indirect electrochemical oxidation of formaldehyde in situ \({\text{HO}}_{2}^{ - }\) generated from O2 in a gas diffusion electrode in alkali medium, Russ. J. Appl. Chem., 2000, vol. 73, p. 1036.]
Chaenko, N.V., Pavlenko N.I., and Kornienko, V.L., Indirect electrochemical oxidation of maleic acid by hydrogen peroxide in situ generated from oxygen in a gas diffusion electrode, Khim. Interesakh Ustoich. Razvit., 2002, vol. 10, p. 497.
Sato, K., Aoki, M., and Noyori, R.A., A “green” route to adipic acid: direct oxidation of cyclohexenes with 30 percent hydrogen peroxide, Science, 1998, vol. 281, no. 5385, p. 1646.
Lyalin, B.V. and Petrosyan, V.A., Electrosynthesis of adipic acid in conditions of diaphragm free electrolysis, Russ. Chem. Bull., 2004, p. 657.
Lyalin, B.V. and Petrosyan, V.A., Oxidation of organic compounds on NiOOH electrode, Russ. J. Electrochem., 2010, vol. 46, p. 1199.
Chaenko, N.V., Kornienko, G.V., Sokolenko, V.V., and Kornienko, V.L., Redox-mediated oxidation of cyclohexanone to adipic acid on oxide-nickel anode, with active forms of oxygen involved, Russ. J. Appl. Chem., 2014, vol. 87, p. 444.
Pillai, U.R. and Sahle-Demessie, Oxidation of alcohols over Fe3+/montmorillonite-K10 using hydrogen peroxide, Appl. Catal., A, 2003, vol. 245, p. 103.
Remorov, B.S., Avrutskaya, I.A., and Fioshin, M.Ya., Effect of electrode material on isobutyl alcohol electrooxidation in alkaline solution, Sov. Electrochem. 1981, vol. 17, p. 1287.
Chaenko, N.V., Kornienko, G.V., Kosheleva, A.M., Maksimov, N.G., and Kornienko, V.L., Indirect electrochemical oxidation of aliphatic alcohols to carboxylic acids by active oxygen forms in aqueous media, Russ. J. Electrochem., 2011, vol. 47, p. 1146.
Kosheleva, A.M., Chaenko, N.M., Kornienko, G.V. Vlasenko, V.I., and Kornienko, V.L., Nonanol-1 oxidation on nickel oxide electrode with the involvement of active oxygen forms, Russ. J. Electrochem., 2013, vol. 49, p. 96.
Kosheleva, A.M., Maksimov, N.G., Kornienko, G.V., and Kornienko, V.L., Studies of kinetics of indirect in situ electrocatalytic oxidation of aliphatic alcohols to carboxylic acids by active forms of oxygen, Russ. J. Electrochem., 2015, vol. 51, p. 1079.
Kornienko, V.L., Kosheleva, A.M., and Kornienko, G.V., Electrosynthesis of carboxylic acids by indirect electrocatalytic oxidation with active forms of oxygen, Khim. Interesakh Ustoich. Razvit., 2016, vol. 24, p. 613.
Llanos, J., Moraleda, I., Sáez, C., Rodrigo, M.A., and Cañizares, P., Optimization of a cell for the electrochemical synergistic production of peroxoacetic acid, Electrochim. Acta, 2018, vol. 260, p. 177.
Kitis, M., Disinfection of wastewater with peracetic acid: a review, Environ. Int., 2004, vol. 30, p. 47.
Saha, M.S., Denggerile, A., Nishiki, Y., and Ohsaka, T., Synthesis of peroxyacetic acid using in situ electrogenerated hydrogen peroxide on gas diffusion electrode, Electrochem. Commun., 2003, vol. 5, p. 445.
Saha, M.S., Nishiki, Y., Furuta, T., and Ohsaka, T., Electrolytic synthesis of peroxyacetic acid using in situ generated hydrogen peroxide on gas diffusion electrodes, J. Electrochem. Soc., 2004, vol. 151, p. 93.
Chaenko, N.V., Kornienko, G.V., and Kornienko, V.L., Indirect electrosynthesis of peracetic acid using hydrogen peroxide generated in situ a gas diffusion electrode, Russ. J. Electrochem., 2011, vol. 47, p. 230.
Kolyagin, G.A., Vasiľeva, I.S., and Kornienko, V.L., Effect of the composition of acid solutions and the presence of organic acid on oxygen electroreduction to hydrogen peroxide in a carbon black gas-diffusion electrode, Russ. J. Electrochem., 2011, vol. 47, p. 282.
Grangaard, D.H., US Patent 3591470, 1971.
Vasil’eva, I.S., Kornienko, V.L., and Kolyagin, G.A., Indirect electrochemical oxidation of formaldehyde by hydrogen peroxide generated from oxygen in the presence of Fe (II) salts in acidic solutions, Khim. Interesakh Ustoich. Razvit., 2001, vol. 9, p. 529.
Vasil′eva, I.S. and Kornienko, V.L., Mineralization of formic acid by intermediates \({\text{O}}{{{\text{H}}}^{● }}\) and \({\text{HO}}_{2}^{● }\) in cell without membrane in electrolytes with different pH, Khim. Interesakh Ustoich. Razvit., 2003, vol. 11, p. 713.
Kornienko, G.V., Kornienko, V.L., Maksimov, N.G., and Pavlenko, N.I., Oxidation of phenol by hydrogen peroxide in situ generated in a gas- diffusion electrode in alkali electrolyte, Khim. Interesakh Ustoich. Razvit., 2001, vol. 9, p. 35.
Kornienko, G.V., Maksimov, N.G., Kornienko, V.L., and Pavlenko, N.I., Destructive oxidation of phenol by hydrogen peroxide in alkali medium in cell without membrane, Khim. Interesakh Ustoich. Razvit., 2002, vol. 10, p. 321.]
Kornienko, G.V., Chaenko, N.V., and Kornienko, V.L., Indirect electrochemical oxidation of phenol by hydrogen peroxide in situ generated in a gas-diffusion electrode in acidic and neutral mediums, Khim. Interesakh Ustoich. Razvit., 2006, vol. 14, p. 23.
Turkay, O., Barisci, S., Ozturk, B., Öztürk, H., and Dimoglo, A., Electro-peroxone treatment of phenol: Process comparison, the effect of operational parameters and degradation mechanism, J. Electrochem. Soc., 2017, vol. 164, p. 180.
Yalfani, M.S., Contreras, S., Medina, F., and Sueiras, J., Phenol degradation by Fenton’s process using catalytic in situ generated hydrogen peroxide, Appl. Catal., 2009, vol. 89, p. 519.
Mousset, E., Frunzo, I., Esposito, G., van Hullebusch, E.D., Oturan, N., and Oturan, M.A., A complete phenol oxidation pathway obtained during electro-Fenton treatment and validated by a kinetic model study, Appl. Catal., B, 2016, vol. 180, p. 189.
Pimentel, M., Oturan, N., Dezotti, M., and Oturan, M.A., Phenol degradation by advanced electrochemical oxidation process electro-Fenton using a carbon felt cathode, Appl. Catal., 2008, vol. 83, p. 140.
Maksimov, V.F., Volf, I.V., and Vinokurova, T.A., Ochistka i rekuperatsiya promyshlennykh vybrosov (Purification and Recuperation of Industrial Emissions), Moscow: Lesnaya Promyshl., 1989
Kornienko, G.V., Chaenko, N.V., Kornienko, V.L., and Maksimov, N.G., Neutralization of N-methyl-p-aminophenol by indirect electrochemical oxidation with reactive oxygen species different pH, Khim. Interesakh Ustoich. Razvit., 2007, vol. 15, p. 171.
Kornienko, V.L., Chaenko, N.V., and Kornienko, G.V., Indirect electrochemical destructive oxidation of aromatic compounds with reactive oxygen species, Russ. J. Electrochem., 2007, vol. 43, p. 1243.]
Kornienko, G.V., Chaenko, N.V., and Kornienko, V.L., Indirect electrochemical oxidation of N-methyl-p-aminophenol by active oxygen species generated in situ from O2, H2O, and H2O2, Russ. J. Appl. Chem. 2008, vol. 81, p. 1364.
Lunar, L., Sicilia, D., Rubio, S., Pérez-Bendito, D., and Nickel, U., Identification of metol degradation products under Fenton’s reagent treatment using liquid chromatography-mass spectrometry, Water Res., 2000, vol. 34, p. 3400.
Aceituno, M., Stalikas, C.D., Lunar, L., Rubio, S., and Pérez-Bendito, D., H2O2/TiO2 photocatalytic oxidation of metol identification of intermediates and reaction pathways, Water Res., 2002, vol. 36, p. 3582.
SAN. PIN. 4630-88, Sanitary rules and regulations for the protection of surface waters from pollutants, Moscow: Ministry of Health USSR, 1988.
Panizza, M., Michaud, P.A., Cerisola, G., and Comninellis, Ch., Anodic oxidation of 2-naphthol at boron-doped diamond electrodes, J. Electroanal. Chem., 2001, vol. 507, p. 206.
Kornienko, G.V., Chaenko, N.V., Maksimov, N.G., and Kornienko, V.L., Oxidation of β-Naphthol by active oxygen species generated in electrochemical cells, Russ. J. Appl. Chem., 2009, vol. 82, p. 1018.
Chaenko, N.V., Kornienko, G.V., and Kornienko, V.L., Electrochemical mineralization of β-Naphthol by in situ active oxygen species, Khim. Interesakh Ustoich. Razvit., 2010, vol. 18, № 2, p. 171.
Panizza, M., Bocca, C., and Cerisola, G., Electrochemical treatment of wastewater containing polyaromatic organic pollutants, Water Res., 2000, vol. 34, p. 2601.
Le Naour, C., Moisy, P., Arpigny, S., and Madic, C., Electro-oxidation of dihydroxymalonic acid on polycrystalline platinum electrode, Electrochim. Acta, 1999, vol. 44, p. 3505.
Oliveira, R.T.S., Salazar-Banda, G.R., Santos, M.C., Calegaro, M. L., Miwa, D.W., Machado, S.A.S., and Avaca, L.A., Electrochemical oxidation of benzene on boron-doped diamond electrodes, Chemosphere, 2007, vol. 66, p. 2152.
Kornienko, G.V., Kenova, T.A., Kornienko, V.L., Maksimov, N.G., and Balhareva, M.Ya., Indirect electrochemical oxidation of aniline in acid electrolyte with active oxygen species, Russ. J. Appl. Chem., 2016, vol. 89, p. 1612.
Kenova, T.A., Kornienko, V.L., and Machova, N.V., in Materials of IV International symposium “Gold of Siberia: geochemistry, technology, economy”, Krasnoyarsk: KNIIG MS, 2006. p. 96.
Kenova, T.A., Kornienko, V.L., and Drozdov, S.V., On electrochemical oxidation of thiocyanates in solutions for cyanidation of gold- containing ores and concentrates, Russ. J. Appl. Chem., 2010, vol. 83, p. 1589.
Titova, K.V., Hydrogen peroxide in synthesis peroxo solvates, Russ. J. Inorg. Chem., 2000, vol. 45, p. 320.
Kolyagin, G.A. and Kornienko, V.L., Electrosynthesis of hydrogen peroxide in solutions of salts that form molecular addition products (peroxo solvates) with it, Russ. J. Electrochem., 2014, vol. 50, p. 798.
Kudenko, Ya.A. and Pavlenko, R.A., RF Patent 2111939, 1998.
Kolyagin, G.A., Kornienko, V.L., Kudenko, Ya.A., Tikhomirov, A.A., and Trifonov, S.V., Electrosynthesis of hydrogen peroxide from oxygen in gas-diffusion electrode in solutions of mineralized exometabolites, Russ. J. Electrochem., 2013, vol. 49, p. 1004.
Kornienko, V.L. and Kolyagin, G.A., Indirect Electrochemical oxidation of brown coal on gas-diffusion electrodes in aqueous electrolyte, Trans. SAEST, 1999, vol. 34, p. 32.
Kolyagin, G.A., Kornienko, V.L., and Vetoshkina, O.V., Indirect electrochemical oxidation of brown coal by hydrogen peroxide generated from oxygen in a gas-diffusion electrode, Russ. J. Appl. Chem., 2000, vol. 73, p. 1734.
ACKNOWLEDGMENTS
We are grateful to the administration of the company “Omskuglerod” for the samples of different types of technological carbon for electrocatalytic tests.
Funding
This complex study was supported by the following grants of the Krasnoyarsk Regional Science Foundation (KRSF) and the Russian Foundation for Basic Research (RFBR): KRSF-RFBR Yenisei no. 07-03-96801, RFBR Siberia no. 09-03-98000, RFBR Siberia no. 11-03- 98000, and also within the frames of the State Grant for the Institute of Chemistry and Chemical Technology of the Siberian Branch of the Russian Academy of Sciences and the Krasnoyarsk Federal Scientific Center of the Siberian Branch of Russian Academy of Sciences (project no. 0356-2016-05 02. V.45.3.3.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of any conflict of interests.
Additional information
Translated by T. Safonova
Published on the basis of materials of the XIX All-Russian Conference “Electrochemistry of Organic Compounds” (EKHOS-2018) (with international participation), Novocherkassk, 2018.
Rights and permissions
About this article
Cite this article
Kornienko, V.L., Kolyagin, G.A., Kornienko, G.V. et al. The Prospects of the in situ and ex situ Use of Aqueous Solutions of Hydrogen Peroxide Electrogenerated from Oxygen. Russ J Electrochem 56, 405–417 (2020). https://doi.org/10.1134/S1023193520050067
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193520050067