Abstract
Potato cultivar Bzura bred in 1986 is one of the few European cultivars outstanding in high level of durable resistance to late blight, but its resistance has not been characterized. The presented study was aimed at clarifying bases of durable resistance in Bzura by testing its resistance and resistance in progeny individuals. In ten-year field experiment Bzura was significantly more resistant than mid-resistant standards Escort and Robijn, and only slightly less resistant than highly resistant Sárpo Mira. Bzura expressed also high resistance in detached leaflet tests when inoculated with specific isolates of Phytophthora infestans. It was found in Bzura progeny that this race-specific resistance segregated in 1:1 ratio and is governed by a major resistance gene R2-like. However, the long-lasting field resistance observed in Bzura could be explained by combination of R2-like gene and specific genetic background of this cultivar rather than by this gene exclusively.
Resumen
La variedad de papa Bzura, de 1986, es una de las pocas variedades europeas sobresalientes en alto nivel de resistencia durable al tizón tardío, pero no se ha caracterizado su resistencia. El presente estudio tuvo como propósito la clarificación de las bases de la resistencia durable en Bzura mediante la prueba de su resistencia y de la de individuos de su progenie. En experimentos de campo de diez años Bzura fue significativamente más resistente que las de resistencia media estándar Escort y Robijn, y solo ligeramente menos resistente que la altamente resistente Sárpo Mira. Bzura expresó también alta resistencia en pruebas de hoja separada cuando se le inoculó con aislamientos específicos de Phytophthora infestans. Se encontró en la progenie de Bzura que esta resistencia específica a razas segregó en una proporción de 1:1 y está gobernada por un gene mayor de resistencia tipo R2. No obstante, la resistencia durable de campo observada en Bzura pudiera explicarse por la combinación de un gene tipo R2 y un respaldo genético específico de esta variedad, en vez de este gene exclusivamente.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polish potato cultivar Bzura was released in 1986, but it is still distinguished by durable resistance to late blight. This is a late maturing, high yielding, starch cultivar. In some regions of Poland Bzura is also grown as table cultivar due to its floury cooking type and good taste. This cultivar was bred in IHAR-PIB, Młochów Research Center, within the frame of program aimed at combination of multiple resistances to various potato diseases. Bzura is resistant to late blight (caused by Phytophthora infestans (Mont.) de Bary), potato viruses Y and X, and potato wart fungus (race D1 and some virulent pathotypes) (Chrzanowska 2000; Douches et al. 2002, 2004; Flis et al. 2005; Przetakiewicz 2010; Tatarowska et al. 2011; Zimnoch-Guzowska et al. 2013). Therefore, this cultivar was widely used in many research and breeding programs in Europe and in both Americas (Ahmadvand 2013; Bendahmane et al. 2000; Bisognin et al. 2002; Haynes et al. 1998; Polkowska-Kowalczyk et al. 2011; Querci et al. 1995). This cultivar is still cultivated in Poland, due to its high level of field resistance to late blight. Resistance against P. infestans of cv. Bzura provides effective protection against late blight in various environments. Results of large-scale field experiments carried out in Poland (four locations) (Tatarowska et al. 2012) as well as in United States (eight locations) (Haynes et al. 1998) confirmed high and stable resistance of this cultivar. There are also reports about effectiveness of Bzura resistance under strong late blight pressure in Toluca Valley (GILB 2001).
Resistance of this cultivar originates probably from S. demissum, since in its pedigree multiple introductions of this species can be found (Swieżyński et al. 1997, Fig. 1). The long lasting effective protection against late blight in field conditions and stable expression of resistance in various environments (parallel to noted susceptibility in the laboratory assay) may indicate an important role of horizontal resistance. The presence of demissum-derived R genes in Bzura is also expected (Dorrance and Inglis 1997; Lebecka et al. 2006; Świeżyński et al. 1991). So far, only the presence of the R1 gene in Bzura was confirmed (Gebhardt et al. 2004). However, it is highly unlikely that the observed resistance is solely due to the presence of R1 gene, since its effectiveness in field conditions is low (Vleeshouwers et al. 2011). In cv. Prosna, the female parent of Bzura, Sieczka (1979) has found an unidentified, highly effective major resistance gene against late blight. This gene could be present also in Bzura, since after inoculation with some (incompatible) isolates leaflets of Bzura show typical hypersensitive reaction (HR). Analysis of virulence profiles of set of P. infestans isolates, which are avirulent to Bzura, indicate possible role of R2 gene (R. Lebecka and A. Michalska - personal communication). These observations suggest presence of a major resistance gene in Bzura, which probably belongs to R2-gene-family and provides resistance to similar spectrum of P. infestans races like the R2 gene.
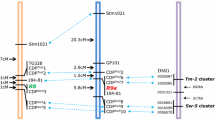
Pedigree of cultivar Bzura - grey color marks S. demissum sources (According to Swieżyński et al. 1997)
Although host resistance is considered as effective and environmentally friendly way of restricting late blight infection, its practical use has had very limited success. There is still a scarcity of highly and durably resistant potato cultivars (Forbes 2012). Among a few Polish potato cultivars with a high level of resistance to late blight, Bzura stands out in terms of stability of this resistance in various environments (Haynes et al. 1998; Tatarowska et al. 2012). Despite of that, the genetic factors underlying the resistance against late blight in Bzura have been never characterized in detail. The objective of presented study was to clarify the bases of Bzura resistance by testing resistance of this cultivar in various conditions and by identification of genetic factors involved in Bzura race-specific resistance observed earlier in detached leaflet. The chromosomal localization and nucleotide sequence of identified gene were also determined.
Materials and Methods
Plant Materials
In field and laboratory experiments, the resistance of Bzura was assessed along with resistance standards: cvs Sárpo Mira (highly resistant), Escort (mid-resistant), Robijn (mid-resistant), and Bintje (susceptible). Set of eleven Black’s differentials was also used to determine the virulence of P. infestans. The field trials were conducted with standard fertilization (120 kg N ha−1, 80 kg P2O5 ha−1,120 kg K2O ha−1) and chemical plant protection excluding fungicide applications. For field experiments, Randomized Complete Block design was applied with 2 blocks and 6 hill plots. To study race-specific resistance, a tetraploid F1 progeny was developed by crossing of Bzura with susceptible male parent cv. Felka Bona. The unselected (Bzura × Felka Bona) F1 consisted of 140 individuals.
Resistance Tests
Field Resistance Tests
Field resistance tests of cvs Bzura, Sárpo Mira, Escort, Robijn, and Bintje were performed during ten consecutive years from 2004 to 2013 in Boguchwała (South-eastern part of Poland) under high, natural infection pressure. In this location the weather conditions are favorable for late blight infection and artificial inoculation was not applied. Each year six tubers per cultivar in two replications were planted. Spreader plants of a semi-susceptible cultivar Alicja or Gawin were used to separate plots and to ensure a reliable source of inoculum during the epidemic period. Disease severity was evaluated weekly from first days of July till the mid-August. The values of rAUDPC (relative Area Under Disease Progress Curve) were calculated from 6 or 7 readings, according to Fry (1978).
Laboratory Assessment
In laboratory conditions, resistance was assessed in detached leaflet tests according to Zarzycka (2001) in three consecutive years 2007, 2008, and 2009. In all 3 years plants of (Bzura × Felka Bona) F1 were tested along with their parents (Bzura and Felka Bona) and standards. Five leaflets per genotype were tested in two replications twice each year. Leaflets were inoculated with a 30 μl droplet of sporangia/zoospore suspension (50 sporangia/μl). Resistance of leaflets was scored after 6 days of incubation, on a 1–9 scale, where 9 was the most resistant.
Isolates of P. infestans
In detached leaflet tests, three isolates MP 725, MP 824, and MP 847 (race: 1, 3, 4, 7, 10, 11) from IHAR-PIB Młochów potato pathogen collection were used in years 2007, 2008, and 2009, respectively. These isolates were collected in various parts of Poland but all were avirulent to Bzura and exhibited the same virulence profiles, therefore in this study were considered as one group of isolates AvrBz (avirulent to Bzura). In all 3 years, a virulent to cv. Bzura isolate MP 324 (race 1, 2, 3, 4, 6, 7, 10, 11) was also used as a control. Isolate MP 324 collected in 1997 in Poland is highly aggressive, metalaxyl resistant and is of A1 mating type. This isolate has frequently been used in late blight resistance tests (Lebecka et al. 2006; Śliwka et al. 2006, 2007, 2013).
Statistical Analysis
To assess effects of genotype, year, and their interactions on the resistance scores, analysis of variance (ANOVA) was applied. Multiple comparisons of means were done by Tukey’ test. The Pearson’s correlation coefficients ware calculated for results of detached leaflet tests between the individual years. The fit of segregation to the expected ratio was checked by the χ 2 test. Statistical analyses were performed with the use of MS Excel 2010 and STATISTICA’98 Edition.
DNA Extraction, Molecular Analysis and Gene Sequencing
Genomic DNA was extracted from 1 g of fresh, young leaves with the GenElute Plant Genomic DNA Miniprep kit (Sigma Aldrich) according to the supplier’s protocol. To verify hypotheses that the major resistance gene present in cv. Bzura is located on potato chromosome IV, the group of locus-specific DNA markers was used. Tested markers were previously used by Park et al. (2005) for mapping the gene Rpi-blb3 (ortholog of R2) on potato chromosome IV. Primers sequences and annealing temperatures are listed in Table 1. A standard PCR conditions were applied: 5 min at 94 °C followed by 35 cycles of 94 °C for 30 s, appropriate Tm for 45 s, 72 °C for 45 s, then followed by 72 °C for 7 min. PCR analysis was performed in 20 μl reaction mix containing 1.5 ng/μl template DNA, 0.2 mM of each dNTP, 0.7 mM of primers and 1U Allegro Taq DNA polymerase in the reaction buffer provided by the manufacturer (Novazym, Poland). PCR products of B10L and 20D11R markers were digested respectively with HinfI and TaqI restriction enzymes and separated on agarose gels stained with ethidium bromide.
To confirm the presence of R2 gene in cultivar Bzura, the PCR marker R2 was applied (Kim et al. 2012). Primers of this marker (Table 1.) are based on sequence of initial and terminal fragments of genes from R2-family, therefore product of its amplification is expected to be a full-length R2 gene. For this marker modified PCR conditions were applied: 5 min at 94 °C followed by 35 cycles of 94 °C for 30 s, 60 °C for 150 s, 72 °C for 90 s, then followed by 72 °C for 7 min. PCR was performed in a total volume of 20 μl containing 0.2 mM dNTPs; 0.6 mM of primers and 1U Marathon Polymerase (A&A Biotechnology, Poland) in the provided reaction buffer. The obtained product of amplification (about 2,5 kb) was extracted from agarose gel (with GenElute Gel Extraction Kit, Sigma-Aldrich) and sequenced using four additional primers (Table 1). Sequencing of each fragment was performed at least two times in Genomed, Poland. The obtained sequences were analyzed using Chromas Lite 2.1.1 (Technelysium Pty Ltd) and SeaView 4.5.1 (Gouy et al. 2010). The search for sequence homology with other potato R genes was carried out using the BLAST - Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Results
Resistance Tests
Field Resistance
The local populations of P. infestans in Boguchwała were virulent, and Black’s differentials R1, R2, R3, R4, R6, R7, R10 and R11 were severely infected in each year of experiments. On differentials R5, R8 and R9, weak symptoms of infection were observed only occasionally. In each year, late blight symptoms were observed on plants of all tested cultivars with exception of Sárpo Mira. ANOVA for rAUDPC values revealed significant effects of cultivar, year and their interaction. The ‘cultivar’ source of variation accounted for more than 80 % of the total sum of squares, while ‘year’ and interaction ‘cultivar × year’ explained only 4.61 and 11.13 % of total variation, respectively.
During 10 years of field experiment we confirmed high and stable level of cv. Bzura resistance to late blight. The mean value of Bzura rAUDPC (0.084) did not differ significantly from mean rAUDPC of highly resistant standard Sárpo Mira (0.027), and it was significantly lower than values of susceptible standard Bintje or mid-resistant Robijn and Escort (Fig. 2, Table 2).
Detached Leaflet Tests – Parental and Standard Cultivars
Susceptible parent Felka Bona proved to be highly susceptible after inoculation with isolates MP 324 and AvrBz. Bzura was susceptible after inoculation with MP 324, but highly resistant when AvrBz isolates were used. The standard cultivar Sárpo Mira was highly resistant, Robijn was rather susceptible and Bintje was highly susceptible, irrespectively to the isolate used. Standard cv. Escort, similarly to Bzura, was moderately susceptible to MP 324 but highly resistant to AvrBz isolates (Table 3).
Detached Leaflet Tests – (Bzura × Felka Bona) F1
Among clones of (Bzura × Felka Bona) F1 tested with isolate MP 324 resistant individuals were not found (mean score 2.9; range 1.8–4.6). However, the same clones inoculated with AvrBz isolates showed two clearly distinguishable types of reaction: no symptoms (or only small necrosis) or large lesions with visible sporulation, which cover large part of leaflet. Intermediate reaction was not observed (Fig. 3). The ratio of highly resistant clones to susceptible ones was 76:64, not significantly deviated from the ratio 1:1 (χ 2 = 1.03, P = 0.311) expected for segregation of a single gene. Results of 3 years tests were highly correlated (r = 0.857 for 2007–2008, r = 0.794 for 2007–2009 and r = 0.776 for 2008–2009; P < 0.001) and each year the same clones were classified in resistant or susceptible group.
According to ANOVA (data not shown), the genotype had the strongest effect on resistance and accounted for 93.7 % of variance in leaflets tests. Effect of year and interaction were also significant, but explained only 1.9 and 2.5 % of variation, respectively.
Molecular Analysis
Resistance segregation in (Bzura × Felka Bona) F1 progeny suggests the presence of single dominant gene. To verify hypothesis, that this gene is located on potato chromosome IV, Bzura and Felka Bona, and all progeny clones were tested for the presence of DNA markers B10L and 20D11R. In our study these markers were polymorphic and associated with resistance. In (Bzura × Felka Bona) F1 progeny, the presence of markers was highly correlated with reaction observed in detached leaflet tests after inoculation with AvrBz isolates (Fig. 4). The percentage of recombination between markers and hypothetical gene R2 from Bzura was 4.3 and 5.0 % for B10L and 20D11R, respectively. Their presence explained respectively 82.9 and 79.6 % of variability observed in resistance.
Bzura and Felka Bona, and all progeny clones were screened also for the presence of gene-specific marker R2. Product of amplification, sized ~2,5 kb, was present in Bzura and all resistant progeny clones, while absent in Felka Bona and all susceptible clones. This product was extracted from gel and sequenced. In result, 2544 bp long sequence was obtained. After comparison with other sequences in the databases using the BLAST algorithm, the sequence of Bzura gene has showed 97 % identity to R2 gene (GeneBank: FJ536325.1), and has proved to be identical with the sequence of R2-like gene (GeneBank: FJ536323.1).
Discussion
Results of presented study showed effectiveness of late blight resistance of cv. Bzura in field conditions. In each of the 10 years of field experiment, Bzura was more resistant than mid-resistant standards Escort and Robijn, and only slightly less resistant than cv. Sárpo Mira. In local populations of P. infestans, races virulent to Bzura were present, since symptoms of infection on this cultivar were observed in each year of experiment. However, progress of late blight symptoms on this cultivar was restricted, and Bzura was always classified as highly resistant (rAUDPC < 0.200). The high level of Bzura field resistance is mainly based on low disease progress rate, probably related to its late maturity, and delay in disease onset (Plich, unpublished data). According to Andrivon et al. (2006) reduction of late blight apparent infection rate is typical for partial resistance, while delay in onset of the disease symptoms is usually attributed to the presence of R gene(s). It may suggest that effective resistance of Bzura results from combined effects of both horizontal and R-gene-based resistance. Until now only the presence of R1 gene has been reported in Bzura (Gebhardt et al. 2004), but efficacy of this gene in protection against late blight is very limited. In this study, the presence of another major resistance gene was confirmed. This gene, present in Bzura and 76 progeny clones, provides resistance for P. infestans infection after inoculation with AvrBz isolates. This gene is located at major late blight resistance locus on potato chromosome IV and belongs to R2-gene-family. Its presence was confirmed with the use of markers B10L, 20D11R, and/or R2. The nucleotide sequence of this gene shares 97 % identity with R2 gene and is identical with R2-like gene. Gene R2-like originally was found in potato clone AM3778-16 (dihaploid from AM78-3778) which, among other wild Solanum species, has S. edinense in its pedigree (Park et al.2005). The same S. edinense, or rather S. demissum source, clone EF II 13 (EF = Edinense Fraglich = dms-hybrid) can be found also in Bzura pedigree (Swieżyński et al. 1997). However, the presence of any R genes in this clones was not confirmed yet.
Several R2 alleles and orthologs from different Solanum species have been already identified (Champouret 2010). They all recognize PiAVR2 effector and confer resistance to similar spectrum of P. infestans races (Lokossou et al. 2009). Strains of P. infestans that are virulent on R2 potatoes either do not express effector Avr2 or express its distinct variant, that evades perception (Gilroy et al. 2011). Gene R2 (or its homologs) has been widely exploited in agriculture and virulent races of P. infestans are present in populations of this pathogen. However, potatoes with R2 gene have been reported to be still resistant to local P. infestans populations in China, Russia, France, and The Netherlands (Li 2012; Pilet et al. 2005; Vleeshouwers et al. 2011; Wang et al. 2012). Also in Poland, the R2 is still fairly effective, since the corresponding virulence factor is only moderately frequent in local populations of P. infestans (Chmielarz et al. 2014). Members of R2-family genes are considered to be components of durable resistance, especially if combined with other elements that complement their efficacy (Gilroy et al. 2011; Jo 2013). In our experiment gene R2-like present in Bzura provide complete resistance for inoculation with AvrBz isolates in detached leaflet tests, but was insufficient to fully protect foliage of this cultivar in field conditions. Slight late blight symptoms were observed on Bzura in each year of the field experiment in the middle or the end of epidemic periods. However, on foliage of other potatoes containing R2 gene (Black’s differential R2 and cv. Escort) the late blight symptoms were observed earlier (data not shown) and were more severe than on Bzura. According to Lokossou and coworkers (2009), the R2-like and R2 genes provide resistance to the same spectrum of P. infestans isolates. Therefore, the observed differences between levels of resistance in Bzura and other R2-containing potatoes could be explained only by the influence of unique genetic background of this cultivar, which is responsible for high level of partial resistance against late blight. Probably such combination of R2-like gene and specific genetic background is also responsible for durability of Bzura field resistance observed in this and earlier studies (Haynes et al. 1998; Tatarowska et al. 2012).
In the case of potato, the achievement of durable resistance against late blight is very difficult, since P. infestans is highly adaptable pathogen (Fry 2008; Haas et al. 2009). Therefore various strategies have been developed to improve durability of potato resistance to P. infestans. Some breeders have focused on the development of cultivars with horizontal resistance (Thurston 1971; Van der Plank 1971), while others expect that stacking of R genes will bring durable resistance (Kim et al. 2012; Zhu 2014). Past experience showed that employment of single or even combined R genes did not bring durable resistance, while level of protection provided by horizontal resistance is most frequently insufficient (Colon 1994). Another strategy to achieve durable resistance is to bring together both types of resistance in one cultivar (Van der Plank 1966). Recently Palloix et al. (2009) and Brun et al. (2010) postulated usefulness of this strategy to facilitate disease control for other pathosystems. Expected benefits of such joint action of two resistance types arise from enhanced disease control provided by R gene(s), slower selection for virulent isolates and satisfactory level of protection (when the R gene is finally overcome) provided by a high level of quantitative resistance. As a consequence, quantitative resistance can increase the durability of R-gene mediated resistance and may enhance disease control and crop protection (Brun et al. 2010). Combining of selected R genes with genetic background, which provides rate reducing resistance, leads to improvement of durability and effectiveness of resistance also in case of potato late blight. The highly effective and durable genes R8 and R9 from S. demissum are background-dependent, and when transferred into susceptible cultivars could fail in providing high resistance (Kim et al. 2012). Also effectiveness of another highly promising resistance gene Rpi-phu1 could be affected be influence of genetic background, since differences in transcript level in diploid and tetraploid breeding lines were found (Śliwka et al. 2013).
Recently, the number of potentially durable R genes, like RB/Rpi-blb1, Rpi-blb3, Rpi-phu1, Rpi-vnt1.1, Rpi-rzc1, Rpi-sto1, and Rpi-Smira2, were found (Lokossou et al. 2009; Jo 2013; Pel et al. 2009; Song et al. 2003; Śliwka et al. 2006; Śliwka et al. 2012; Vleeshouwers et al. 2008). Potato resistance breeding will greatly benefit from the using of these promising R genes. However, to enhance durability of these new resistance sources they should be introduced into potato breeding pool carefully. Potato cultivars/clones, in which the durability of resistance has been already proved, are excellent breeding component for broad spectrum R genes. Example of cultivar Bzura confirms that proper combination of R gene(s) and genetic background could provide the benefits of improving the effectiveness of resistance and its durability.
Products of PCR amplification of CAPS markers: B10L (a) and 20D11R (b) after digestion with appropriate restriction enzymes for Bzura, Felka Bona, and individuals from (Bzura × Felka Bona) F1. M – DNA ladder; PR – resistant parent – cv. Bzura; PS – susceptible parent – cv. Felka Bona; R – resistant clones from (Bzura × Felka Bona) F1; S – susceptible clones from (Bzura × Felka Bona) F1. Rectangle marks susceptible clone from this progeny in which amplification product of 20D11R was found (recombinant)
References
Ahmadvand, R. 2013. Analysis of resistance genes in potato with special attention to expressional approaches. PhD thesis. University of Pannonia, Georgikon Faculty. Keszthely, Hungary.
Andrivon, D., R. Pelle, and D. Ellisseche. 2006. Assessing resistance types and levels to epidemic diseases from the analysis of disease progress curves: Principles and application to potato late blight. American Journal of Potato Research 83: 455–461.
Bendahmane, A., M. Querci, K. Kanyuka, and D.C. Baulcombe. 2000. Agrobacterium transient expression system as a tool for the isolation of disease resistance genes: Application to the Rx2 locus in potato. The Plant Journal 21(1): 73–81.
Bisognin, D.A., D.S. Douches, K. Jastrzębski, and W.W. Kirk. 2002. Half-sib progeny evaluation and selection of potatoes resistant to the US8 genotype of Phytophthora infestans from crosses between resistant and susceptible parents. Euphytica 125: 129–138.
Brun, H., A.-M. Chevre, B.D.L. Fitt, S. Powers, A.-L. Besnard, M. Ermel, V. Huteau, B. Marquer, M. Renard, and D. Andrivon. 2010. Quantitative resistance increases the durability of qualitative resistance to Leptospheria maculans in Brassica napus. New Phytologist 185: 285–299. doi:10.1111/j.1469-8137.2009.03049.x.
Champouret, N. 2010. Functional Genomics of Phytophthora infestans Effectors and Solanum Resistance Genes. PhD thesis. Wageningen University.
Chmielarz, M., S. Sobkowiak, K. Dębski, D.E.L. Cooke, M.B. Brurberg, and J. Śliwka. 2014. Diversity of Phytophthora infestans from Poland. Plant Pathology 63(1): 203–211.
Chrzanowska, M. 2000. Krańcowa odporność na wirusy Y i X ziemniaka oraz polowa odporność na wirus S ziemniaka w polskich odmianach ziemniaka. Biuletyn IHAR 214: 231–238.
Colon, L.T. 1994. Resistance to Phytophthora infestans in Solanum tuberosum and wild Solanum species. PhD Thesis, Wageningen University.
Dorrance, A.E., and D.A. Inglis. 1997. Assessment of greenhouse and laboratory screening methods for evaluating potato foliage for resistance to late blight. Plant Disease 81: 1206–1213.
Douches, D.S., W.W. Kirk, M.A. Bertram, J.J. Coombs, and B.A. Niemira. 2002. Foliar and tuber assessment of late blight (Phytophthora infestans (Mont.) de Bary) reaction in cultivated potato (Solanum tuberosum L.). Potato Research 45(2–4): 215–224.
Douches, D.S., J. Coombs, K. Flecher, and W.W. Kirk. 2004. Foliar reaction to Phytophthora infestans in inoculated potato field trials in Michigan. American Journal of Potato Research 81: 443–448.
Flis, B., J. Hennig, D. Strzelczyk-Żyta, C. Gebhardt, and W. Marczewski. 2005. The Ry-f sto gene from Solanum stoloniferum for extreme resistance to Potato virus Y maps to potato chromosome XII and is diagnosed by PCR marker GP122718 in PVY resistant potato cultivars. Molecular Breeding 15: 95–101.
Forbes, G.A. 2012. Using host resistance to manage potato late blight with particular reference to developing countries. Potato Research 55: 205–216.
Fry, W.E. 1978. Quantification of general resistance of potato cultivars and fungicide effects for integrated control of potato late blight. Phytopathology 68: 1650–1655.
Fry, W.E. 2008. Phytophthora infestans: The plant (and R gene) destroyer. Molecular Plant Pathology 9(3): 385–402.
Gebhardt, C., A. Ballvora, B. Walkemeier, P. Oberhageman, and K. Schuler. 2004. Assessing genetic potential in germplasm collections of crop plants by market-trait association: a cause for potatoes with quantitative variation of resistance to late blight and maturity type. Molecular Breeding 13: 93–102.
GILB (Global Initiative on Late Blight) Annual Report. 2001. Annex 1B. Standard International Field Trials. pp. 15–16.
Gilroy, E.M., S. Breen, S.C. Whisson, J. Squires, I. Hein, M. Kaczmarek, D. Turnbull, P.C. Boevink, A. Lokossou, L.M. Cano, J. Morales, A.O. Avrova, L. Pritchard, E. Randall, A. Lees, F. Govers, P. van West, S. Kamoun, V.G.A.A. Vleeshouwers, D.E.L. Cooke, and P.R.J. Birch. 2011. Presence/absence, differential expression and sequence polymorphisms between PiAVR2 and PiAVR2-like in Phytophthora infestans determine virulence on R2 plants. New Phytologist 191: 763–776.
Gouy, M., S. Guindon, and O. Gascuel. 2010. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution 27(2): 221–224.
Haas, B.J., S. Kamoun, M.C. Zody, R.H. Jiang, R.E. Handsaker, L.M. Cano, M. Grabherr, C.D. Kodira, S. Raffaele, T. Torto-Alalibo, T.O. Bozkurt, A.M. Ah-Fong, L. Alvarado, V.L. Anderson, M.R. Armstrong, A. Avrova, L. Baxter, J. Beynon, P.C. Boevink, S.R. Bollmann, J.I. Bos, V. Bulone, G. Cai, C. Cakir, J.C. Carrington, M. Chawner, L. Conti, S. Costanzo, R. Ewan, N. Fahlgren, M.A. Fischbach, J. Fugelstad, E.M. Gilroy, S. Gnerre, P.J. Green, L.J. Grenville-Briggs, J. Griffith, N.J. Grunwald, K. Horn, N.R. Horner, C.H. Hu, E. Huitema, D.H. Jeong, A.M. Jones, J.D. Jones, R.W. Jones, E.K. Karlsson, S.G. Kunjeti, K. Lamour, Z. Liu, L. Ma, D. Maclean, M.C. Chibucos, H. McDonald, J. McWalters, H.J. Meijer, W. Morgan, P.F. Morris, C.A. Munro, K. O’Neill, M. Ospina-Giraldo, A. Pinzon, L. Pritchard, B. Ramsahoye, Q. Ren, S. Restrepo, S. Roy, A. Sadanandom, A. Savidor, S. Schornack, D.C. Schwartz, U.D. Schumann, B. Schwessinger, L. Seyer, T. Sharpe, C. Silvar, J. Song, D.J. Studholme, S. Sykes, M. Thines, P.J. van de Vondervoort, V. Phuntumart, S. Wawra, R. Weide, J. Win, C. Young, S. Zhou, W. Fry, B.C. Meyers, P. van West, J. Ristaino, F. Govers, P.R. Birch, S.C. Whisson, H.S. Judelson, and C. Nusbaum. 2009. Genome sequence and comparative analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461: 393–398.
Haynes, K.G., D.H. Lambert, B.J. Christ, D.P. Weingartner, D.S. Douches, J.E. Backlund, G. Secor, W. Fry, and W. Stevenson. 1998. Phenotypic stability of resistance to late blight in potato clones evaluated at eight sites in the United States. American Journal of Potato Research 75: 211–217.
Jo, K.R. 2013. Unveiling and deploying durability of late blight resistance in potato – from natural stacking to cisgenic stacking. PhD thesis. Wageningen University.
Kim, H.J., H.R. Lee, K.R. Jo, S.M. Mahdi Mortazavian, D.J. Huigen, B. Evenhius, G. Kessel, R.G.F. Visser, E. Jacobsen, and J.H. Vossen. 2012. Broad spectrum late blight resistance in potato differential set plants MaR8 and MaR9 is conferred by multiple stacked R genes. Theoretical and Applied Genetics 124: 923–935.
Lebecka, R., S. Sobkowiak, and E. Zimnoch-Guzowska. 2006. Resistance of potato tubers to a highly aggressive isolate of Phytophthora infestans in relation to tuber age. Potato Research 49: 99–107.
Li, Y. 2012. Multiplex SSR analysis of Phytophthora infestans in different countries and the importance for potato breeding. PhD thesis. Wageningen University.
Lokossou, A.A., T.H. Park, G. van Arkel, M. Arens, C. Ruyter-Spira, J. Morales, S.C. Whisson, P.R.J. Birch, R.G. Visser, and E. Jacobsen. 2009. Exploiting knowledge of R/Avr genes to rapidly clone a new LZ-NBSLRR family of late blight resistance genes from potato linkage group IV. Molecular Plant-Microbe Interaction 22: 630–641.
Palloix, A., V. Ayme, and B. Moury. 2009. Durability of plant major resistance genes to pathogens depends on the genetic background, experimental evidence and consequences for breeding strategies. New Phytologist 183: 190–199.
Park, T.H., V.G.A.A. Vleeshouwers, D.J. Huigen, E.A.G. van der Vossen, H.J. van Eck, and R.G.F. Visser. 2005. Characterization and high-resolution mapping of a late blight resistance locus similar to R2 in potato. Theoretical and Applied Genetics 111(3): 591–597.
Pel, M.A., Foster, S.J., Park, T.H., Rietman, H., van Arkel, G., Jones J.D.G., Van Eck H.J., Jacobsen E., Visser R.G.F., Van der Vossen E.A.G. 2009. Mapping and Cloning Resistance Genes from Solanum ventorii Using a Intraspecific Candidate Gene Approach. Molecular Plant-Microbe Interactions Volume 22, Number 5: 601–615.
Pilet, F., R. Pelle, D. Elisseche, and D. Andrivon. 2005. Efficacy of the R2 resistance gene as a component for the durable management of potato late blight in France. Plant Pathology 54: 723–732.
Polkowska-Kowalczyk, L., B. Wielgat, and U. Maciejewska. 2011. Involvement of phospholipase A2 in the response of Solanum species to an elicitor from Phytophthora infestans. Acta Physiologiae Plantarum 33: 2521–2531.
Przetakiewicz, J. 2010. Resistance of Polish cultivars of potato to virulent pathotypes of Synchytrium endobioticum (Schilb.) Per.: 2(Ch1) and 3(M1). Biuletyn IHAR 257/258: 207–214.
Querci, M., D. Baulcombe, R.W. Goldbach, and L.F. Salazar. 1995. Analysis of the resistance-breaking determinants of Potato Virus X strain HB on different potato genotypes expressing extreme resistance to PVX. Molecular Plant Pathology 85(9): 1003–1010.
Sieczka, M.T. 1979. Próba optymalizacji warunków selekcji ziemniaków pod kątem widzenia odporności polowej na Phytophthora infestans (Mont.) de Bary. PhD thesis (in Polish). Instytut Ziemniaka. Bonin.
Śliwka, J., H. Jakuczun, R. Lebecka, W. Marczewski, C. Gebhardt, and E. Zimnoch-Guzowska. 2006. The novel, major locus Rpi-phu1 for late blight resistance maps to potato chromosome IX and is not correlated with long vegetation period. Theoretical and Applied Genetics 113: 685–695.
Śliwka, J., H. Jakuczun, R. Lebecka, W. Marczewski, C. Gebhardt, and E. Zimnoch-Guzowska. 2007. Tagging QTLs for late blight resistance and plant maturity from diploid wild relatives in a cultivated potato (Solanum tuberosum) background. Theoretical and Applied Genetics 115: 101–112.
Śliwka, J., H. Jakuczun, M. Chmielarz, A. Hara-Skrzypiec, I. Tomczyńska, A. Kilian, and E. Zimnoch-Guzowska. 2012. Late blight resistance gene from Solanum ruiz-ceballosii is located on potato chromosome X and is linked to violet flower color. BCM Genetics 13: 11.
Śliwka, J., M. Świątek, I. Tomczyńska, E. Stefańczyk, M. Chmielarz, and E. Zimnoch-Guzowska. 2013. Influence of genetic background and plant age on expression of the potato late blight resistance gene Rpi-phu1 during incompatible interaction with Phytophthora infestans. Plant Pathology 62: 1072–1080.
Song, J., J.M. Bradeen, S.K. Naess, J.A. Raasch, S.M. Wielgus, J.T. Haberlach, J. Liu, H. Kuang, S. Austin-Phillips, C.R. Buell, J.P. Helgeson, and J. Jiang. 2003. Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proceedings of the National Academy of Science of the United States of America 100: 9128–9133.
Świeżyński, K.M., Osiecka, M., Sieczka, M.T., Sujkowski, L.S., Zarzycka, H., Zimnoch-Guzowska, E. 1991. Perspektywy postępu hodowli ziemniaków odpornych na grzyb Phytophthora infestans. Instytut Ziemniaka. Bonin. 198–202.
Swieżyński, K.M., K.G. Haynes, R.C.B. Hutten, M.T. Sieczka, P. Watts, and E. Zimnoch-Guzowska. 1997. Pedigree of European and North-American Potato Varieties. Plant Breeding and Seed Science 41(Supplement 1): 3–149.
Tatarowska, B., B. Flis, and J. Plich. 2011. Application of Scheffé - Caliński mixed model in analysis of the stability of resistance to Phytophthora infestans (Mont.) de Bary in potato cultivars. Biuletyn IHAR 262: 141–154.
Tatarowska, B., B. Flis, and E. Zimnoch-Guzowska. 2012. Biological stability of resistance to Phytophthora infestans (Mont.) de Bary in 22 Polish potato cultivars evaluation in field experiments. American Journal of Potato Research 89: 73–81.
Thurston, H.D. 1971. Relationship of general resistance: Late blight of potato. Phytopathology 61: 620–626.
van der Plank, J.E. 1966. Horizontal (polygenic) and vertical (olygogenic) resistance against blight. American Potato Journal 43: 43–52.
van der Plank, J.E. 1971. Stability of resistance to Phytophthora infestans in cultivars without R genes. Potato Research 14(4): 263–270.
Vleeshouwers, V.G.A.A., H. Rietman, P. Krenek, N. Champouret, C. Young, S.-K. Oh, M. Wang, K. Bouwmeester, B. Vosman, R.G.F. Visser, E. Jacobsen, F. Govers, S. Kamoun, and E.A.G. van der Vossen. 2008. Effector genomics accelerates discovery and functional profiling of potato disease resistance and Phytophthora infestans avirulence genes. PLoS ONE 3: e2875.
Vleeshouwers, V.G.A.A., S. Raffaele, J. Vossen, N. Champouret, R. Oliva, M.E. Segretin, H. Reitman, L.M. Cano, A. Lokossou, G. Kessel, M.A. Pel, and S. Kamoun. 2011. Understanding and exploiting late blight resistance in the age of effectors. Annual Review of Phytopathology 49: 25.1–25.25.
Wang, D.D., M. Guo, F.X. Min, Y.F. Gao, F.F. Xu, S. Yang, and D.Q. Lu. 2012. Virulence complexity and high levels of fungicide resistance suggest population change of Phytophthora infestans in the Heilongjiang province of China. Potato Research 55: 217–224.
Zarzycka, H. 2001. Ocena odporności na zarazę ziemniaka w teście listkowym. Sporządzanie inokulum. Instytut Hodowli i Aklimatyzacji Roślin, Radzików, IHAR Monografie i Rozprawy Naukowe 10/2001: 77–80.
Zhu, S. 2014. R gene stacking by trans- and cisgenesis to achieve durable late blight resistance in potato. PhD thesis. Wageningen University.
Zimnoch-Guzowska, E., Z. Yin, M. Chrzanowska, and B. Flis. 2013. Sources and effectiveness of potato PVY resistance in IHAR’s breeding research. American Journal of Potato Research 90: 21–27.
Acknowledgments
The authors wish to thank Sylwester Sobkowiak for preparing P. infestans cultures and suspensions for detached leaflet tests and Emil Stefańczyk for help in preparing samples for sequencing and sequence data analyses. This study was partly financed be Polish Ministry of Science and Higher Education / National Science Center, grant N N310 162738. Sequencing of the R2-like gene homolog from potato cv. Bzura was funded from Norway Grants in the Polish-Norwegian Research Programme operated by the National Centre for Research and Development, grant POTPAT, number: Pol-Nor/202448/28/2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plich, J., Tatarowska, B., Lebecka, R. et al. R2-like Gene Contributes to Resistance to Phytophthora infestans in Polish Potato Cultivar Bzura. Am. J. Potato Res. 92, 350–358 (2015). https://doi.org/10.1007/s12230-015-9437-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-015-9437-9