Abstract
Using allele-specific primers and hybridological analysis, the allelic composition of the VRN and PPD loci was determined in common wheat lines derived from the Bezostaya 1 (Bez1) cultivar. In lines of the Bez1 cultivar carrying different dominant alleles of the VRN genes and their combinations, the duration of certain developmental phases was examined. It was demonstrated that, in lines with the combination of two dominant alleles of the VRN-1 locus (Bez1Vrn-A1aVrn-B1a and Bez1Vrn-A1aVrn-B1c), the duration of the “tillering–first node” and “shoots–heading” periods was statistically significantly decreased compared to the initial isogenic lines (i:Bez1Vrn-A1a, i:Bez1Vrn-B1a, and i:Bez1Vrn-B1c). In addition, the presence of two dominant alleles led to the reduction in the time span of the organogenesis stages, as shown by studying the dynamics of shoot apex size and morphology in common wheat lines of the Bez1 cultivar. The productivity analysis in the lines of the Bez1 cultivar showed that the i:Bez1Vrn-B1c line was characterized by highest productivity among isogenic lines, while the Bez1Vrn-A1a Vrn-B1c line was more productive than the Bez1Vrn-A1a Vrn-B1a line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Common wheat (T. aestivum L.) is one of principal cereals grown in the world. It is adapted to a wide range of climatic conditions and is therefore grown in different agroecological zones. Cultivated wheat varieties, in addition to high grain yield and resistance to adverse environmental conditions, should correspond to the climatic zone for the duration of growing season and its individual phases. The study of genes that control the growing season duration in common wheat, in particular, heading and flowering time, is of great practical importance, since allelic diversity of these genes largely determines wide adaptation of wheat to environmental conditions.
In wheat, there are a number of genetic systems that determine the switch from the vegetative to reproductive phase, the main ones of which are vernalization response genes (VRN) and photoperiod sensitivity genes (PPD) [1, 2].
Vernalization is a mechanism of prolonged exposure to low above-zero temperatures, required for the switch of winter plants from the vegetative phase of development to the reproductive phase. The need for vernalization of winter plants is an important adaptive mechanism that allows their wintering in the regions with low winter temperatures, preventing damage to the apical meristem sensitive to low temperatures [3, 4]. The genetic diversity of wheat in heading and flowering time is determined by four major VRN genes, VRN-1, VRN-2, VRN-3, and VRN-4. The VRN-1 locus, which encodes MADS-box transcription factor, determining the switch of apical meristem cells to reproductive development, is represented by three homeologous genes, VRN-A1, VRN-B1, and VRN-D1, mapped to the long arms of chromosomes 5A, 5B, and 5D, respectively [1, 3]. The VRN-3 locus, which is orthologous to the FT gene of Arabidopsis is mapped to the short arm of chromosome 7B [5]. The VRN-4 gene (formerly known as VRN-D4, VRN-D5) is a duplication of the chromosome 5A long arm region with the VRN-A1 gene in the short arm of chromosome 5D [6]. The spring type of wheat development is determined by the presence of at least one dominant VRN-1, VRN-3, or VRN-4 gene, while the winter type of development is determined by recessive alleles of these loci.
A different effect of homeologous VRN-1 genes on the sensitivity to vernalization was demonstrated. The least plant sensitivity to vernalization is determined by the VRN-A1 gene, and genotypes with the dominant VRN-B1 and VRN-D1 genes are more sensitive to vernalization [7], which correlates with the relative expression levels of these genes [8]. To date, a series of dominant alleles of the VRN-A1, VRN-B1, and VRN-D1 loci, which, unlike recessive alleles, determine the absence of the need for vernalization, has been described. Allelic diversity at the VRN-1 locus was found to be determined by the insertions and/or deletions in two regulatory regions, the promoter and the first intron regions [9]. For instance, most of the dominant VRN-A1 alleles described so far, including Vrn-A1a and Vrn-A1b, characteristic of common wheat cultivars, are associated with mutations in the promoter region [10–13]. On the contrary, the dominant alleles of the VRN-B1 and VRN-D1 loci are mainly associated with structural changes in the first intron [14–19].
Analysis of structural features provided the design of allele-specific primers [10, 14, 16, 17]. The use of these primers allows for rapid identification of the allelic composition in common wheat cultivars and lines. They also played an important role in studying the geographical distribution patterns of different alleles of the VRN-1 locus.
For instance, it is known that the Vrn-A1a allele is widely distributed among spring varieties from Northern and Eastern Europe, the greater part of Russia and Western Siberia [20–25], Canada [26], the United States, Argentina, and China [10, 15, 27].
Dominant allele Vrn-B1a is typical of cultivars from Argentina, California [14], Pakistan [28], Canada [26], the United States [15], and European countries [20, 22]. The Vrn-B1c allele is less common than Vrn-B1a. In addition to Russian cultivars [21, 23, 24, 29], this allele was found mainly among the cultivars from Eastern and Central Europe and Ukraine [16, 22, 30].
The photoperiod is also one of the factors regulating the growing season duration, which controls the beginning of heading and flowering depending on the plant response to the day length. Sensitivity to the day length (photoperiod) is an adaptation owing to which plants grow in the regions with different day lengths. Photoperiod sensitivity is controlled by the PPD genes. In wheat, the most important photoperiod sensitivity genes (PPD-1), PPD-D1, PPD-B1, and PPD-A1 (formerly PPD1, PPD2, and PPD3), were mapped to the short arms of the second homeologous group chromosomes: 2D, 2B, and 2A, respectively [1, 31, 32]. In addition, one more PPD gene, PPD-B2, was mapped to the short arm of chromosome 7B [33].
The majority of known dominant alleles of wheat PPD-1 locus, unlike recessive alleles, were found to contain deletions or insertions in the promoter region [34, 35]. The photoperiod insensitivity is controlled by the dominant PPD alleles and determines the reduction of the vegetation period under both short- and long-day conditions, while photoperiod sensitive cultivars do not switch to reproductive development until the day length reaches a particular value [36].
The dominant PPD genes differ in expressivity. The highest photoperiod insensitivity is provided by the dominant PPD-D1 gene, and then follow the PPD-B1 and PPD-A1 genes [36]. In regions with a hot and arid climate, it is most advantageous to cultivate photoperiod-insensitive wheat cultivars, which makes it possible for the plants to mature prior to the beginning of high summer temperatures, providing high yields. In turn, photoperiod-sensitive cultivars are most adapted for cultivation in the regions with a cooler and more humid climate [32, 37].
It is important to study the effect of different alleles associated with structural changes in the regulatory regions of the VRN-1 genes on the beginning of heading and flowering of common wheat. It was demonstrated that the genotypes bearing the vernalization-insensitive dominant allele Vrn-A1a were the earliest ripening. In the carriers of the Vrn-A1b allele, heading occurs later than in the carriers of the Vrn-A1a allele [38]. Using a series of near isogenic lines of the winter cultivar Bezostaya 1 (Bez1) developed at the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences [39], it was demonstrated that the Vrn-A1a allele determined earlier heading than the Vrn-B1a and Vrn-B1c alleles, while Vrn-B1c allele, in turn, reduced the duration of the shoots–heading period compared to the Vrn-B1a allele [40], which is consistent with the data on transcription of these dominant alleles obtained in [41].
In addition to the effect of the individual VRN and PPD genes on the growing season duration, the effect of the combination of different VRN and PPD alleles is worth exploring. In a number of studies, differences in heading time were studied in spring cultivars carrying different combinations of the VRN and PPD genes [22–25, 29]. At the same time, the production and study of genotypes carrying combinations of different VRN and PPD alleles in the same genotypic environment helps to shed light on the genetic effects of VRN and PPD loci.
It was demonstrated that different VRN genes and their combinations affecting the growing season duration and heading time could also affect the productivity of common wheat. Cultivars with two dominant genes VRN-A1 and VRN-B1 were earlier ripening and more productive than cultivars with a single VRN gene [7, 42].
Given that the development type of most modern Russian and Western Siberian cultivars is determined by two dominant alleles, Vrn-A1a and either Vrn-B1a or Vrn-B1c [21, 23–25], we have developed two common wheat lines carrying Vrn-A1a in combination with Vrn-B1a or Vrn-B1c against the genetic background of the winter cultivar Bez1 [43].
This study presents the data on the effect of different dominant alleles of VRN-1 loci and their combinations on the duration of certain developmental phases, the dynamics of shoot apex formation, and productivity of wheat lines of the winter Bez1 cultivar in the forest-steppe zone of Novosibirsk oblast.
MATERIALS AND METHODS
Plant Material
Wheat lines of the winter cultivar Bez1 with the combination of two alleles of the VRN-1 loci were examined: Bez1Vrn-A1a Vrn-B1a and Bez1Vrn-A1a Vrn-B1c. These lines were obtained by crossing two near isogenic lines (i:Bez1Vrn-B1a and i:Bez1Vrn-B1c) with isogenic line i:Bez1Vrn-A1a. Homozygous plants carrying two dominant alleles of the VRN genes were isolated among F2 plants using known allele-specific primers for the VRN-A1 and VRN-B1 genes [43].
In addition, isogenic common wheat lines of the Bez1 cultivar with dominant alleles of VRN genes, i:Bez1Vrn-A1a (Triple Dirk D isogenic line, donor of the dominant Vrn-A1a allele), i:Bez1Vrn-B1a and i:Bez1Vrn-B1c (dominant alleles from Diamant II and Saratovskaya 29 cultivars, respectively), and i:Bez1VRN-D4 (donor, accession k-5498 of T. sphaerococcum Persiv. from the VIR collection) (Table 1), were included in the study. The schemes for obtaining the isogenic lines carrying the Vrn-B1a and Vrn-B1c alleles were described previously [39]. The isogenic lines with the Vrn-A1a and VRN-D4 genes were obtained using similar technique.
Two lines with combination of the alleles of VRN loci were genotyped using the isogenic lines of the Triple Dirk cultivar obtained by A.T. Pugsley (TD D line with the dominant VRN-A1 gene, TD B line with the VRN-B1 gene, TD E line with VRN-D1, and TD F line with VRN-D4), as well as the isogenic lines of the Bez1 cultivar with the dominant Vrn-A1a, Vrn-B1a, Vrn-B1c, and VRN-D4 alleles. The Filatovka winter cultivar was used as a recessive form.
DNA Extraction and PCR
Genomic DNA was extracted from the leaves of adult plants according to [44]. PCR was performed in a total volume of 25 μL reaction mixture containing 50–100 ng of DNA template, 1× reaction buffer (67 mM Tris HCl (pH 8.8), 1.5 mM MgCl2, 18 mM (NH4)2SO4, 0.01% Tween 20), 200 μM of dNTPs, 0.25 μM of forward and reverse primers, 1 unit of Taq DNA polymerase (Medigen, Russia), and H2O, up to 25 μL.
The structure of the used primers and PCR conditions were consistent with the published protocols (Table 2). The reaction was run on a Bio Rad T100 Thermal Cycler (United States). The amplification products were separated by electrophoresis on a 1.5% agarose gel in 1× ТАЕ buffer with the addition of ethidium bromide. After electrophoresis, the gel was photographed in ultraviolet light using the Doc-Print II gel documentation system (Vilber Lourmat, France).
Analysis of the Duration of Developmental Phases
Analysis of the duration of certain developmental phases in common wheat lines with dominant alleles of VRN-1 loci was carried out during spring sowing of 2017 and 2018 on the experimental field of the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, under natural long photoperiod (55°2 N, 82°56 E; day length for the May–August period, 17 h) and in the greenhouse of the LIVR Common Use Center of the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, in 2019 during spring vegetation.
The duration of the following developmental phases was studied: shoots–tillering, tillering–first node (or shoots–first node), first node–heading, and shoots–heading. Tillering was recorded on the day when a secondary shoot developed off the main shoot. The first node phase was recorded when the first node appeared on the main shoot at a height of 1 cm above the soil surface. The stem elongation phase was recorded on the day when the first node rose to a height of about 4 cm and the second node began to form at the soil surface. Heading was recorded when the head had fully emerged from the flag leaf [45]. The dates of the beginning of developmental phases were recorded for each plant individually, and the mean value was calculated. A total of 25–35 plants of each line were examined.
In the analysis of segregation in F2 hybrids obtained from crossing the Bez1Vrn-A1a Vrn-B1a and Bez1Vrn-A1a Vrn-B1c lines with the tester isogenic lines of the Triple Dirk cultivar and the isogenic lines of the Bez1 cultivar, the number of spring and winter plants was determined. Plants in which heads were not fully developed or stem elongation were not formed 100 days after germination were assigned to winter wheat.
Analysis of the Dynamics of Shoot Apex Development
The shoot apex was examined under an Altami PSO745 stereo microscope and photographed with an Altami FireWire 1340R7 1/2CCD camera (Russia). For analysis, the upper part of the stem was first cut off about 1–2 cm above the node and then the shoot apex was freed from the covering leaves with a microscopic needle and examined under a microscope at magnification of 10×. The dynamics of the shoot apex development was monitored from June 9 to June 26, 2017, with the interval of 3–4 days, starting from tillering phase and ending with booting phase.
Analysis of the Productivity Traits
Plant productivity was studied in 2018 upon growing on the experimental field of the Institute of Cytology and Genetics, Siberian Branch, Russian Academy of Sciences. To perform structural analysis, the 25 best accessions from each line were selected. The productivity components of the main spike (spike length, number of spikelets, grain number and weight) and plant (spike number, grain number and weight) were examined.
Statistical Treatment of the Data
Statistical treatment of the obtained data was carried out using Microsoft Excel 2013. To assess the statistical significance of the differences between the mean values, Student’s test was used (t-test).
RESULTS
Determination of Allele Composition at the VRN-A1, VRN-B1, and PPD-D1 Loci in the Lines of Bez1 Cultivar Using PCR Analysis
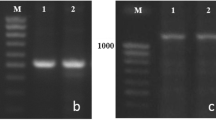
Wheat lines of the Bez1 cultivar were genotyped using PCR analysis with allele-specific primers shown in Table 2. Using allele-specific primers VRN1AF and VRN1R, in the examined lines, two fragments 650 and 750 bp in size characteristic of the dominant Vrn-A1a allele were amplified (Fig. 1a). Using multiplex PCR with four primers, Ex1/B/F3, Intr1/B/F, Intr1/B/R3, and Intr1/B/R4, in one of the lines, the PCR product was represented by two fragments of 709 and 1235 bp characteristic of the dominant Vrn-B1a allele. In another line, a fragment of 849 bp was amplified, indicating the presence of the dominant Vrn-B1c allele (Fig. 1b). Thus, the genotype of line 1 (L1) is Vrn-A1a Vrn-B1a, and the genotype of line 2 (L2) is Vrn-A1a Vrn-B1c.
It is known that, in addition to VRN genes, PPD genes that control the photoperiod sensitivity also have a considerable effect on the duration of developmental phases in common wheat. Therefore, our task was to identify alleles of PPD genes in the isogenic lines and lines with the VRN allele combinations derived from the Bez1 cultivar.
Using multiplex PCR with three primers, Ppd-D1_F, Ppd-D1_R1, and Ppd-D1_R2, in the Bez1 cultivar and the lines created on its basis with different dominant VRN alleles, a fragment of 288 bp was amplified, which indicated the presence of the Ppd-D1 allele insensitive to day length (Fig. 2).
Determination of VRN Genotypes in Spring Wheat Lines of Bez1 Cultivar on the Basis of Genetic Segregation in F2 Hybrids with Tester Isogenic Lines
To support the results of molecular analysis, an additional hybridological analysis was performed with A.T. Pugsley’s isogenic lines of the Bez1 cultivar (i:Bez1Vrn-A1a, i:Bez1Vrn-B1a, i:Bez1Vrn-B1c, and i:Bez1VRN-D4) and the winter cultivar Filatovka. In F2 hybrids obtained from crosses of the Bez1Vrn-A1a Vrn-B1a and Bez1Vrn-A1a Vrn-B1c lines with the tester TD D (VRN-A1) and TD B (VRN-B1) lines and the i:Bez1Vrn-A1a, i:Bez1Vrn-B1a, and i:Bez1Vrn-B1c isogenic lines, segregation was absent and all plants were of spring type. When these lines were crossed with the tester TD E (VRN-D1) and TD F (VRN-D4) lines and the i:Bez1VRN-D4 isogenic line, segregation into spring and winter wheat close to 63 : 1 was observed. In the case of crosses with the winter Filatovka cultivar, which carries only recessive vrn genes, the segregation corresponded to a digenic one (15 : 1) (Table 3).
Thus, the data obtained showed the presence of two dominant VRN-A1 and VRN-B1 genes and recessive vrn-D1 and vrn-D4 genes in the genotypes of the studied lines. Hence, the results of genetic analysis were consistent with those obtained in molecular analysis.
Determination of the Effect of Different Dominant Alleles of the VRN-1 Loci and Their Combinations on the Duration of Certain Developmental Phases in the Forest-Steppe Zone of Novosibirsk Oblast
The duration of certain developmental phases in common wheat lines of the Bez1 cultivar with two dominant alleles of the VRN-1 loci, Bez1Vrn-A1a Vrn-B1a and Bez1Vrn-A1a Vrn-B1c, was studied. The isogenic lines derived from the Bez1 cultivar and carrying the dominant Vrn-A1a, Vrn-B1a, Vrn-B1c, and VRN-D4 alleles were used as controls. The results are presented in Table 4. Under a natural long photoperiod, the effect of the PPD genes is weak, which facilitates more accurate determination of the effects of the VRN genes.
First, it was demonstrated that lines with the combination of two alleles (Bez1Vrn-A1a Vrn-B1a and Bez1Vrn-A1a Vrn-B1c) headed earlier than other lines (in 40–41 days). Compared to the Bez1Vrn-A1a isogenic line, in which the shoots–heading phase lasted 42–43 days, the difference was 2 days (P < 0.001). In addition, statistically significant differences were observed between lines with two dominant alleles and VRN-B1 isogenic lines. In particular, the beginning of the heading stage in the Bez1Vrn-A1a Vrn-B1a line was observed about 10 days earlier compared to the i:Bez1Vrn-B1a line (the shoots–heading phase lasted 49–52 days), while in the Bez1Vrn-A1a Vrn-B1c line, the beginning of the heading stage occurred about 8 days earlier compared to i:Bez1Vrn-B1c (45–51 days) (P < 0.001). A similar trend was also observed upon cultivation of these lines in the greenhouse of the LIVR Common Use Center of the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences. During spring vegetation of 2019, in the lines with two dominant alleles, heading occurred within 43 days, and the difference from the lines with the Vrn-A1a, Vrn-B1a, and Vrn-B1c alleles was 3, 10, and 13 days, respectively.
In the isogenic lines with dominant alleles of the VRN-B1 locus, heading occurred later compared to the isogenic line with the Vrn-A1a allele. Comparison of the VRN-B1 isogenic lines with each other showed that the i:Bez1Vrn-B1c isogenic line headed by about 3 days earlier than i:Bez1Vrn-B1a line (P < 0.001). In the i:Bez1VRN-D4 isogenic line, the phase duration was 48 days, and it was intermediate between that in the i:Bez1Vrn-A1a and i:Bez1Vrn-B1a lines and did not differ from that in i:Bez1Vrn-B1c.
The duration of shoots–tillering phase (the organogenesis stages I–III) in all studied lines of the Bez1 cultivar was approximately the same and constituted 11–12 days. Moreover, the duration of this phase in the Bez1Vrn-A1a Vrn-B1a line was the shortest among the studied lines of Bez1 cultivar.
In lines with two dominant alleles, the duration of the tillering–first node phase was 10–11 days, and it was on average 2–3 days shorter compared to the i:Bez1Vrn-A1a isogenic line, in which this phase lasted 13 days (P < 0.001). In the Bez1Vrn-A1a Vrn-B1a line, the duration of this phase was 11 days, and it was reduced by 9 days (P < 0.001) compared to the i:BezVrn-B1a isogenic line, in which the duration of this phase was 20 days; and in the Bez1Vrn-A1aVrn-B1c line, compared to i:Bez1Vrn-B1c, in which this period lasted 18 days, there was a reduction of 8 days, respectively (P < 0.001). In the i:Bez1VRN-D4 isogenic line, the duration of this phase was 16 days, and by the duration of this phase, this line occupies an intermediate position between the isogenic lines with the dominant Vrn-A1a allele and dominant alleles of the VRN-B1 locus. In general, the period from shoots to the first node in the lines with two dominant alleles, according to two-year data averaged 21–22 days and was on average 1–2 days shorter compared to the i:Bez1Vrn-A1a isogenic line. Compared to the i:Bez1Vrn-B1a isogenic line, in which the duration of this phase was 32 days, in the Bez1Vrn-A1a Vrn-B1a line, there was a 10-day reduction (P < 0.001), and in the Bez1Vrn-A1a Vrn-B1c line, compared to i:Bez1Vrn-B1c, in which this period lasted 29 days, there was an 8-day reduction, respectively (P < 0.001).
The duration of first node–heading period (the organogenesis stages IV–VII) in lines with two dominant alleles increased slightly relative to the isogenic lines with the Vrn-A1a, Vrn-B1a, and Vrn-B1c alleles (P < 0.01–0.001) (by 1–2 days). It is known that in late-ripening genotypes, the duration of further developmental phases is slightly reduced; therefore, the duration of the period after the appearance of the first node is slightly reduced compared to earlier ripening genotypes.
It should be noted that the duration of developmental phases varied depending on the weather conditions of the growing year. For instance, in 2018, compared to 2017, the plants were later ripening and the duration of the shoots–heading period increased to a greater extent because of the increased time span of the first node–heading period.
Thus, a two-year study of common wheat lines derived from the Bez1 cultivar and carrying the combinations of dominant alleles of the VRN-A1 and VRN-B1 loci in Novosibirsk oblast showed a considerable decrease in the duration of the shoots–first node (or tillering–first node) and shoots–heading periods in comparison with initial i:Bez1Vrn-A1a, i:BezVrn-B1a, and i:Bez1Vrn-B1c isogenic lines.
Dynamics of the Shoot Apex Growth and Development in Common Wheat Lines Derived from Bez1 Cultivar
The developmental phases and stages of organogenesis were also examined using an approach that consisted in monitoring the shoot apex growth and development [45].
In this study, dynamics of the organogenesis stages was analyzed by monitoring the shoot apex growth and development in the isogenic lines derived from the Bez1 cultivar and carrying different dominant alleles of the VRN loci, as well as the lines with dominant allele combinations (Vrn-A1a Vrn-B1a and Vrn-A1a Vrn-B1c). The photos in Fig. 3 illustrate the dynamics of shoot apex development. The tillering phase corresponds to the organogenesis stages II–III, and the first node–stem elongation phase corresponds to stages IV–V.
The beginning of the tillering phase in all lines was recorded approximately at the same time, May 31–June 1. The first examination of shoot apex was carried out on June 9, which corresponded to the tillering phase. In particular, in the lines with the dominant Vrn-A1a allele (i:Bez1Vrn-A1a, Bez1Vrn-A1aVrn-B1a, and Bez1Vrn-A1a Vrn-B1c) during the period corresponding to the middle tillering–first node phase, an increase in the size of the shoot apex to 1.5 mm was observed along with noticeable shoot apex segmentation with the formation of spikelet primordia, since from this moment, the differences in the development rate of genotypes differing in allelic composition of the VRN genes arise. In later ripening lines of the Bez1 cultivar, segmentation was weakly expressed. The appearance of the first node in the lines with two dominant alleles was observed on June 11–12; in the i:Bez1Vrn-A1a line, on June 14; in the lines with the Vrn-B1c and VRN-D4 alleles, on June 18; and in the i:Bez1Vrn-B1a line, on June 22.
Examination of June 12 showed further growth of the shoot apical meristem along with more noticeable segmentation in the earliest ripening lines. At the same time, the lines with two dominant alleles demonstrated a noticeable increase in the shoot apex size compared to the line with the dominant Vrn-A1a allele. Later ripening lines, i:Bez1Vrn-B1c and i:Bez1Vrn-D4, demonstrated the beginning of shoot apical meristem segmentation, while in the i:Bez1Vrn-B1a line, segmentation was poorly expressed.
Moreover, the lines with two dominant alleles at all subsequent stages of organogenesis were ahead of other lines in their development in terms of shoot apex differentiation and size. The period of spikelet primordia formation in these lines was finished earlier compared to the lines with the dominant Vrn-B1a and Vrn-B1c alleles, as well as the Vrn-A1a allele. In addition, at this stage, developmental differences between the lines with different VRN-B1 alleles were observed. In particular, the i:Bez1Vrn-B1c line was ahead of the i:Bez1Vrn-B1a line in development. In the i:BezVrn-B1c line, higher shoot apex extension and segmentation was observed at the organogenesis stages III and IV.
Thus, differences in the dynamics of the shoot apex length and morphology between the lines of the Bez1 cultivar were identified. Differences began to appear during the tillering–first node phase.
The Effect of Different Dominant Alleles of VRN Loci and Their Combinations on Productivity of Common Wheat Lines of Bez1 Cultivar
The challenge of wheat breeding now is to produce high-yielding forms. In this regard, it is of interest to determine the effect of different dominant alleles of the VRN loci and their combinations on the productivity components. Since in this study lines containing different VRN alleles in the same genotypic environment are used, this will make it possible to study the contribution of different alleles of the VRN genes and their combinations to productivity.
The following are the results of studying the productivity of the main spike and plant as a whole in lines with the allele combinations of the VRN-A1 and VRN-B1 loci (Bez1Vrn-A1a Vrn-B1a and Bez1Vrn-A1a Vrn-B1c), as well as in isogenic lines with the dominant Vrn-A1a, Vrn-B1a, Vrn-B1c, and VRN-D4 alleles. The results are presented in Table 5. Comparative analysis of the lines with the combination of dominant alleles showed that the Bez1Vrn-A1a Vrn-B1c line was more productive than the i:Bez1Vrn-A1a Vrn-B1a line. A considerable increase in the productivity index values was observed for the spike length, grain weight per spike, and grain weight per plant (P < 0.001). An increase in absolute values of other productivity indices was also observed. In addition, the values of the main spike productivity indices in the Bez1Vrn-A1a Vrn-B1c line did not differ from the values obtained for the i:Bez1Vrn-A1a line, while the value of the grain weight per main spike index was higher in the first line (by 0.43 g, P < 0.001). The plant productivity indices (spike number, grain number, and grain weight) in this line were higher than in the control i:Bez1Vrn-A1a line (P < 0.01–0.001).
Among the isogenic lines, one line, i:Bez1Vrn-B1c, was distinguished by plant productivity. This line had the maximum number of spikelets per spike (21.68 pcs), the maximum number of spikes per plant (7.72 pcs), the maximum number of grains per plant (274.16 pcs), and the highest grain weight per plant (11.69 g). The least productive of the four lines was the i:Bez1Vrn-A1a line. The highest grain weight per spike was observed in the i:Bez1Vrn-B1a and i:Bez1Vrn-B1c lines (2.19 and 2.28 g).
DISCUSSION
Using molecular and genetic analysis, two common wheat lines derived from the Bez1 cultivar were genotyped. The results showed the presence of two dominant VRN-A1 and VRN-B1 genes. One of the lines carries the Vrn-A1aVrn-B1a alleles, and the other line carries the Vrn-A1aVrn-B1c alleles. In addition, both of these lines, as well as the isogenic lines of the Bez1 cultivar carry the allele Ppd-D1a insensitive to day length.
It is known that early ripeness of modern commercial common wheat cultivars cultivated in temperate countries, including Russia, is ensured by the presence of the dominant Vrn-A1a allele, which has the highest effect on heading acceleration [10, 15, 22–24, 26]. It was demonstrated that the combination of two alleles, Vrn-A1a with Vrn-B1a or Vrn-B1c, can reduce the growing season duration in common wheat cultivars relative to cultivars bearing the single Vrn-A1a allele [22–24]. That is why, in climatic conditions of the most part of Russia and Western Siberia, cultivars, the spring type of development of which is determined by the combination of dominant alleles, Vrn-A1a and Vrn-B1c or Vrn-B1a, are the most prevalent [21, 23–25].
Since heading time in cultivars with the same VRN genotype may differ depending on the genetic background, in the present study, the effects of allele combinations at the same genetic background were examined. The data obtained in this study are consistent with the earlier obtained data on earlier ripeness of genotypes with two dominant alleles, Vrn-A1a Vrn-B1a and Vrn-A1a Vrn-B1c.
Experimentally, it was found that the constructed lines with two dominant alleles of the VRN-1 locus in the conditions of Novosibirsk oblast were earlier ripening than the initial isogenic lines, i:Bez1Vrn-A1a, i:Bez1Vrn-B1a, and i:Bez1Vrn-B1c. Differences in the duration of the shoots–heading period were to a greater extent associated with the reduction of the period before the first node formation, in particular, of the tillering–first node period (the organogenesis stages II–IV), which is the key stage determining the duration of the common wheat growing season. This finding is supported by the results of other authors [40, 46, 47].
Studying the features of developmental biology is especially important for cultivating common wheat cultivars corresponding to the climatic conditions of a particular zone. For instance, if the organogenesis stages III–IV pass too quickly or in adverse conditions (water deficit, high temperatures), the number of spikelets and the spike length decreases, which eventually affects the end-use quality of the plant. And, conversely, under favorable conditions, stronger spikes are formed, which increases the plant productivity [45]. Therefore, it is important to accurately determine the time of the organogenesis stages III–IV and to select genotypes that are optimal for each growing region, taking into account the time limits of sowing and harvesting.
It was demonstrated that the duration of growing season, as well as timing and the duration of individual developmental phases, determined to a greater extent by the VRN genes, was closely associated with tolerance to biotic and abiotic factors and productivity [1, 37, 45, 48, 49]. This is the basis for the selection of appropriate wheat cultivars that carry certain VRN alleles (or combinations thereof) for cultivation in a particular climatic zone. Thus, in temperate climates, cultivars carrying two dominant alleles, VRN-A1 and VRN-B1, have an advantage and are more productive than cultivars with a single VRN gene [7, 42], which makes it possible to avoid frost damage in late spring and early autumn.
In the present study, the i:Bez1Vrn-B1c line demonstrated the highest productivity among isogenic lines derived from the Bez1 cultivar. Analysis of productivity in the lines derived from the Bez1 cultivar and carrying the combination of dominant alleles showed that the line carrying Vrn-A1a in combination with Vrn-B1c was more productive. The data obtained support the idea that, for Western Siberia, early ripening and mid-ripening cultivars of spring common wheat, which can realize their potential in these conditions, are most suitable. Early ripening cultivars pass through the organogenesis stages II–IV more rapidly (genotype Vrn-A1a Vrn-B1c). The mid-ripening cultivars (genotype Vrn-B1c genotype) are characterized by rather long duration of the organogenesis stages I–III and medium duration of stages IV–V.
Thus, in this study, new data on the effect of different dominant VRN alleles and combinations of two dominant alleles of the VRN-1 locus against the same genetic background (winter Bez1 cultivar) on the duration of certain developmental phases and wheat productivity in the forest-steppe zone of Novosibirsk oblast were obtained.
REFERENCES
Snape, J.W., Butterworth, K., Whitechurch, E., and Worland, A.J., Waiting for fine times: genetics of flowering time in wheat, Euphytica, 2001, vol. 119, pp. 185—190. https://doi.org/10.1023/A:1017594422176
Kamran, A., Iqbal, M., and Spaner, D., Flowering time in wheat (Triticum aestivum L.): a key factor for global adaptability, Euphytica, 2014, vol. 197, pp. 1—26. https://doi.org/10.1007/s10681-014-1075-7
Yan, L., Loukoianov, A., Tranquilli, G., et al., Positional cloning of wheat vernalization gene VRN1,Proc. Natl. Acad. Sci. U.S.A., 2003, vol. 100, pp. 6263—6268. https://doi.org/10.1073/pnas.0937399100
Trevaskis, B., Hemming, M.N., Peacock, W.J., and Dennis, E.S., HvVRN2 responds to day length, whereas HvVRN1 is regulated by vernalization and developmental status, Plant Physiol., 2006, vol. 140, pp. 1397—1405. https://doi.org/10.1104/pp.105.073486
Yan, L., Fu, D., Li, C., et al., The wheat and barley vernalization gene VRN3 is an orthologue of FT,Proc. Natl. Acad. Sci. U.S.A., 2006, vol. 103, pp. 19581—19586. https://doi.org/10.1073/pnas.0607142103
Yoshida, T., Nishida, H., Zhu, J., et al., Vrn-D4 is a vernalization gene located on the centromeric region of chromosome 5D in hexaploid wheat, Theor. Appl. Genet., 2010, vol. 120, pp. 543—552. https://doi.org/10.1007/s00122-009-1174-3
Stelmakh, A.F., Genetic effects of Vrn genes on heading date and agronomic traits in bread wheat, Euphytica, 1993, vol. 65, pp. 53—60.
Loukoianov, A., Yan, L., Blechl, A., et al., Regulation of VRN-1 vernalization genes in normal and transgenic polyploid wheat, Plant Physiol., 2005, vol. 138, pp. 2364—2373. https://doi.org/10.1104/pp.105.064287
Shi, C., Zhao, L., Zhang, X., et al., Gene regulatory network and abundant genetic variation play critical roles in heading stage of polyploidy wheat, BMC Plant Biol., 2019, vol. 19, no. 1, p. 6. https://doi.org/10.1186/s12870-018-1591-z
Yan, L., Helguera, M., Kato, K., et al., Allelic variation at the VRN-1 promoter region in polyploid wheat, Theor. Appl. Genet., 2004, vol. 109, pp. 1677—1686. https://doi.org/10.1007/s00122-004-1796-4
Dubcovsky, J., Loukoianov, A., Fu, D., et al., Effect of photoperiod on the regulation of wheat vernalization genes VRN1 and VRN2,Plant Mol. Biol., 2006, vol. 60, no. 4, pp. 469—480. https://doi.org/10.1007/s11103-005-4814-2
Golovnina, K.A., Kondratenko, E.Y., Blinov, A.G., and Goncharov, N.P., Molecular characterization of vernalization loci VRN1 in wild and cultivated wheats, BMC Plant Biol., 2010, vol. 10, pp. 168—182. https://doi.org/10.1186/1471-2229-10-168
Muterko, A., Kalendar, R., and Salina, E., Novel alleles of the VERNALIZATION1 genes in wheat are associated with modulation of DNA curvature and flexibility in the promoter region, BMC Plant Biol., 2016, vol. 16, pp. 65—81. https://doi.org/10.1186/s12870-015-0691-2
Fu, D., Szucs, P., Yan, L., et al., Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat, Mol. Genet. Genomics, 2005, vol. 273, pp. 54—65. https://doi.org/10.1007/s00438-004-1095-4
Santra, D.K., Santra, M., Allan, R.E., et al., Genetic and molecular characterization of vernalization genes Vrn-A1, Vrn-B1 and Vrn-D1 in spring wheat germplasm from the Pacific Northwest region of the USA, Plant Breed., 2009, vol. 128, pp. 576—584. https://doi.org/10.1111/j.1439-0523.2009.01681.x
Milec, Z., Tomková, L., Sumíková, T., and Pánková, K., A new multiplex PCR test for the determination of Vrn-B1 alleles in bread wheat (Triticum aestivum L.), Mol. Breed., 2012, vol. 30, pp. 317—323. https://doi.org/10.1007/s11032-011-9621-7
Shcherban, A.B., Efremova, T.T., and Salina, E.A., Identification of a new Vrn-B1 allele using two near-isogenic wheat lines with difference in heading time, Mol. Breed., 2012, vol. 29, pp. 675—685. https://doi.org/10.1007/s11032-011-9581-y
Zhang, J., Wang, Y., Wu, S., et al., A single nucleotide polymorphism at the Vrn-D1 promoter region in common wheat is associated with vernalization response, Theor. Appl. Genet., 2012, vol. 125, pp. 1697—1704. https://doi.org/10.1007/s00122-012-1946-z
Muterko, A., Balashova, I., Cockram, J., et al., The new wheat vernalization response allele Vrn-D1s is caused by DNA transposon insertion in the first intron, Plant Mol. Biol. Rep., 2015, vol. 33, pp. 294—303. https://doi.org/10.1007/s11105-014-0750-0
Nowak, M. and Kowalczyk, K., Allelic variation at the Vrn-1 locus of Polish cultivars of common wheat (Triticum aestivum L.), Acta Biol. Cracov., Ser. Bot., 2010, vol. 52, no. 2, pp. 86—91. https://doi.org/10.2478/v10182-010-0028-2
Shcherban, A.B., Emtseva, M.V., and Efremova, T.T., Molecular genetical characterization of vernalization genes Vrn-A1, Vrn-B1 and Vrn-D1 in spring wheat germplasm from Russia and adjacent regions, Cereal Res. Commun., 2012, vol. 40, no. 3, pp. 425—435. https://doi.org/10.1556/CRC.40.2012.3.4
Shcherban, A.B., Börner, A., and Salina, E.A., Effect of VRN-1 and PPD-1 genes on heading time in European bread wheat cultivars, Plant Breed., 2014, vol. 134, pp. 49—55. https://doi.org/10.1111/pbr.12223
Efremova, T.T., Chumanova, E.V., Trubacheeva, N.V., et al., Prevalence of VRN1 locus alleles among spring common wheat cultivars cultivated in Western Siberia, Russ. J. Genet., 2016, vol. 52, no. 2, pp. 146—153. https://doi.org/10.1134/S102279541601004X
Likhenko, I.E., Stasyuk, A.I., Shcherban, A.B., et al., Study of allelic composition of Vrn-1 and Ppd-1 genes in early–ripening and middle–early varieties of spring soft wheat in Siberia, Russ. J. Genet.: Appl. Res., 2014, vol. 5, no. 3, pp 198—207. https://doi.org/10.1134/S2079059715030107
Yankovskaya, A.A., Fisenko, A.V., Dragovich, A.Yu., et al., The genetic diversity of spring soft wheat cultivars in European Russia at the VRN and PPD genes, defining the earing time, Genetika (Moscow), 2018, vol. 54, no. 13, suppl., pp. S32—S36. https://doi.org/10.1134/S0016675818130209
Iqbal, M., Navabi, A., Yang, R.C., et al., Molecular characterization of vernalization response genes in Canadian spring wheat, Genome, 2007, vol. 50, pp. 511—516. https://doi.org/10.1139/G07-028
Zhang, X.K., Xiao, Y.G., Zhang, Y., et al., Allelic variation at the vernalization genes Vrn-A1, Vrn-B1, Vrn-D1, and Vrn-B3 in Chinese wheat cultivars and their association with growth habit, Crop Sci., 2008, vol. 48, pp. 458—470. https://doi.org/10.2135/cropsci2007.06.0355
Iqbal, M., Shahzad, A., and Ahmed, I., Allelic variation at the Vrn-A1, Vrn-B1, Vrn-D1, Vrn-B3 and Ppd-D1a loci of Pakistani spring wheat cultivars, Electron. J. Biotechnol., 2011, vol. 14, no. 1, pp. 1—8. https://doi.org/10.2225/vol14-issue1-fulltext-6
Potokina, E.K., Koshkin, V.A., Alekseeva, E.A., et al., The combination of the Ppd and Vrn gene alleles determines the heading date in common wheat varieties, Russ. J. Genet.: Appl. Res., 2012, vol. 2, no. 4, pp. 311—318. https://doi.org/10.1134/S2079059712040089
Milec, Z., Sumíková, T., Tomková, L., and Pánková, K., Distribution of different Vrn-B1 alleles in hexaploid spring wheat germplasm, Euphytica, 2013, vol. 192, pp. 371—378. https://doi.org/10.1007/s10681-013-0863-9
Law, C.N., Sutka, J., and Worland, A.J., A genetic study of day-length response in wheat, Heredity, 1978, vol. 41, no. 2, pp. 185—191.
Worland, A.J., Börner, A., Korzun, V., et al., The influence of photoperiod genes on the adaptability of European winter wheats, Euphytica, 1998, vol. 100, pp. 385—394.
Khlestkina, E.K., Giura, A., Roder, M.S., and Borner, A., A new gene controlling the flowering response to photoperiod in wheat, Euphytica, 2009, vol. 165, pp. 579—585. https://doi.org/10.1007/s10681-008-9783-5
Beales, J., Turner, A., Griffiths, S., et al., A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.), Theor. Appl. Genet., 2007, vol. 115, no. 5, pp. 721—733. https://doi.org/10.1007/s00122-007-0603-4
Nishida, H., Yoshida, T., Kawakami, K., et al., Structural variation in the 5′ upstream region of photoperiod-insensitive alleles Ppd-A1a and Ppd-B1a identified in hexaploid wheat (Triticum aestivum L.), and their effect on heading time, Mol. Breed., 2013, vol. 31, pp. 27—37. https://doi.org/10.1007/s11032-012-9765-0
Scarth, R. and Law, C.N., The control of the day length response in wheat by the group 2 chromosomes, Z. Pflanzenzücht., 1984, vol. 93, pp. 140—150.
Cockram, J., Jones, H., Leigh, F.J., et al., Control of flowering time in temperate cereals: genes, domestication, and sustainable productivity, J. Exp. Bot., 2007, vol. 58, no. 6, pp. 1231—1244. https://doi.org/10.1093/jxb/erm042
Koval, S.F. and Goncharov, N.P., Multiple allelism at the VRN1 locus of common wheat, Acta Agron. Hung., 1998, vol. 46, no. 2, pp. 113—119.
Efremova, T.T., Arbuzova, V.S., Leonova, I.N., and Makhmudova, K., Multiple allelism in the Vrn-B1 locus of common wheat, Cereal Res. Commun., 2011, vol. 39, no. 1, pp. 12—21. https://doi.org/10.1556/CRC.39.2011.1.2
Emtseva, M.V., Efremova, T.T., and Arbuzova, V.S., The influence of Vrn-B1a and Vrn-B1c alleles on the length of developmental phases of substitution and nearisogenic lines of common wheat, Russ. J. Genet., 2013, vol. 49, no. 5, pp. 545—552. https://doi.org/10.1134/S1022795413050050
Shcherban, A.B., Khlestkina, E.K., Efremova, T.T., and Salina, E.A., The effect of two differentially expressed wheat VRN-B1 alleles on the heading time is associated with structural variation in the first intron, Genetica, 2013, vol. 141, pp. 133—141. https://doi.org/10.1007/s10709-013-9712-y
Goncharov, N.P., Response to vernalization in wheat: its quantitative or qualitative nature, Cereal Res. Commun., 2004, vol. 32, pp. 323—330.
Chumanova, E.V., Efremova, T.T., Kruchinina, Y.V., and Pershina, L.A., Development and investigation of common wheat lines of winter cultivar Bezostaya 1 with combinations of dominant alleles of VRN-1 loci, Vavilovskii Zh. Genet. Sel., 2018, vol. 22, no. 8, pp. 951—956. https://doi.org/10.18699/VJ18.437
Sharp, P.J., Kreis, M., Shewry, P.R., and Gale, M.D., Location of β-amylase sequences in wheat and its relatives, Theor. Appl. Genet., 1988, vol. 75, pp. 286—290. https://doi.org/10.1007/BF00303966
Kuperman, F.M., Rzhanova, E.I., Murashev, V.V., et al., Biologiya razvitiya kul’turnykh rastenii (Developmental Biology of Cultivated Plants), Moscow: Vyssh. Shkola, 1982.
Voronin, A.N. and Stel’makh, A.F., Stages of organogenesis in common wheat lines nearly isogenic for Vrn1–3 loci, Nauch.-Tekhn. Byull. Vses. Sel.-Genet. Inst., 1985, no. 1 (55), pp. 19—23.
Pánková, K. and Košner, J., Chromosome substitutions with dominant loci Vrn-1 and their effect on developmental stages of wheat, Czech J. Genet. Plant Breed., 2004, vol. 40, no. 2, pp. 37—44.
Stelmakh, A.F., Geographic distribution of Vrn genes in landraces and improved varieties of spring bread wheat, Euphytica, 1990, vol. 45, pp. 113—118.
Worland, A.J., The influence of flowering time genes on environmental adaptability in European wheats, Euphytica, 1996, vol. 89, pp. 49—57. https://doi.org/10.1007/BF00015718
Funding
This study was supported by the Russian Foundation for Basic Research (grant no. 18-34-00146 mol_a) and budget financing on the state contract with the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences (grant no. 0324-2019-0039).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by N. Maleeva
Rights and permissions
About this article
Cite this article
Chumanova, E.V., Efremova, T.T. & Kruchinina, Y.V. The Effect of Different Dominant VRN Alleles and Their Combinations on the Duration of Developmental Phases and Productivity in Common Wheat Lines. Russ J Genet 56, 822–834 (2020). https://doi.org/10.1134/S1022795420070029
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795420070029