Abstract
Hyaluronic acid finds expanding application in the pharmaceutical and cosmetic industries, resulting in an increasing need for the high-quality substance. The main production processes to obtain hyaluronic acid in commercial quantities are extraction from animal tissues and bacterial fermentation using opportunistic Streptococcus strains. The production by recombinant bacteria that are safe for humans seems to be an efficient and economically viable way to obtain hyaluronic acid. The recombinant producer strains constructed on the basis of the Bacillus subtilis platform make it possible to obtain the yield and quality of the product comparable to those of commercially developed Streptococcus strains. By varying genetic, biochemical, and biotechnological factors, it becomes possible to obtain products with different target molecular weights. Despite the results achieved, the potential of the B. subtilis platform for the construction of recombinant hyaluronic acid producer strains has not been exhausted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
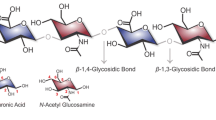
Hyaluronic acid (HA) is a high molecular weight linear non-sulfated glycosaminoglycan, consisting of repeating disaccharide units connected by β-1,4-glycosidic bonds. The disaccharide unit consists of D‑glucuronic acid and N-acetyl-D-glucosamine fragments connected by a β-1,3-glycosidic bond [1, 2]. The presence of numerous sulfated groups in relative glycosaminoglycans is the reason for the existence of numerous isomers, which is not observed in hyaluronic acid, which is always chemically identical, regardless of the methods and sources of production. In aqueous solution, HA is stabilized into a secondary structure in the form of a single-strand left-handed helix. Helix duplexes form a tertiary structure in the form of an extensive network, the properties of which depend on the molecular weight (MW) and HA concentration [3]. Structural characteristics and polyelectrolyte nature determine the unique rheological properties of HA solutions [4, 5].
In the human body, HA is one of the main components of extracellular matrix. Considerable amounts of HA were found in the dermis and epidermis of the skin, synovial fluid, hyaline cartilage, and vitreous humor of the eye [6]. HA functions both as a structural and signaling molecule. Molecular weight is a key factor determining the biological functions of HA [7]. High molecular weight HA (≥106 Da) serves as lubricating factor in the synovial fluid, maintains water and electrolyte balance and the extracellular matrix structure [8], has an anti-angiogenic effect, and participates in the processes of inflammation and tissue injury and repair through interaction with fibrinogen and control of the immune cell activation, regulation of cytokines, and stem cell migration [9, 10]. In a number of pathological conditions, such as asthma, pulmonary fibrosis, and rheumatoid arthritis, low molecular weight HA (104–106 Da) is formed, which demonstrates proinflammatory and proangiogenic activity. Low molecular weight HA stimulates the production of proinflammatory cytokines [7] and also provokes the extracellular matrix rearrangement [11]. The HA fragments and oligosaccharides (≤104 Da), depending on the tissue type and physiological state, demonstrate both proinflammatory [12] and anti-inflammatory effects [13].
Hyaluronic acid of different MW can be used in the construction of delivery vehicles for therapeutic agents, in the treatment of cancer and diseases of the eyes, joints, lungs, upper respiratory tract, and urinary system, and in aesthetic medicine [2]. The HA-based medicines and products, including synovial fluid prostheses, agents for the treatment of injuries and skin burns, viscoelastic substances for cataract surgery and eye drops, agents for the treatment of rhinitis, and dermal fillers are widely used in everyday practice [14]. The pronounced aesthetic and supportive effect warrants widespread use of HA in cosmetics and dietary supplements.
The review analyzes the data obtained during the development of recombinant HA producer strains based on the Bacillus subtilis platform.
METHODS FOR COMMERCIAL PRODUCTION OF HA
In commercial quantities, HA is obtained in two ways: by extraction from animal tissues and by fermentation using natural microbial producer strains. The method for obtaining HA from animal tissues is a proven technology that makes it possible to obtain a product with high MW and reasonable costs. Raw materials for large-scale production are rooster combs and bovine vitreous humor [15]. The disadvantage of this method is the low yield of the product with high variability in MW, which is associated with low concentration of the polymer in the tissue, uncontrolled degradation by endogenous hyaluronidases, and harsh extraction conditions. The product obtained by this method may contain infectious agents, i.e., viruses or prions, as well as trace amounts of proteins and nucleic acids that can cause allergic reactions [16, 17].
Fermentation of natural producer strains is the main commercial process for obtaining HA [18]. The main producers are selective strains of Streptococcus equi subsp. zooepidemicus and S. equi subsp. equi, which under optimal conditions can produce 6–7 g/L HA with the MW of 2.0–3.5 MDa [19]. The main disadvantage of this method is the use of strains constructed on the basis of streptococci pathogenic for farm animals and conditionally pathogenic for humans. The target product must go through many stages of purification to avoid contamination with endo- and exotoxins, which negatively affects the economic characteristics.
Identification of HA biosynthesis genes made it possible to carry out work on the construction of recombinant producers on various platforms devoid of disadvantages of using streptococci with the achievement of the same productivity and MW. HA producer strains have been constructed both on the basis of commercial platforms (Escherichia coli) and on the basis of platforms with GRAS status (Corynebacterium glutamicum, Lactobacillus acidophilus, Lactococcus lactis, B. subtilis) [18, 20]. A promising method is chemoenzymatic biosynthesis of HA, which makes it possible to obtain high purity monodisperse fractions [21]. However, despite the results achieved, there are currently no products on the market based on the HA substance obtained by fermentation of recombinant strains other than streptococci or by the chemoenzymatic method. Therefore, production of high-quality HA with high yield and low cost is an urgent problem in the field of molecular genetics and biotechnology.
HYALURONIC ACID BIOSYNTHETIC PATHWAY
Natural producers of HA are strains of gram-positive bacteria Streptococcus pyogenes, S. uberis, S. equi subsp. zooepidemicus, S. equi subsp. equi, S. iniae, S. equisimilis, and Bacillus cereus strain G9241 and gram-negative bacteria Pasteurella multocida [19, 22–25]. All natural producers of HA are pathogenic and opportunistic microorganisms that cause diseases in animals and humans. HA forms the basis of the cell capsule and acts as the virulence factor, making it possible for microorganisms to avoid recognition and counteraction of the immune system, and also promotes colonization of mucous surfaces [26]. Despite obvious benefits for enhancing virulence, only a few bacterial species have acquired the ability to synthesize capsular HA.
Genes involved in the HA biosynthesis are part of an operon in which the key gene is that for the hyaluronan synthase (EC 2.4.1.212), an enzyme that synthesizes HA from activated forms of the UDP-glucuronate and UDP-N-acetylglucosamine monomers. There are two classes of bacterial hyaluronan synthases that differ in molecular structure and amino acid sequence [27]. The most common is Class 1, which is responsible for HA biosynthesis in streptococci and vertebrates, and is a membrane enzyme [28]. Class 2 is represented only by the hyaluronan synthase encoded by the operon of P. multocida and which is a membrane-associated enzyme [25]. The streptococcal operon of HA biosynthesis also includes two to four genes participating in the biosynthesis of activated monomer precursors, and in the case of P. multocida, it also includes genes responsible for the translocation of growing HA chain to the cell exterior.
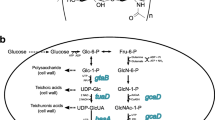
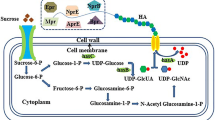
The pathway of HA biosynthesis was studied in detail in streptococci [29, 30]. UDP-glucuronate and UDP-N-acetylglucosamine are derivatives of glucose-6-phosphate and fructose-6-phosphate, respectively. The HA biosynthetic pathway is shown in Fig. 1. The first reaction in the biosynthesis of UDP-glucuronate is the reversible conversion of glucose-6-phosphate to glucose-1-phosphate by α-phosphoglucomutase (EC 5.4.2.2). Next, glucose-1-phosphate uridylyl transferase (EC 2.7.7.9) catalyzes the formation of UDP-glucose from UTP and glucose-1-phosphate. UDP-glucuronic acid is then formed in the reaction of oxidation of the primary alcohol group of UDP-glucose by UDP-glucose dehydrogenase (EC 1.1.1.22).
Hyaluronic acid biosynthesis pathway and associated biochemical pathways. PPP, pentose phosphate pathway; TA, teichoic acids; TUA, teichuronic acids; GL, glycolysis; PG, peptidoglycan. Homologous genes are demonstrated: underlined superscripts, from the Streptococcus genome; subscripts, from the B. subtilis genome.
The first reaction of the UDP-N-acetylglucosamine biosynthesis is the amino group transfer from glutamine to fructose-6-phosphate by amidotransferase (EC 2.6.1.16) to form glucosamine-6-phosphate. The phosphate groups are then rearranged by mutase (EC 5.4.2.10) to form glucosamine-1-phosphate. Next, the acetyl group transfer by acetyltransferase (EC 2.3.1.157) occurs with the formation of N-acetylglucosamine-1-phosphate, and pyrophosphorylase (EC 2.7.7.23) in the reaction with UTP synthesizes UDP-N-acetylglucosamine.
UDP-N-acetylglucosamine, UDP-glucose, and glucose-1-phosphate are involved in the biosynthesis of peptidoglycan and other cell wall components, which provokes obvious competition for the HA biosynthetic pathway. To meet the metabolic needs of the cell in nucleotide sugars, the streptococcal genome contains additional genes encoding glucose-1-phosphate uridylyltranspherase (hasC2), α-phosphoglucomutase (pgm1, pgm2), and acetyltransferase/pyrophosphorylase (gcaD). At the same time, the UDP-glucose dehydrogenase encoding gene (hasB) is represented by a single copy in the HA biosynthesis operon. Thus, streptococcal metabolism is able to support the synthesis of a large amount of HA in the extracellular capsule.
The pathways of UDP-N-acetylglucosamine and UDP-glucose biosynthesis in streptococci and B. subtilis are biochemically identical. The B. subtilis genome contains homologs of all genes for the biosynthesis of HA precursors. This makes it possible for the B. subtilis genes to be used to construct efficient artificial operons, since it is known that native genes are better expressed than homologous alien ones.
RECOMBINANT B. SUBTILIS STRAINS PRODUCING HYALURONIC ACID
The recombinant strains of B. subtilis producing hyaluronic acid obtained to date are represented in Tables 1 and 2.
The first study on heterologous production of HA in B. subtilis confirmed the high potential of the platform [31, 32]. The ability of recombinant strains to secrete HA into the cell exterior and accumulate the product in the culture medium was demonstrated. The characteristics of the obtained HA corresponded to those synthesized by natural microbial producers developed on the basis of Streptococcus strains. Heterologous expression of only seHas hyaluronan synthase, in contrast to E. coli strains, had no negative effect on the growth rate of B. subtilis and did not lead to HA biosynthesis without additional expression of UDP-glucuronate biosynthesis genes [33]. The combination of two precursor biosynthesis genes in the operon made it possible to identify the biosynthesis-limiting stage, which was the synthesis of UDP-glucuronate. The operon composed of seHasA and its own UDP-glucose dehydrogenase tuaD gene is sufficient for efficient HA production. Supplementation of the operon with the genes involved in the biosynthesis of UDP-N-acetylglucosamine (gcaD) and UDP-glucuronate (gtaB) increases the yield of HA by 10–20%. Placement of the seHasA and tuaD-gtaB genes into different operons of the B. subtilis chromosome does not lead to noticeable changes in the yield or MW of the product compared to their location in a single seHasA–tuaD-gtaB operon. The artificial operon composed of its own precursor synthesis genes in B. subtilis showed higher yield efficiency than that developed on the basis of HA biosynthesis genes from the operon of natural producer S. equisimilis. An unexpected effect was the deletion of the cat chloramphenicol resistance gene and that of the cytochrome C450 family oxidase, involved in the synthesis of red pigment (cypX), which was expressed as the increase in the yield of high molecular weight HA.
The HA biosynthesis by both streptococcal strains and recombinant B. subtilis producers is an energy-consuming process. The energy metabolism of a bacterial cell can be intensified by increasing oxygen availability with the help of bacterial hemoglobin [34]. In the recombinant B. subtilis strain producing HA, heterologous VHb expression had a positive effect on culture properties of the strain and the product yield [35]. The strain with vhb demonstrated a specific prolonged lag period and increased growth rate and reached 25% higher final cell density (7.5 versus 6.2 OD). The HA yield doubled from 0.9 to 1.8 g/L. In addition, experimental results supporting the effect of expression of the characteristic precursor biosynthesis genes on the product yield were obtained. Strains with operons consisting of the hyaluronan synthase gene in combination with the UDP-glucose dehydrogenase genes of different origin showed differences in productivity. The UDP-glucose dehydrogenase activity was 3 times higher in the strain with its own tuaD gene than in the strain with heterologous hasB. The difference in activity led to increase in the HA yield by 36%.
The use of an inducible promoter for the expression of the HA biosynthetic operon made it possible to obtain HA with different MW [36]. The fermentation conditions and cultivation time were critical for the MW and HA yield. For example, fermentation for 80–160 h resulted in a product with MW of 0.1–0.5 MDa; fermentation for 40–80 h resulted in a product of 0.5–1 MDa, and fermentation for 12–40 h resulted in a product of 1.0–2.0 MDa. However, effective and cell-safe operon expression required the transformant screening on the IPTG gradient and experimental determination of the optimal inductor concentration.
Two-step controlled expression with different inductors also made it possible to vary the MW of HA [37]. Expression of the integrated PmHAS hyaluronan synthase is controlled by the inducible Pxyl promoter, while the plasmid operon is controlled by the inducible Pspac promoter. In the case of simultaneous induction of the cassette and operon at the second hour from the beginning of the TPG223 strain cultivation, the HA production reaches 6.8 g/L at MW of 3.38–4.55 MDa. Induction of the PmHAS cassette at the eighth hour and of the tuaD-gtaB operon at the second hour demonstrates a decrease in the HA production to 3.1 g/L with a considerable decrease in MW, to 0.006–0.008 MDa.
In a systematic study, the effect of overexpression of genes involved in the biosynthesis of UDP-glucuronic acid and UDP-N-acetylglucosamine precursors on the HA yield and MW was examined [38]. The best performance with the yield of 2.7 g/L and MW of 1.61 MDa was demonstrated by the strain E168A/pP43-DU-PBMS, the operons of which contained the genes participating in the complete biosynthetic pathway of UDP-glucuronic acid (tuaD-gluM) and UDP-N-acetylglucosamine (gtaB-glmM-glmS). Positive correlation between the expression of the UDP-N-acetylglucosamine biosynthesis genes and increase in the product MW (which was not detected for the UDP-glucuronic acid biosynthesis genes) was revealed.
For the first time, the possibility of increasing the productivity of producer strains by reducing the consumption of UDP-N-acetylglucosamine precursor frutose-6-phosphate in the glycolysis pathway was demonstrated. Decrease in the expression of 6-phosphofructokinase gene pfkA, which is the first step of fructose-6-phosphate conversion in the glycolysis pathway, was reached through the replacement of the ATG start codon by TTG and GTG variants. The E168T/pP43-DU-PBMS strain, which differs from E168A/pP43-DU-PBMS only in the substitution of the ATG codon by the pfkA TTG codon, demonstrated increased HA yield (from 2.7 to 3.2 g/L) compared to the latter at a similar growth rate and MW of the product.
The possibility of obtaining low molecular weight HA using heterologous hyaluronidase expression in a HA producer strain was also demonstrated. Low molecular weight fractions and oligosaccharides of HA with a given weight were previously obtained by in vitro enzymatic hydrolysis by recombinant leech hyaluronidase LHyal [39]. The degree of HA depolymerization depends on the hyaluronidase concentration; therefore, to obtain fractions with a given weight, the level of LHyal hyaluronidase expressed from the constitutive Plepa promoter was varied using a library of ribosome binding sites [40]. Cassettes with LHyal were integrated into the genome of the E168T/pP43-DU-PBMS strain, which contained a modification of the pfkA start codon and complete precursor biosynthetic pathways. This resulted in the rise of the HA yield along with the corresponding decrease in MW, constituting 4.35, 2.9, and 3.3 g/L at 0.0022, 0.0026, and 0.003 MDa for different variants, respectively. The fermentation scaling supported the stepwise nature of the change in the molecular weights of the obtained fractions and the rise in the HA yield. Higher cell density of strains with hyaluronidase was observed, which was associated with better conditions for oxygen availability.
Expression of leech hyaluronidase from the temperature-sensitive plasmid pKSV7 in the WmB producer strain led to the production of HA with different molecular weight at different temperature [41, 42]. Cultivation at a permissive temperature of 32°C led to a decrease in the HA MW from 0.392 to 0.00861 MDa.
Increased HA yield was also observed in case of the promoter replacement in the genomic copies of precursor biosynthesis genes, which resulted in their increased expression and made it possible to dispense with the need to expand the recombinant operon [43, 44]. The replacement of the gtaB and gcaD promoters with the strong tandem Prpsf-gsib promoter made it possible to achieve productivity of 2.35 and 3.21 g/L without expanding the minimal seHasA-tuaD operon upon cultivation for 24 and 48 h, respectively. The use of the Prpsf-gsib tandem promoter makes it possible to provide a more efficient target gene expression profile and, accordingly, more efficient production of the target product. The PrpsF promoter is one of the strongest promoters in the B. subtilis genome and provides the highest level of transcription at the logarithmic growth phase. Transcription initiation of the gsiB gene occurs with the participation of the alternative σB sigma subunit and reaches its maximum at the stationary growth phase. Increased expression of precursor biosynthesis genes has a positive effect on the yield of the product, which supports and complements the conclusions of previous studies.
Further development of the approach with partial diversion of metabolic fluxes from the pentose phosphate pathway and glycolysis to the HA biosynthesis demonstrated a considerable potential of this approach for increasing the HA yield [45]. Glucose-6-phosphate is consumed in the pentose-phosphate pathway through conversion by glucose-6-phosphate-1-dehydrogenase, encoded by the zwf gene, to 6-phosphogluconolactone. Fructose-6-phosphate is consumed in the glycolysis pathway through conversion by 6-phosphofructokinase, encoded by the pfkA gene, to fructose-1,6-bisphosphate. Inactivation of the zwf gene leads to a considerable diversion of the metabolic flux to the glycolysis pathway, and inactivation of the pfkA gene is lethal for B. subtilis [46]. Regulated zwf and pfkA gene silencing was achieved using the CRISPR interference method, which makes it possible to vary the transcription efficiency over a wide range [47]. Silencing of the pfkA gene increased the HA yield to 50% compared to the original strain with slight decrease in the HA MW and cell density. Silencing of the zwf gene increased the HA yield by 44 and 74% only in the case of two variants of the guide RNA, AW014-3 and AW016-3, respectively, while the remaining guide RNAs reduced the yield and MW of the product.
Simultaneous silencing of the pfkA and zwf genes had a considerable effect on the B. subtilis strain productivity. Compared to the basic AW009 strain, the strains with simultaneous pfkA and zwf gene silencing, AW018-3 and AW019-3, demonstrated an increase in the HA yield by 98 and 108%, respectively, along with slight increase in the MW. The growth rate of strains AW018-3 and AW019-3 did not differ from that of AW009, but was higher than that of strains with either pfkA or zwf silencing. In addition, the pfkA and zwf silencing in strains AW018-3 and AW019-3 led to the decrease in acetoin accumulation in the culture medium compared to strain AW009 by 167 and 118%, respectively, which reflected the decrease in metabolic flux through the glycolysis pathway. The pfkA and zwf silencing in strain AW009 was much more effective than heterologous expression of the precursor biosynthesis genes pgcA and glmS.
HA precursors are also consumed during bacterial cell wall synthesis. UDP-N-acetylglucosamine is involved in the synthesis of teichoic acids and peptidoglycan components with the help of enzymes encoded by the tagO and murAA genes. The silencing of the tagO and murAA genes by RNA interference led to a considerable decrease in the strain growth rates, genetic instability, and the loss of the characteristic mucoid phenotype.
Creating conditions for effective functioning of membrane hyaluronan synthases using membrane engineering makes it possible to raise the productivity of the HA producer strains [48]. The type 1 hyaluronan synthases require the presence of cardiolipin phospholipid in the membrane, with which the enzyme forms functional complex in vivo and in vitro [49]. Cardiolipin is a minor part of the B. subtilis cell membrane lipids. Cardiolipin biosynthesis occurs with the help of cardiolipin synthase (ClsA) from phosphatidylglycerol, which, in turn, is synthesized with the help of phosphatidylglycerol synthase (PgsA) [50]. The B. subtilis strain AW001-4 with constitutive expression of the pgsA and clsA genes demonstrated an increase in the HA yield by 32%, increase in MW to 2.06 MDa, and increase in the final cell density by 83% compared to the control strain AW008. Fluorescence microscopy showed an increase in the cardiolipin content at the cell pole and septal regions. Silencing of genes for the biosynthesis of other cell membrane lipids, phosphatidylethanolamine (pssA) and neutral glycolipids (ugtP), had no effect on the productivity and growth rate of the control strain AW008.
Localization of cardiolipin synthase ClsA occurs in septal region and depends on the tubulin homolog FtsZ [51]. Suppression of FtsZ expression can potentially lead to the distribution of ClsA and increase the cardiolipin concentration over the entire membrane [52]. The ftsZ silencing in strain AW001-4 with strong constitutive expression of pgsA and clsA led to genetic instability. Only strain AW004-4 retained the mucoid phenotype after recovery from glycerol stock and synthesized 25% more HA than AW001-4 with a slight decrease in MW. The ftsZ silencing in the control strain AW008 by the same guide RNA variant as in AW004-4 resulted in an increase in the HA yield by 204%, increase in final cell density, and decrease in MW to 1.67 MDa. Strain AW007-4 with weakened constitutive expression of pgsA and clsA was used for engineering of genetically stable strains with four guide RNA variants. Strains AW009-4 and AW011-4 demonstrated an increase in yield, MW, and final cell density by 10–15% compared to strain AW007-4.
Silencing of sporulation factor genes affects the HA yield and MW. Strain WmA with a deletion of the sigma factor gene sigF, which is involved in the activation of the early sporulation gene network, demonstrates 30% higher HA yield compared to strain WA without the deletion [41]. Producer strains constructed on the basis of strain WB600 demonstrated a higher HA yield compared to producer strains constructed on the basis of B. subtilis 168. Specifically, under optimal conditions, strain WmB with the sigF deletion constructed on the basis of strain WB600 accumulated 3.21 g/L of the product in the culture medium, while strain 1B constructed on the basis of strain 168 accumulated only 1.7 g/L. An interesting effect of cultivation temperature on the HA MW was found. Cultivation at increased temperature of 47°С led to a sharp increase in the HA MW to 6.973 MDa. Cultivation at 32°C, at which the largest amount of the product was observed, led to the synthesis of HA with MW up to 0.392 MDa.
Inactivation of the ability to sporulate in strains obtained by undirected mutagenesis has a positive effect on the HA production. For example, strain 3NA containing a mutation in the spo0A sporulation initiation gene is able to reach the cell density of 75 g/L under fed-batch fermentation conditions, which makes it an excellent candidate for industrial use [53]. Optimization of the cultivation process and nutrient medium made it possible for strain KCNHA10 containing the recombinant operon with the szHasA, tuaD, gtaB, and gcaD genes to reach the cell density of 29.4 g/L in 12 h and synthesize 7 g of HA with the MW of 1 MDa per liter of culture. Technical and economic analysis of this production process demonstrated higher economic efficiency compared to the process based on streptococcal strains.
FACTORS AFFECTING PRODUCER STRAIN AND PRODUCT CHARACTERISTICS AND THE WAYS OF FURTHER DEVELOPMENT OF PRODUCER STRAINS
The data obtained from the construction of bacterial HA producer strains made it possible to draw conclusions on the influence of different genetic factors on the yield and technical characteristics of the product.
Basic Strain and Its Modifications
Recombinant producer strains were constructed using different strains of B. subtilis, the most common of which was strain 168 and its derivatives (Table 3). The important advantages of strain 168 are the ability to grow on simple media, the studied biochemistry and genetics, ease of genetic manipulations, and high transformation efficiency. Strains WB600 and WB800N were constructed on the basis of strain 168 as a platform for efficient heterologous protein expression [54]. To increase the secreted protein stability, in strain WB800N, deletions in the genes of eight extracellular proteases, nprE, aprE, epr, bpr, mpr, nprB, vpr, and wprA, were introduced, and in strain WB600, the genes of six proteases, nprE, aprE, epr, bpr, mpr, and nprB, were deleted. Such modifications make it possible to increase the secretion and stability of recombinant proteins, which is also relevant for membrane proteins like HasA. The only direct comparison of the efficiency of strains described in the literature showed the advantage of the deficiency in extracellular protease activity of strain WB600, carrying the sigF deletion, over strain 168 in the HA production [41]. Strain 3NA is a mutant version of strain 168 with a frameshift mutation in the spo0A gene and is characterized by the absence of sporulation, low protease expression, and the ability to achieve high cell density under fed-batch fermentation conditions [53]. Strain 168 and strains with mutations of sporulation sigma factors on a synthetic medium reach the cell density of 5–15 g/L, which is considerably lower than that of 3NA [55]. In comparison with strain 168, the genome of strain 3NA contains 425 genetic variations; therefore, it is impossible to unambiguously conclude that the spo0A mutation is the reason for acquiring improved technological properties.
At the same time, B. subtilis strain 168 is a model laboratory strain, and in the process of construction, it underwent numerous stages of undirected mutagenesis, which led to the accumulation of numerous mutations in its genome [56]. The genetic instability of strain 168 with the Pgrac-seHas-tuaD operon integrated into the genome has been reported [48]. For some unknown reason, being restored from cryopreservation, this strain formed segregated populations with the majority of cells of the wild nonmucoid phenotype. The same genetic instability was demonstrated by the producer strain constructed on the basis of strain BGSC 1A786 (amyE::cat, lacA::spec, leuC8, metA4, hsd(RI)R+M–). Only the BGSC 1A751-based producers showed genetic stability of the characteristic mucoid phenotype. Strain 1A751, which is a derivative of strain DB104, contains mutations in the nprE and aprE protease genes, as well as deletions of the endo-β-1,3-1,4-glucanase bglS and endo-β-1,4-glucanase bglC genes [57].
Strains other than B. subtilis 168 may have properties that would suggest possible beneficial effect on the HA production. For example, commercial strain A164∆5 used for recombinant protein production is characterized by improved growth characteristics and the high yield of secreted proteins [32]. The A164∆5 strain is an improved modification of strain ATCC 6051a, which also is superior to B. subtilis 168 in the production of recombinant proteins [58]. Modifications that improved the technological properties of strain ATCC 6051a include the deletion of the following genes: surfactin biosynthesis srfC (reduced foaming), sigma factor F SpoIIAC (sporulation blockage), nprE and aprE proteases, and amyE alpha-amylase. The disadvantage of strain A164Δ5 was low transformation efficiency, which led to the need to use the intermediate strain B. subtilis 16844 for genetic engineering manipulations [32].
Hyaluronan Synthase
The choice of the hyaluronan synthase gene affects the HA production and MW. When creating strain-producers, the hyaluronan synthase gene was chosen on the basis of either the data from a few studies on the enzyme catalytic properties [31, 45, 48] or the productivity characteristics of natural producers [38, 53]. For instance, the recombinant seHAS synthase from S. equisimilis demonstrates a twofold higher elongation rate than the recombinant spHAS synthase from S. pyogenes [23, 59].
Direct comparison of the effectiveness of hyaluronan synthases from Streptococcus was carried out in vitro and in vivo using L. lactis as a platform [22]. Under the same conditions, hyaluronan synthases differ in the MW of synthesized HA. The maximum MW of HA synthesized by recombinant L. lactis strains in vivo correlates with the data obtained in vitro and constitutes about 2.2 MDa for suHAS from S. uberis and spaHAS from S. parauberis, about 1.4 MDa for szHAS from S. zooepidemicus, and about 0.4 MDa for spHAS. The HA MW value is in good agreement with the phylogenetic grouping of hyaluronan synthases according to the amino acid sequences. For instance, suHAS and spaHAS form one phylogenetic group, distinct from szHAS and spHAS. However, the authors failed to obtain results with the hyaluronan synthase genes from S. equi subsp. equi and S. iniae using the Lactococcus platform in vivo, while the strains of S. equi subsp. equi are known to be efficient HA producers.
Interesting data were obtained with type 2 hyaluronan synthase from P. multocida [37]. Unlike the synthases from streptococci, PmHAS hyaluronan synthase is not a membrane protein and it contains a C‑terminal anchor domain that holds the enzyme at the inner face of plasma membrane. The P. multocida HA biosynthesis operon includes additional genes, hexA, hexB, hexC, and hexD, the products of which are homologous to membrane transport proteins [60]. In P. multocida, the functions of HA synthesis and transmembrane transport of the HA chain to the cell exterior are divided between PmHAS synthase and hexA, hexB, hexC, and hexD proteins [61, 62]. Type 1 hyaluronan synthases, which include streptococcal synthases, combine the functions of the HA synthesis and translocation of the HA chain to the cell exterior [63]. It was suggested that the ABC transporter complex is involved in the HA translocation to the cell exterior of S. pyogenes [64]. However, this hypothesis is disproved both by the data of in vitro studies [65, 66] and by successful heterologous production of HA by type 1 hyaluronan synthases using different microbial platforms [18]. PmHAS hyaluronan synthase was used to construct the HA producer strains using E. coli [67], Agrobacterium sp. [68], and Synechococcus sp. [69]. PmHAS expression in E. coli and Agrobacterium sp. led to an increase in the broth viscosity, which indicated extracellular accumulation of HA. The distribution of the synthesized HA between the extracellular, surface-absorbed, and intracellular fractions was elucidated by studying the producer strains constructed on the basis of the Synechococcus cyanobacterium. It was demonstrated that from 42 to 88% of total HA accumulated in the cell exterior of these strains. Intracellular accumulation of HA was one of the reasons for the decrease in the producer strain growth rates. The mechanism of the HA export to the cell exterior in recombinant strains expressing PmHAS remains obscure. PmHAS hyaluronan synthase has high biotechnological potential and is capable of synthesizing high molecular weight HA. Deletion of the anchor domain makes it possible to obtain PmHAS in soluble form, which makes it an apparent candidate for the cell-free HA biosynthesis, which makes it possible to obtain a monodisperse high molecular weight product [21]. In addition, the PmHAS enzyme is superior to streptococcal hyaluronan synthases in terms of kinetic characteristics. In particular, the KM values are two times lower than the streptococcal ones and constitute 75 and 20 μM for UDP-N-acetylglucosamine and UDP-glucuronic acid, respectively [70]. To elucidate the role of transport systems in HA translocation to the cell exterior both in P. multocida strains and in PmHAS-based recombinant strains on various platforms, further studies are required.
The possibility of obtaining PmHAS in soluble form facilitates the task of improving the enzyme characteristics by evolutionary and rational engineering methods. For example, the combination of four amino acid substitutions found using the KnowVolution evolutionary method made it possible to obtain a PmHAS variant capable of synthesizing HA with molecular weight up to 4.7 MDa [71]. Rational engineering of improved variants of type 1 hyaluronan synthases is complicated by unavailability of the 3-D structure of the protein, although this gap is partially filled by numerical modeling methods [72]. To date, only one study on the improvement of szHAS hyaluronan synthase characteristics using evolutionary method is known [73]. Using in vivo selection, in B. subtilis strains, it was possible to identify the szHAS variant, which demonstrated the increase in the HA yield from 1.22 to 2.24 g/L with the MW increase from 1.20 to 1.36 MDa.
Precursor Biosynthesis Pathway
The genome of B. subtilis contains all genes for the biosynthesis of UDP-glucuronate and UDP-N-acetylglucosamine; however, heterologous expression of only hyaluronan synthase does not lead to the synthesis of HA. Efficient biosynthesis of HA by recombinant producers can be carried out only from a minimal operon, which includes, in addition to hyaluronan synthase gene, the UDP-glucose dehydrogenase gene, which catalyzes the last step of UDP-glucuronate biosynthesis. The addition of a minimal operon with the genes for UDP-N-acetylglucosamine biosynthesis leads to a considerably lower effect of increasing the yield of HA compared to the addition of the UDP-glucose dehydrogenase encoding gene [31]. The need for heterologous expression of UDP-glucose dehydrogenase is associated with the absence of the tuaD gene (a homolog of the ugd gene from E. coli and hasB from Streptococcus) expression, which is part of the teichuronic acid biosynthesis operon tuaABCEDFGH. Teichuronic acid is an anionic polymer the synthesis of which is activated under conditions of phosphate starvation to replace phosphorus-rich teichoic acid in the cell wall [74]. The fundamental importance of UDP-glucose dehydrogenase is evidenced by the obligatory presence of the hasB gene in the operons of HA biosynthesis in representatives of the genus Streptococcus, where the minimum operon consists of the hasA and hasB genes (as in S. uberis). It was hypothesized that the HA biosynthesis operon appeared in the ancestral strain as the hasA/hasB pair, to which the remaining precursor biosynthesis genes (hasC, hasD, hasE) were added in the course of evolution [75]. The hasB gene deletion in S. zooepidemicus leads to complete inactivation of HA biosynthesis and has a small effect on the strain growth properties [30]. A number of studies demonstrated the HA accumulation by B. subtilis producer strains with recombinant operons that do not contain the tuaD gene or its homolog [37, 38]. Taking into account the role of UDP-glucose dehydrogenase and the nature of its expression, it seems necessary to conduct further studies on this issue.
Extension of the recombinant operon with precursor biosynthesis genes leads to an increase in the HA yield in recombinant strains developed on the basis of the B. subtilis, C. glutamicum, and L. lactis platforms [18]. The combination of different genes leads to an increase in the HA yield with different efficiency. The inclusion of the gtaB, glmM, and gcaD genes into the artificial operon containing szHas and tuaD genes leads to an increase in the HA yield by 30% [38].
The extended HA biosynthesis operon has a considerable influence on the HA production from natural producers. For instance, the S. equi and S. zooepidemicus strains containing the hasABCDE operon in the genome produce considerably more HA than S. uberis and S. pyogenes containing hasABC and hasAВ, respectively [75]. It seems likely that the inclusion of additional genes in the operons of the S. equi subspecies occurred under the selective pressure for HA production, which led to the ability to direct up to 10% of incoming sugars to HA biosynthesis [76].
An important aspect affecting the strain productivity and MW of the product is the expression level of hyaluronan synthase and the precursor biosynthesis genes. High expression level of tuaD, which is toxic to E. coli and complicates the construction of genetically engineered constructs [38], may also have a negative influence on the physiology of B. subtilis [36]. In particular, a decrease in the efficiency of the Pgrac promoter of the seHas-tuaD operon led to an increase in the HA yield by 100%, decrease in MW by 17%, and increase in the final cell density by 47% [45].
The expression level of hyaluronan synthase and precursor biosynthesis genes, which depends on the composition of recombinant operon and the promoter strength, determines the balance of UDP-glucuronate and UDP-N-acetylglucosamine in the cell and, finally, the MW of the product. Increased hasA expression relative to hasB leads to a decrease in the MW of HA synthesized by the recombinant L. lactis strain [77]. Overexpression of genes in the UDP-glucuronate biosynthetic pathway in S. equi reduces the MW of HA, while overexpression of genes in the UDP-N-acetylglucosamine biosynthetic pathway leads to an increase in MW from 1.8 to 3.4 MDa. A correlation between the MW of HA and the UDP-N-acetylglucosoamine concentration was revealed. The biosynthesis of high molecular weight HA is the result of the balance of precursor concentrations, which can be brought to the optimal value by genetic methods [78]. For recombinant producer strains constructed on the L. lactis platform, optimal conditions for the synthesis of high molecular weight HA were equimolar intracellular concentrations of UDP-N-acetylglucosoamine and UDP-glucuronate and increased expression of hasB relative to hasA [79]. The addition of N-acetylglucosamine to the culture medium has a similar effect of increasing the MW of HA, both in the case of natural producers and in the case of recombinant producer strains constructed on the basis of B. subtilis [45].
Energy and Basic Metabolism
The HA biosynthesis is an energy-consuming process. To obtain 1 mol of UDP-N-acetylglucosamine and 1 mol of UDP-glucuronate, the cell spends 2 mol of glucose, 3 mol of ATP, 2 mol of UTP, and 1 mol of acetyl-CoA. Promising approaches to increase the HA yield are the inactivation of pathways for the competitive utilization of precursors and increase in the level of ATP synthesis.
Homolactic fermentation, which is based on glycolysis, is the main source of energy for lactic acid bacteria such as S. zooepidermicus and L. lactis. The result of this process is the formation of two pyruvate molecules, two NAD·Н2 molecules, and two ATP molecules per glucose molecule. The regeneration of NAD+ is carried out by the transfer of two electrons from NAD·H2 to the pyruvate molecule, which leads to the lactate formation. However, under aerobic conditions, lactic acid bacteria, the cells of which are devoid of electron transport chain, regenerate NAD+ using the NAD·Н2 oxidase (NOX) enzyme, which leads to the diversion of the metabolic flux toward the formation of acetate and the formation of an additional ATP molecule per glucose molecule in reactions catalyzed by acetate kinase (AK) [80]. Synthesis of additional ATP in the acetate kinase reaction and increase in the NAD+ regeneration rate due to NOX correlate with increase in the level of HA synthesis by producer strains constructed on the basis of lactic acid bacteria. Under aerobic conditions, S. zoopidemicus not only grew faster and accumulated biomass but also demonstrated a higher MW and level of HA biosynthesis compared to anaerobic conditions [81, 82]. However, direct overexpression of NOX in the S. zooepidemicus strain, despite the rise in the level of ATP synthesis by 33% and the biomass yield by 15%, did not lead to a rise in the HA yield or a change in MW. Probably, in this case, the main limiting factor was the activity of the pyruvate dehydrogenase complex, which converts pyruvate to acetyl-CoA [83]. At the same time, recombinant HA producers constructed on the basis of the L. lactis strain with the ldh deletion demonstrated a threefold increase in the HA production and MW [84].
Unlike streptococci, which regenerate NAD+ with the help of NOX in the presence of oxygen and synthesize additional ATP with the help of the AK enzyme, B. subtilis uses the electron transport chain and the tricarboxylic acid cycle [85]. In the case of aerobic cultivation of recombinant HA producer strains constructed on the basis of B. subtilis, acetate and acetoin were byproducts of fermentation [45]. However, a decrease in the level of acetate synthesis by inactivation of the pyruvate dehydrogenase complex creates a deficiency of acetyl-CoA for the tricarboxylic acid cycle, which leads to a decrease in ATP production [86]. Inactivation of the 2-acetolactate decarboxylase alsD gene, which is involved in the synthesis of acetoin, negatively affects the growth of B. subtilis strains, although elucidation of the mechanism of this phenomenon requires further investigation [86].
Increasing oxygen availability for the cells is an effective strategy for activating HA biosynthesis, despite the fact that the mechanism of the effect differs for lactic acid bacteria and bacilli. The oxygen concentration in the culture medium is a limiting factor of the HA biosynthesis by S. zooepidemicus and B. subtilis strains owing to the low solubility of gaseous oxygen and the high viscosity of the HA solutions [87, 88]. The technological solution to this issue is to increase the oxygen capacity of the medium by optimizing the agitation rate and using oxygen vectors, i.e., hydrophobic liquids in which oxygen has higher solubility than in water. This approach increases the HA yield by S. zooepidemicus strains [89]. A substantial increase in the HA yield and increase in the cell density of the recombinant B. subtilis strain culture was observed with the use of n-heptane, n-hexadiene, perfluoromethyldecalin, and perfluoro-1,3-dimethylcyclohexane. Optimization of the fermentation conditions, i.e., the concentration of the oxygen vector, the time of its addition, and the agitation rate, made it possible to achieve the HA concentration of 4.5 g/L in just 10 h of cultivation [88].
In the construction of recombinant producer strains, bacterial hemoglobin VHb from the Gram-negative bacterium Vitreoscilla is actively used [34]. Hemoglobin VHb enhances the oxygen flux to terminal oxidases under hypoxic conditions; therefore, VHb overexpression leads to an increase in the cell density and increase in the oxidative metabolism and the yield of the target product, especially under conditions of limited oxygen availability. Expression of VHb in B. subtilis led to an increase in protein secretion and increase in the yield of alpha-amylase and neutral protease [90]. Heterologous VHb expression by the HA producer S. zooepidemicus strain ATCC 39920 increased the HA yield from 1.61 to 2.16 g/L upon the decrease in MW from 1.8 to 1.6 MDa [91]. At the same time, there was a decrease in the lactic acid production by 35% against the background of reduced ldh lactate dehydrogenase activity by 41% and a rise in the activity of acetate kinase and NOX by 9 and 106%, respectively. More impressive results were obtained using the producer strain constructed on the basis of B. subtilis; however, the effect of VHb coexpression on the MW of HA was not examined in this study [35].
UDP-glucuronate and UDP-N-acetylglucosamine are used by the cell in the biosynthesis of cell wall components, and their precursors glucose-6-phosphate and fructose-6-phosphate are consumed in the pentose phosphate pathway and glycolysis. Inactivation of the pathways for the competitive utilization of UDP-glucuronate and UDP-N-acetylglucosamine had a greater effect on the growth characteristics of B. subtilis strains and the yield of HA than overexpression of the genes of the precursor biosynthesis pathway [38, 45]. In addition, strains with decreased pfkA and zwf activity demonstrated a lower level of acetate and acetoin production, which indicated an effective diversion of the metabolic flux from biosynthetic byproducts to HA [45].
Sugar Utilization
The way to improve producer strains is to increase the transport and metabolism of energy sources. The function of sucrose metabolism genes was studied and the stages restricting the HA yield in the S. zooepidemicus strain were identified [92]. The first stages of sucrose utilization are membrane transport with phosphorylation via the phosphoenolpyruvate-dependent phosphotransferase system (encoded by the scrA gene) followed by hydrolysis of sucrose-6-phosphate by sucrose-6-phosphate hydrolase (encoded by the scrB gene) to fructose and glucose-6-phosphate. Overexpression of scrB, in contrast to the reverse effect of scrA overexpression, increases the biomass by 26% and the HA yield by 30%. Shifting the metabolic flux to fructose-6-phosphate through the deletion of the fruA fructose transport gene or the fruK phosphofructokinase gene increased the HA yield by 22 and 27%, respectively, without the effect on cell growth. Overexpression of scrB in strains with either fruA or fruK deletion increased the HA yield by 44 and 55%, respectively.
The main system of sucrose transport and utilization in B. subtilis is identical to that in S. zooepidemicus and consists of an operon encoding SacP phosphotransferase and SacA sucrose-6-phosphate hydrolase [93]. Replacement of the B. subtilis own sucrose transport and metabolism system with an energetically more favorable heterologous system was demonstrated using the polyglutamic acid and 2,3-butanediol producer strains. The combination of the sucrose permease cscB gene from E. coli and sucrose phosphorylase sucP gene from Bifidobacterium adolescentis demonstrated the rise in sucrose consumption by 49.4% and polyglutamic acid production by 38.5% compared with the unchanged strain [94]. The combination of the sucrose permease cscB gene from E. coli and the sucrose phosphorylase gtfA gene from the Streptococcus mutans demonstrated a 36% increase in product yield compared to unmodified strains [95].
CONCLUSIONS
At present, on the basis of the B. subtilis platform, a panel of recombinant HA producer strains was constructed. Important advantages of these strains are GRAS (Generally Recognized as Safe) status, low cost of industrial fermentation, ease of genetic manipulation, and the absence of endo- and exotoxins. Recombinant HA producer strains constructed on the basis of B. subtilis make it possible to obtain a yield and MW of the product comparable to that of commercially developed streptococcal strains. At the same time, the studies carried out revealed platform limitations that can be overcome with the involvement of the experience of constructing HA producer strains on the basis of other microbial platforms. Analysis of the published data suggests that the conditions for the construction of an industrial technology for the HA production on the basis of B. subtilis recombinant producer strains have been formed.
REFERENCES
Weissmann, B. and Meyer, K., The structure of hyalobiuronic acid and of hyaluronic acid from umbilical cord, J. Am. Chem. Soc., 1954, vol. 76, no. 7, pp. 1753—1757. https://doi.org/10.1021/ja01636a010
Fallacara, A., Baldini, E., Manfredini, S., et al., Hyaluronic acid in the third millennium, Polymers, 2018, vol. 10, no. 7. https://doi.org/10.3390/polym10070701
Scott, J.E. and Heatley, F., Hyaluronan forms specific stable tertiary structures in aqueous solution: a 13C NMR study, Proc. Natl. Acad. Sci. U.S.A., 1999, vol. 96, no. 9, pp. 4850—4855. https://doi.org/10.1073/pnas.96.9.4850
Rwei, S.P., Chen, S.W., Mao, C.F., et al., Viscoelasticity and wearability of hyaluronate solutions, Biochem. Eng. J., 2008, vol. 40, no. 2, pp. 211—217.https://doi.org/10.1016/j.bej.2007.12.021
Pisárčik, M., Bakoš, D., and Čeppan, M., Non-Newtonian properties of hyaluronic acid aqueous solution, Colloids Surf., A, 1995, vol. 97, no. 3, pp. 197—202. https://doi.org/10.1016/0927-7757(95)03097-W
Laurent, T.C. and Fraser, J.R.E., Hyaluronan, FASEB J., 1992, vol. 6, no. 7, pp. 2397—2404. https://doi.org/10.1096/fasebj.6.7.1563592
Cyphert, J.M., Trempus, C.S., and Garantziotis, S., Size matters: molecular weight specificity of hyaluronan effects in cell biology, Int. J. Cell Biol., 2015, vol. 2015, p. 563818. https://doi.org/10.1155/2015/563818
Robert, L., Robert, A.M., and Renard, G., Biological effects of hyaluronan in connective tissues, eye, skin, venous wall: role in aging, Pathol.—Biol., 2010, vol. 58, no. 3, pp. 187—198. https://doi.org/10.1016/j.patbio.2009.09.010
Girish, K.S. and Kemparaju, K., The magic glue hyaluronan and its eraser hyaluronidase: a biological overview, Life Sci., 2007, vol. 80, no. 21, pp. 1921—1943. https://doi.org/10.1016/j.lfs.2007.02.037
Jiang, D., Liang, J., and Noble, P.W., Hyaluronan in tissue injury and repair, Annu. Rev. Cell Dev. Biol., 2007, vol. 23, pp. 435—461. https://doi.org/10.1146/annurev.cellbio.23.090506.123337
Heldin, P., Lin, C.Y., Kolliopoulos, C., et al., Regulation of hyaluronan biosynthesis and clinical impact of excessive hyaluronan production, Matrix Biol., 2019, vols. 78—79, pp. 100—117. https://doi.org/10.1016/j.matbio.2018.01.017
Campo, G.M., Avenoso, A., D’Ascola, A., et al., 4-mer hyaluronan oligosaccharides stimulate inflammation response in synovial fibroblasts in part via TAK-1 and in part via p38-MAPK, Curr. Med. Chem., 2013, vol. 20, no. 9, pp. 1162—1172. https://doi.org/10.2174/0929867311320090005
Toole, B.P., Ghatak, S., and Misra, S., Hyaluronan oligosaccharides as a potential anticancer therapeutic, Curr. Pharm. Biotechnol., 2008, vol. 9, no. 4, pp. 249—252. https://doi.org/10.2174/138920108785161569
Dovedytis, M., Liu, Z.J., and Bartlett, S., Hyaluronic acid and its biomedical applications: a review, Eng. Regener., 2020, vol. 1, pp. 102—113. https://doi.org/10.1016/j.engreg.2020.10.001
Boeriu, C.G., Springer, J., Kooy, F.K., et al., Production methods for hyaluronan, Int. J. Carbohydr. Chem., 2013, vol. 2013, pp. 1—14. https://doi.org/10.1155/2013/624967
Shiedlin, A., Bigelow, R., Christopher, W., et al., Evaluation of hyaluronan from different sources: Streptococcus zooepidemicus, rooster comb, bovine vitreous, and human umbilical cord, Biomacromol., 2004, vol. 5, no. 6, pp. 2122—2127. https://doi.org/10.1021/bm0498427
Zagorulko, Y.Y. and Zagorulko, E.Y., Features of hyaluronic acid solutions for intra-articular introduction and recent trends in their development (review), Drug Dev. Regist., 2020, vol. 9, no. 2, pp. 45—54. https://doi.org/10.33380/2305-2066-2020-9-2-45-54
Oliveira, J.D. de Carvalho, L.S., Gomes, A.M.V., et al., Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms, Microb. Cell Fact., 2016, vol. 15, no. 1, p. 119. https://doi.org/10.1186/s12934-016-0517-4
Liu, L., Liu, Y., Li, J., et al., Microbial production of hyaluronic acid: current state, challenges, and perspectives, Microb. Cell Fact., 2011, vol. 10, p. 99. https://doi.org/10.1186/1475-2859-10-99
Gunasekaran, V., Gowdhaman, D., and Ponnusami, P., Role of membrane proteins in bacterial synthesis of hyaluronic acid and their potential in industrial production, Int. J. Biol. Macromol., 2020, vol. 164, pp. 1916—1926. https://doi.org/10.1016/j.ijbiomac.2020.08.077
Gottschalk, J. and Elling, L., Current state on the enzymatic synthesis of glycosaminoglycans, Curr. Opin. Chem. Biol., 2021, vol. 61, pp. 71—80. https://doi.org/10.1016/j.cbpa.2020.09.008
Schulte, S., Doss, S.S., Jeeva, P., et al., Exploiting the diversity of streptococcal hyaluronan synthases for the production of molecular weight-tailored hyaluronan, Appl. Microbiol. Biotechnol., 2019, vol. 103, no. 18, pp. 7567—7581. https://doi.org/10.1007/s00253-019-10023-w
Kumari, K. and Weigel, P.H., Molecular cloning, expression, and characterization of the authentic hyaluronan synthase from group C Streptococcus equisimilis, J. Biol. Chem., 1997, vol. 272, no. 51, pp. 32539—32546. https://doi.org/10.1074/jbc.272.51.32539
Oh, S.Y., Budzik, J.M., Garufi, G., et al., Two capsular polysaccharides enable Bacillus cereus G9241 to cause anthrax-like disease, Mol. Microbiol., 2011, vol. 80, no. 2, pp. 455—470. https://doi.org/10.1111/j.1365-2958.2011.07582.x
DeAngelis, P.L., Jing, W., Drake, R.R., et al., Identification and molecular cloning of a unique hyaluronan synthase from Pasteurella multocida, J. Biol. Chem., 1998, vol. 273, no. 14, pp. 8454—8458. https://doi.org/10.1074/jbc.273.14.8454
Schmidt, K.H., Günther, E., and Courtney, H.S., Expression of both M protein and hyaluronic acid capsule by group A streptococcal strains results in a high virulence for chicken embryos, Med. Microbiol. Immunol., 1996, vol. 184, no. 4, pp. 169—173. https://doi.org/10.1007/BF02456131
DeAngelis, P.L., Hyaluronan synthases: fascinating glycosyltransferases from vertebrates, bacterial pathogens, and algal viruses, Cell. Mol. Life Sci., 1999, vol. 56, pp. 670—682. https://doi.org/10.1007/s000180050461
Weigel, P.H. and DeAngelis, P.L., Hyaluronan synthases: a decade-plus of novel glycosyltransferases, J. Biol. Chem., 2007, vol. 282, no. 51, pp. 36777—36781. https://doi.org/10.1074/jbc.R700036200
Chong, B.F., Blank, L.M., Mclaughlin, R., et al., Microbial hyaluronic acid production, Appl. Microbiol. Biotechnol., 2005, vol. 66, no. 4, pp. 341—351. https://doi.org/10.1007/s00253-004-1774-4
Zhang, Y., Luo, K., Zhao, Q., et al., Genetic and biochemical characterization of genes involved in hyaluronic acid synthesis in Streptococcus zooepidemicus, Appl. Microbiol. Biotechnol., 2016, vol. 100, no. 8, pp. 3611—3620. https://doi.org/10.1007/s00253-016-7286-1
Widner, B., Behr, R., von Dollen, S., et al., Hyaluronic acid production in Bacillus subtilis, Appl. Environ. Microbiol., 2005, vol. 71, no. 7, pp. 3747—3752. https://doi.org/10.1128/AEM.71.7.3747-3752.2005
Sloma, A., Behr, R., Widner, W., et al., United States Patent 8093036, 2012.
DeAngelis, P.L., Papaconstantinou, J., and Weigel, P.H., Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes, J. Biol. Chem., 1993, vol. 268, no. 26, pp. 19181—19184. https://doi.org/10.1016/S0021-9258(19)36494-4
Stark, B.C., Pagilla, K.R., and Dikshit, K.L., Recent applications of Vitreoscilla hemoglobin technology in bioproduct synthesis and bioremediation, Appl. Microbiol. Biotechnol., 2015, vol. 99, no. 4, pp. 1627—1636. https://doi.org/10.1007/s00253-014-6350-y
Chien, L.J. and Lee, C.-K., Enhanced hyaluronic acid production in Bacillus subtilis by coexpressing bacterial hemoglobin, Biotechnol. Prog., 2007, vol. 23, no. 5, pp. 1017—1022. https://doi.org/10.1021/bp070036w
Corsa, V., Negro, A., Vaccaro, S., and Messina, L., United States Patent 9290785, 2012.
Jia, Y., Zhu, J., Chen, X., et al., Metabolic engineering of Bacillus subtilis for the efficient biosynthesis of uniform hyaluronic acid with controlled molecular weights, Bioresour. Technol., 2013, vol. 132, pp. 427—431. https://doi.org/10.1016/j.biortech.2012.12.150
Jin, P., Kang, Z., Yuan, P., et al., Production of specific-molecular-weight hyaluronan by metabolically engineered Bacillus subtilis 168, Metab. Eng., 2016, vol. 35, pp. 21—30. https://doi.org/10.1016/j.ymben.2016.01.008
Jin, P., Kang, Z., Zhang, N., et al., High-yield novel leech hyaluronidase to expedite the preparation of specific hyaluronan oligomers, Sci. Rep., 2014, vol. 4, p. 4471. https://doi.org/10.1038/srep04471
Yuan, P., Lv, M., Jin, P., et al., Enzymatic production of specifically distributed hyaluronan oligosaccharides, Carbohydr. Polym., 2015, vol. 129, pp. 194—200. https://doi.org/10.1016/j.carbpol.2015.04.068
Li, Y., Li, G., Zhao, X., et al., Regulation of hyaluronic acid molecular weight and titer by temperature in engineered Bacillus subtilis, 3 Biotech, 2019, vol. 9, no. 6, p. 225. https://doi.org/10.1007/s13205-019-1749-x
Li, Y., Shi, Z., Shao, Y., et al., Temperature-controlled molecular weight of hyaluronic acid produced by engineered Bacillus subtilis, Biotechnol. Lett., 2021, vol. 43, no. 1, pp. 271—277. https://doi.org/10.1007/s10529-020-03001-0
Mironov, A.S., Errais, L.L., Korol’kova, N.V., et al., RF Patent 2719140, 2020.
Mironov, A.S., Errais, L.L., Baeva, O.V., et al., RF Patent 2723722, 2020.
Westbrook, A.W., Ren, X., Oh, J., et al., Metabolic engineering to enhance heterologous production of hyaluronic acid in Bacillus subtilis, Metab. Eng., 2018, vol. 47, pp. 401—413. https://doi.org/10.1016/j.ymben.2018.04.016
Fischer, E. and Sauer, U., Large-scale in vivo flux analysis shows rigidity and suboptimal performance of Bacillus subtilis metabolism, Nat. Genet., 2005, vol. 37, no. 6, pp. 636—640. https://doi.org/10.1038/ng1555
Westbrook, A.W., Moo-Young, M., and Chou, C.P., Development of a CRISPR-Cas9 tool kit for comprehensive engineering of Bacillus subtilis, Appl. Environ. Microbiol., 2016, vol. 82, no. 16, pp. 4876—4895. https://doi.org/10.1128/AEM.01159-16
Westbrook, A.W., Ren, X., Moo-Young, M., et al., Engineering of cell membrane to enhance heterologous production of hyaluronic acid in Bacillus subtilis, Biotechnol. Bioeng., 2018, vol. 115, no. 1, pp. 216—231. https://doi.org/10.1002/bit.26459
Triscott, M.X. and van de Rijn, I., Solubilization of hyaluronic acid synthetic activity from streptococci and its activation with phospholipids, J. Biol. Chem., 1986, vol. 261, no. 13, pp. 6004—6009. https://doi.org/10.1016/S0021-9258(17)38485-5
Salzberg, L.I. and Helmann, J.D., Phenotypic and transcriptomic characterization of Bacillus subtilis mutants with grossly altered membrane composition, J. Bacteriol., 2008, vol. 190, no. 23, pp. 7797—7807. https://doi.org/10.1128/JB.00720-08
Kawai, F., Shoda, M., Harashima, R., et al., Cardiolipin domains in Bacillus subtilis Marburg membranes, J. Bacteriol., 2004, vol. 186, no. 5, pp. 1475—1483. https://doi.org/10.1128/jb.186.5.1475-1483.2004
Nishibori, A., Kusaka, J., Hara, H., et al., Phosphatidylethanolamine domains and localization of phospholipid synthases in Bacillus subtilis membranes, J. Bacteriol., 2005, vol. 187, no. 6, pp. 2163—2174. https://doi.org/10.1128/JB.187.6.2163-2174.2005
Cerminati, S., Leroux, M., Anselmi, P., et al., Low cost and sustainable hyaluronic acid production in a manufacturing platform based on Bacillus subtilis 3NA strain, Appl. Microbiol. Biotechnol., 2021, vol. 105, no. 8, pp. 3075—3086. https://doi.org/10.1007/s00253-021-11246-6
Wu, S.C., Yeung, J.C., Duan, Y., et al., Functional production and characterization of a fibrin-specific single-chain antibody fragment from Bacillus subtilis: effects of molecular chaperones and a wall-bound protease on antibody fragment production, Appl. Environ. Microbiol., 2002, vol. 68, no. 7, pp. 3261—3269. https://doi.org/10.1128/aem.68.7.3261-3269.2002
Reuß, D.R., Schuldes, J., Daniel, R., et al., Complete genome sequence of Bacillus subtilis subsp. subtilis strain 3NA, Genome Announce., 2015, vol. 3, no. 2. https://doi.org/10.1128/genomeA.00084-15
Zeigler, D.R., Prágai, Z., Rodriguez, S., et al., The origins of 168, W23, and other Bacillus subtilis legacy strains, J. Bacteriol., 2008, vol. 190, no. 21, pp. 6983—6995. https://doi.org/10.1128/JB.00722-08
Wolf, M., Geczi, A., Simon, O., et al., Genes encoding xylan and beta-glucan hydrolysing enzymes in Bacillus subtilis: characterization, mapping and construction of strains deficient in lichenase, cellulase and xylanase, Microbiology (Reading, England), 1995, vol. 141, part 2, pp. 281—290. https://doi.org/10.1099/13500872-141-2-281
Kabisch, J., Thürmer, A., Hübel, T., et al., Characterization and optimization of Bacillus subtilis ATCC 6051 as an expression host, J. Biotechnol., 2013, vol. 163, no. 2, pp. 97—104. https://doi.org/10.1016/j.jbiotec.2012.06.034
Tlapak-Simmons, V.L., Baggenstoss, B.A., Kumari, K., et al., Kinetic characterization of the recombinant hyaluronan synthases from Streptococcus pyogenes and Streptococcus equisimilis, J. Biol. Chem., 1999, vol. 274, no. 7, pp. 4246—4253. https://doi.org/10.1074/jbc.274.7.4246
Chung, J.Y., Zhang, Y., and Adler, B., The capsule biosynthetic locus of Pasteurella multocida A:1, FEMS Microbiol. Lett., 1998, vol. 166, no. 2, pp. 289—296. https://doi.org/10.1111/j.1574-6968.1998.tb13903.x
Chung, J.Y., Wilkie, I., Boyce, J.D., et al., Role of capsule in the pathogenesis of fowl cholera caused by Pasteurella multocida serogroup A, Infect. Immun., 2001, vol. 69, no. 4, pp. 2487—2492. https://doi.org/10.1128/IAI.69.4.2487-2492.2001
Zhang, Y.F., Construction and characterization of an acapsular mutant of Pasteurella multocida strain P-1059 (A:3), J. Vaccines Vaccination, 2013, vol. 4, no. 4, p. 184. https://doi.org/10.4172/2157-7560.1000184
Hubbard, C., McNamara, J.T., Azumaya, C., et al., The hyaluronan synthase catalyzes the synthesis and membrane translocation of hyaluronan, J. Mol. Biol., 2012, vol. 418, nos. 1—2, pp. 21—31. https://doi.org/10.1016/j.jmb.2012.01.053
Ouskova, G., Spellerberg, B., and Prehm, P., Hyaluronan release from Streptococcus pyogenes: export by an ABC transporter, Glycobiology, 2004, vol. 14, no. 10, pp. 931—938. https://doi.org/10.1093/glycob/cwh115
Kumari, K., Baggenstoss, B.A., Parker, A.L., et al., Mutation of two intramembrane polar residues conserved within the hyaluronan synthase family alters hyaluronan product size, J. Biol. Chem., 2006, vol. 281, no. 17, pp. 11755—11760. https://doi.org/10.1074/jbc.M600727200
Medina, A.P., Lin, J., and Weigel, P.H., Hyaluronan synthase mediates dye translocation across liposomal membranes, BMC Biochem., 2012, vol. 13, p. 2. https://doi.org/10.1186/1471-2091-13-2
Mao, Z., Shin, H.D., and Chen, R.A., A recombinant E. coli bioprocess for hyaluronan synthesis, Appl. Microbiol. Biotechnol., 2009, vol. 84, no. 1, pp. 63—69. https://doi.org/10.1007/s00253-009-1963-2
Mao, Z. and Chen, R.R., Recombinant synthesis of hyaluronan by Agrobacterium sp., Biotechnol. Progr., 2007, vol. 23, no. 5, pp. 1038—1042. https://doi.org/10.1021/bp070113n
Zhang, L., Toscano Selão, T., Nixon, P.J., et al., Photosynthetic conversion of CO2 to hyaluronic acid by engineered strains of the cyanobacterium Synechococcus sp. PCC 7002, Algal Res., 2019, vol. 44, p. 101702. https://doi.org/10.1016/j.algal.2019.101702
DeAngelis, P.L., Enzymological characterization of the Pasteurella multocida hyaluronic acid synthase, Biochemistry, 1996, vol. 35, no. 30, pp. 9768—9771. https://doi.org/10.1021/bi960154k
Mandawe, J., Infanzon, B., Eisele, A., et al., Directed evolution of hyaluronic acid synthase from Pasteurella multocida towards high-molecular-weight hyaluronic acid, ChemBioChem, 2018, vol. 19, no. 13, pp. 1414—1423. https://doi.org/10.1002/cbic.201800093
Agarwal, G., Krishnan, K.V., Prasad, S.B., et al., Biosynthesis of hyaluronic acid polymer: dissecting the role of sub structural elements of hyaluronan synthase, Sci. Rep., 2019, vol. 9, no. 1, p. 12510. https://doi.org/10.1038/s41598-019-48878-8
Zhang, L., Huang, H., Wang, H., et al., Rapid evolution of hyaluronan synthase to improve hyaluronan production and molecular mass in Bacillus subtilis, Biotechnol. Lett., 2016, vol. 38, no. 12, pp. 2103—2108. https://doi.org/10.1007/s10529-016-2193-1
Soldo, B., Lazarevic, V., Pagni, M., et al., Teichuronic acid operon of Bacillus subtilis 168, Mol. Microbiol., 1999, vol. 31, no. 3, pp. 795—805. https://doi.org/10.1046/j.1365-2958.1999.01218.x
Blank, L.M., Hugenholtz, P., and Nielsen, L.K., Evolution of the hyaluronic acid synthesis (has) operon in Streptococcus zooepidemicus and other pathogenic streptococci, J. Mol. Evol., 2008, vol. 67, no. 1, pp. 13—22. https://doi.org/10.1007/s00239-008-9117-1
Blank, L.M., McLaughlin, R.L., and Nielsen, L.K., Stable production of hyaluronic acid in Streptococcus zooepidemicus chemostats operated at high dilution rate, Biotechnol. Bioeng., 2005, vol. 90, no. 6, pp. 685—693. https://doi.org/10.1002/bit.20466
Sheng, J.Z., Ling, P.X., Zhu, X.Q., et al., Use of induction promoters to regulate hyaluronan synthase and UDP-glucose-6-dehydrogenase of Streptococcus zooepidemicus expression in Lactococcus lactis: a case study of the regulation mechanism of hyaluronic acid polymer, J. Appl. Microbiol., 2009, vol. 107, no. 1, pp. 136—144. https://doi.org/10.1111/j.1365-2672.2009.04185.x
Chen, W.Y., Marcellin, E., Hung, J., et al., Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Streptococcus zooepidemicus, J. Biol. Chem., 2009, vol. 284, no. 27, pp. 18007—18014. https://doi.org/10.1074/jbc.M109.011999
Hmar, R.V., Prasad, S.B., Jayaraman, G., et al., Chromosomal integration of hyaluronic acid synthesis (has) genes enhances the molecular weight of hyaluronan produced in Lactococcus lactis, Biotechnol. J., 2014, vol. 9, no. 12, pp. 1554—1564. https://doi.org/10.1002/biot.201400215
Berezina, O.V., Jurgens, G., Zakharova, N.V., et al., Evaluation of carbon and electron flow in Lactobacillus brevis as a potential host for heterologous 1-butanol biosynthesis, Adv. Microbiol., 2013, vol. 03, no. 5, pp. 450—461. https://doi.org/10.4236/aim.2013.35061
Fong Chong, B. and Nielsen, L.K., Aerobic cultivation of Streptococcus zooepidemicus and the role of NADH oxidase, Biochem. Eng. J., 2003, vol. 16, no. 2, pp. 153—162. https://doi.org/10.1016/S1369-703X(03)00031-7
Armstrong, D.C. and Johns, M.R., Culture conditions affect the molecular weight properties of hyaluronic acid produced by Streptococcus zooepidemicus, Appl. Environ. Microbiol., 1997, vol. 63, no. 7, pp. 2759—2764. https://doi.org/10.1128/AEM.63.7.2759-2764.1997
Chong, B. and Nielsen, L., Amplifying the cellular reduction potential of Streptococcus zooepidemicus, J. Biotechnol., 2003, vol. 100, no. 1, pp. 33—41. https://doi.org/10.1016/s0168-1656(02)00239-0
Kaur, M. and Jayaraman, G., Hyaluronan production and molecular weight is enhanced in pathway-engineered strains of lactate dehydrogenase-deficient Lactococcus lactis, Metab. Eng. Commun., 2016, vol. 3, pp. 15—23. https://doi.org/10.1016/j.meteno.2016.01.003
García Montes de Oca, L.Y.J., Chagolla-López, A., González de Vara, L., et al., The composition of the Bacillus subtilis aerobic respiratory chain supercomplexes, J. Bioenerg. Biomembr., 2012, vol. 44, no. 4, pp. 473—486. https://doi.org/10.1007/s10863-012-9454-z
Li, S., Huang, D., Li, Y., et al., Rational improvement of the engineered isobutanol-producing Bacillus subtilis by elementary mode analysis, Microb. Cell Fact., 2012, vol. 11, p. 101. https://doi.org/10.1186/1475-2859-11-101
Huang, W.C., Chen, S.J., and Chen, T.L., The role of dissolved oxygen and function of agitation in hyaluronic acid fermentation, Biochem. Eng. J., 2006, vol. 32, no. 3, pp. 239—243. https://doi.org/10.1016/j.bej.2006.10.011
Westbrook, A.W., Ren, X., Moo-Young, M., et al., Application of hydrocarbon and perfluorocarbon oxygen vectors to enhance heterologous production of hyaluronic acid in engineered Bacillus subtilis, Biotechnol. Bioeng., 2018, vol. 115, no. 5, pp. 1239—1252. https://doi.org/10.1002/bit.26551
Lai, Z.W., Rahim, R.A., Ariff, A.B., et al., Biosynthesis of high molecular weight hyaluronic acid by Streptococcus zooepidemicus using oxygen vector and optimum impeller tip speed, J. Biosci. Bioeng., 2012, vol. 114, no. 3, pp. 286—291. https://doi.org/10.1016/j.jbiosc.2012.04.011
Kallio, P.T. and Bailey, J.E., Intracellular expression of Vitreoscilla hemoglobin (VHb) enhances total protein secretion and improves the production of alpha-amylase and neutral protease in Bacillus subtilis, Biotechnol. Progr., 1996, vol. 12, no. 1, pp. 31—39. https://doi.org/10.1021/bp950065j
Lu, J.F., Zhu, Y., Sun, H.L., et al., Highly efficient production of hyaluronic acid by Streptococcus zooepidemicus R42 derived from heterologous expression of bacterial haemoglobin and mutant selection, Lett. Appl. Microbiol., 2016, vol. 62, no. 4, pp. 316—322. https://doi.org/10.1111/lam.12546
Zhang, X., Wang, M., Li, T., et al., Construction of efficient Streptococcus zooepidemicus strains for hyaluoronic acid production based on identification of key genes involved in sucrose metabolism, AMB Express, 2016, vol. 6, no. 1, p. 121. https://doi.org/10.1186/s13568-016-0296-7
Reid, S.J. and Abratt, V.R., Sucrose utilisation in bacteria: genetic organisation and regulation, Appl. Microbiol. Biotechnol., 2005, vol. 67, no. 3, pp. 312—321. https://doi.org/10.1007/s00253-004-1885-y
Feng, J., Gu, Y., Quan, Y., et al., Construction of energy-conserving sucrose utilization pathways for improving poly-γ-glutamic acid production in Bacillus amyloliquefaciens, Microb. Cell Fact., 2017, vol. 16, no. 1, p. 98. https://doi.org/10.1186/s12934-017-0712-y
Feng, J., Gu, Y., Yan, P.-F., et al., Recruiting energy-conserving sucrose utilization pathways for enhanced 2,3-butanediol production in Bacillus subtilis, ACS Sustainable Chem. Eng., 2017, vol. 5, no. 12, pp. 11221—11225. https://doi.org/10.1021/acssuschemeng.7b03636
ACKNOWLEDGMENTS
We thank O.V. Berezina for the help in preparation of the manuscript.
Funding
This study was supported by the Ministry of Science and Higher Education of the Russian Federation (contract in the electronic budget system no. 075-10-2021-113, project ID: RF—193021X0001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by N. Maleeva
Rights and permissions
About this article
Cite this article
Rykov, S.V., Battalova, I.Y. & Mironov, A.S. Construction of Recombinant Bacillus subtilis Strains Producing Hyaluronic Acid. Russ J Genet 58, 507–527 (2022). https://doi.org/10.1134/S1022795422050088
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795422050088