Abstract
Alterations in the photochemical responses of Zea mays L. and Oryza sativa L. were analyzed to compare the tolerance level of these two crops towards Cd and Zn toxicity. To achieve this, photosynthetic efficiency of maize and rice plants was probed on the 8 days of CdCl2 and ZnSO4 exposure using chlorophyll a fluorescence kinetics, a noninvasive method suitable to examine the impact of different toxic compounds on photochemistrys. Analysis of induction curves and phenomenological energy pipeline models showed that both Cd and Zn reduced the efficiency of electron transport and associated photosystem II (PSII) functionality. A strong negative correlation was found between the accumulation of reactive oxygen species and ascorbate with the photosynthetic efficiency in both crops, but rice was more tolerant than maize towards Cd and Zn toxicity. Further, we found that Cd strongly affected the early photochemical reactions of maize, which can be considered as the reason for the higher vulnerability of this crop towards Cd. Our study suggests that the non-specific toxic effects of Cd and Zn resulted in chlorophyll degradation, reduction in efficiency of PSII and the disturbance in the electron transport process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The agricultural lands are under a big threat of getting polluted with heavy metals and metalloids such as cadmium (Cd), zinc (Zn), arsenic (As), copper (Cu) and lead (Pb) due to the mismanaged agricultural practices, industrial waste disposal, smelting and mining (Anjum et al. 2015). This heavy metal contamination is a growing concern of the human population due to their biomagnification potential in plants and subsequent transfer to humans. Biomagnification potential is defined as the ability of a xenobiotic compound to progressively increase its concentration through the different trophic level of a food chain (Barwick and Maher, 2003). Among these metals, Cd and Zn are potentially toxic to plants and animals because of their increased bioavailability.
Oryza sativa and Zea mays are widely grown cereal crops and more than half of the world population depends on these crops for daily consumption. In the present scenario, efforts are being taken to enhance the global production of these crops to cope up with the demand of the increasing population (Kong et al. 2018). At the same time, many reports have indicated to an alarming concentration of heavy metals in the arable lands and the farmers are forced to use these contaminated lands to increase the crop production (Kong et al. 2018; Mishra and Mishra 2018). So an examination in the HM tolerance level of these two crops is really needed.
In maize and rice, Cd and Zn toxicity induce many physiological, biochemical and morphological alterations that affect normal metabolism, especially photosynthesis (Chao et al. 2010). Cd can induce a reduction in light use efficiency and photodamage avoidance ability and it significantly damages the photosystem I and II (Song et al. 2019) Elevated Zn concentrations also affect the photosystem II (PSII) efficiency and electron transport in noncyclic photophosphorylation (Szopiński et al. 2019). As per the previous reports, Cd and Zn can damage the water-splitting complex and thus reduce net electron flux to linear electron transport (Szopiński et al. 2019). The oxidative stress caused by the overproduction of reactive oxygen species (ROS) is a major threat to photosynthesis (Kanu et al. 2017). The major source of ROS in plants is photosynthetic dissipation (dissipation of excess excitation energy absorbed by chlorophyll through various pathways within the chloroplasts) in the electron transport chain (ETC) (Björkman and Demmig-Adams 1995; Moradi and Ismail 2007). Cd induces outburst of superoxide (O2·−), hydrogen peroxide (H2O2) and hydroxyl radical (HO·−) and subsequent membrane degradation, while Cd and Zn induce structural and functional modifications of the photosynthetic unit (Paunov et al. 2018). Replacement of divalent metals ions (Mg2+ and Mn2+) involved in photosynthesis by Cd and Zn affects the chlorophyll biosynthesis and water oxidizing system of PSII (Küpper et al. 2002). Cd and Zn also cause a reduction in the activity of RuBisCO (Ribulose-1,5-bisphosphate carboxylase/oxygenase), which reflects in the net carbohydrate production of the plant.
Chlorophyll a fluorescence analysis has been applied by many scientists to study the effects of heavy metals on plants. The analysis of transient induction curves and JIP-test parameters, gives informations related to the changes in the structural and functional efficiency of the photosynthetic apparatus (Bussotti et al. 2010). The output of studies using chlorophyll a fluorescence has proved the capability of these metals to damage the photosynthetic machinery (Paunov et al. 2018; Szopiński et al. 2019).
The present study compares the photosynthetic responses of Zea mays and Oryza sativa towards Cd and Zn toxicity. This should help to quickly analyze the tolerance potential of these important crops towards these metal stresses. Further, this study also analyses the correlation between photosynthetic efficiency, ROS production and antioxidant defence of two major crop plants on exposure to Cd and Zn toxicity.
Materials and methods
Plant materials and growth conditions
Maize (variety CoHM 6) seeds were collected from Centre for Plant Breeding and Genetics, Department of millets, Tamil Nadu Agriculture University (TNAU), Coimbatore, India. Rice (variety Varsha) seeds were collected from Regional Agricultural Research Station (RARS) of Kerala Agricultural University, Pattambi, Kerala, India.
Maize and rice seeds were surface sterilized with 0.1% HgC12 (w/v) solution for 5 min and placed at 8 cm below the sterilized soil (soil: sand in 1:1) filled in polythene bags (18 × 13 cm). Soil sterilization was done according to the method of Raj and Sharma (2009). These polythene bags were kept in polyhouse maintained at 60 ± 2% relative humidity, 25 ± 2 °C temperature and daylight (12 h light and 12 h dark) of 800 ± 100 μmol/m2/s. Three sets (5 plants per each set) of rice and maize plants were maintained for each treatment, i.e. from each set, three healthy plants were chosen and considered one as control, second and third for CdCl2 and ZnSO4 treatment, respectively. After 45 days of growth, plants were treated with 40 mL (field capacity of the soil) solutions of 1.95 g Zn Kg−1 soil as ZnSO4 and 0.45 g Cd Kg−1 soil as CdCl2 (1.95 g Zn Kg−1 soil and 0.45 g Cd Kg−1 soil were selected as stress-imparting concentrations because these concentrations caused ~ 50% growth reduction in both plants on 8 days of exposure and 50% growth reduction was determined based on the enhancement in the MDA content and reduction in the total chlorophyll and tissue moisture content). The second lower healthy leaves of the maize and rice plants were taken for various analyses on 8 days of the treatment.
Analysis of photosynthetic pigment content and chlorophyll a fluorescence parameters
Chlorophyll a and b and total chlorophyll were estimated (Arnon 1949) with carotenoid content (Lichtenthaler and Wellburn 1983) in rice and maize leaves using 80% acetone solution.
Chl a fluorescence analysis was performed using Plant Efficiency Analyzer (Handy PEA; Hansatech Ltd., King’s Lynn, Norfolk, UK), which is a portable fluorometer having high resolutions (Strasser et al. 2004). All measurements were performed on the upper surfaces of the second lower leaves of rice and maize after dark adaptation for 20 min using the light exclusion clips and later by illuminating with a continuous red light of high intensity (1500 µmol m−2·s−1). All the measurements were recorded up to 1 s and the average values from 30 measurements recorded on the second lower leaves of the plant for each treatment were used for the analysis. The radar plot and energy pipeline model was deduced using the Biolyzer HP3 software (Chl a fluorescence analyzing program by Bioenergetics Laboratory, University of Geneva, Switzerland).
Different parameters are used to analyse the photosynthetic efficiency of rice and maize such as FO (minimal fluorescence/First step of chl a fluorescence transient), FJ (intermediate step in the chl a fluorescence transient at 2 ms), FI (intermediate step in the chl a fluorescence transient at 30 ms), FM (maximal fluorescence level/ final step of chl a fluorescence transient), K-peak (intermediate step in the chl a fluorescence transient at 0.3 ms), Area (area above the fluorescence induction curve) and tFM (time taken to reach FM). Other important JIP parameters selected for the study are FV (maximal variable fluorescence (FM-FO)), FV/FM (maximum quantum yield of PSII), FV/FO (maximum efficiency of water splitting complex), SM (multiple turnover of QA reductions), N (turn over number of QA), SFI ABS (an indicator of PS II structure and functioning), PI ABS (performance index of PS II on absorption basis), PI TOTAL (performance index of electron flux to the final PS I electron acceptors), RC/CSM (density of active PS II reaction centers per cross section) and DFABS (PSII-relative driving force index on an absorption basis). The yield parameters mentioned in the present study include the following; φPo (maximum quantum yield of primary PSII photochemistry), φ (Do) quantum yield of energy dissipation, φ(Eo) (quantum yield (at t = 0) for electron transport from QA− to plastoquinone) and ψo (probability that a trapped exciton moves an electron into the electron transport chain beyond QA).
Specific energy fluxes such as ABS/RC (absorption flux per RC corresponding directly to its apparent antenna size), TRo/RC (trapping flux leading to QA reduction per RC at t = 0), ETo/RC (electron transport flux from QA− to plastoquinone per RC at t = 0) and DIo/RC (dissipated energy flux per RC at the initial moment of the measurement) were also analysed in this the study. ABS/CSm (absorption of energy per excited cross-section approximated by FM), TRo/CSm (excitation energy flux trapped by PSII of a photosynthesizing sample cross-section approximated by FM), ETo/CSm (electron flux transported by PSII of a photosynthesizing sample cross-section approximated by FM) and DIo/CSm (heat dissipation of excitation energy by PSII of a photosynthesizing sample cross-section approximated by FM) were the phenomenological energy fluxes scrutinized during Cd and Zn stress (Strasser et al. 2004; Tóth et al. 2007; Kalaji et al. 2011; de Carvalho et al. 2016; Paunov et al. 2018).
Analysis of PSI and PSII activity
The photosynthetic O2 evolution and consumption were recorded polarographically by a Clark-type oxygen electrode system (Hansatech). The thylakoids were isolated from the leaves of rice and maize plants exposed to CdCl2 and ZnSO4 and subsequently, PSI as well as PSII activities were measured according to the procedure of Mirshad and Puthur (2016).
Isolation of thylakoid membranes
500 mg of fresh leaf tissue was homogenized in 6 mL of ice-cold isolation buffer (pH 7.8) containing 400 mM sucrose, 20 mM tricine and 10 mM NaCl with a chilled mortar and pestle. The resulting homogenate was filtered through six layers of cheese cloth and the filtrate was centrifuged at 5000 rpm for 6 min at 4 °C. The supernatant was discarded and the remaining pellet was suspended in 250 µL suspension buffer (pH 7.5) containing 10 mM NaCl, 20 mM HEPES [N-(2-Hydroxyethyl) piperazine- N-(2-Ethanesulphonic acid)], 100 mM sucrose and 2 mM MgCl2 and was transferred to a clean test tube. This thylakoid suspension was stored at 4 °C.
Analysis of thylakoid electron transport activities
PSI activity was measured in terms of oxygen consumption by using artificial electron donor 2, 6-dichlorophenolindophenol (DCPIP) and methyl viologen (MV) as the exogenous electron acceptor. The reaction mixture contained reaction buffer, ascorbate (600 μM), methyl viologen (MV) (500 μM), sodium azide (NaN3) (1 mM), 3- (3, 4-dichlorophenyl)-1, 1-dimethyl urea (DCMU) (5 μM) and DCPIP (0.1 mM). Chloroplast suspension equivalent to 20 μg chlorophyll was added and the volume was made up to 2 mL with reaction buffer. PSII activity was measured in terms of oxygen evolution by using pBQ (phenyl-p-benzoquinone) as the artificial electron acceptor. The reaction mixture consisting of reaction buffer, pBQ (500 μM), and chloroplast suspension equivalent to 20 μg chlorophyll was finally made up to 2 mL with reaction buffer. The chlorophyll content of the thylakoid samples was estimated according to the method of Arnon (1949).
ROS quantification
Estimation of superoxide
Superoxide content in the leaves of rice and maize plants was estimated and sodium nitrate was used as the standard (Doke 1983). 200 mg of leaf sample was cut into 1 × 1 mm size and immersed in 0.01 M potassium phosphate buffer (pH 7.8) containing 0.05% NBT and 10 mM NaN3. The mixture was kept in a water bath (85 °C) for 15 min. After incubation, the mixture was quickly transferred to the ice bath for lowering the temperature. After cooling, the absorbance of the mixture was measured at 580 nm.
Estimation of hydrogen peroxide
Hydrogen peroxide content in leaves of rice and maize plants was estimated as described by Junglee et al. (2014). 200 mg of tissue was weighed and homogenized in 5 ml of 0.1% ice cold TCA. The homogenate was centrifuged at 12,000 rpm for 15 min. 500 mL of the supernatant was mixed with 0.5 ml of potassium phosphate buffer (pH 7), to which 1 mL of 1 M potassium iodide was added. The absorbance of the mixture was measured at 390 nm. Hydrogen peroxide was used as standard.
Estimation of malondialdehyde (MDA)
Malondialdehyde (MDA) content was estimated as described by Heath and Packer (1968). 200 mg of leaf tissue was weighed and homogenized in 5 ml of 5% trichloro acetic acid (TCA). The homogenate was centrifuged at 12,000 rpm for 15 min. The supernatant was collected and used for the estimation of MDA. 2 mL of the supernatant was mixed with an equal aliquot of 0.5% of thiobarbituric acid (TBA) in 20% TCA. The solution was heated at 95 °C for 24 min, cooled and then centrifuged at 3000 rpm for 2 min. The absorbance of the supernatant was measured at 532 and 600 nm and then the MDA content was calculated using its extinction coefficient of 155 mM−1 cm−1.
Estimation of ascorbate content
500 mg of leaf tissue was homogenized with 5 ml of 5% (w/v) TCA and centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatant was collected and the estimation of ASA content was done according to the method of Chen and Wang (2002).
Ascorbate peroxidase (APX) activity assay
APX activity was assayed by the method of Nakano and Asada (1981) with slight modifications. The 3-ml assay system consisted of 0.5 mM ascorbate and 0.1 mM EDTA in 50 mM sodium phosphate buffer (pH 7.0). Twenty μL of enzyme extract was added to the buffer, and the enzyme reaction was initiated by adding 10 μL of 100 mM H2O2 to reach a concentration of 0.1 mM H2O2 in the final reaction mixture. H2O2-dependent oxidation of ascorbate was followed by monitoring the decrease in absorbance at 290 nm.
Quantitative estimation of Zn, Cd and Mg
Samples for quantifying Zn, Cd and Mgwere prepared according to the method of Allan (1969) and were analysed using atomic absorption spectrophotometer. Leaf tissues of treatments and control were sampled and dried at 60 °C in a hot air oven. Known weights of the dried samples were digested by refluxing in a mixture of nitric acid and perchloric acid in the ratio of 10:4 until the solution became colourless using Kjeldahl’s flask heated in a heating mantle. Then the digest was filtered and further transferred to a standard flask and the volume was made up to 50 mL and stored in screw-capped containers. Atomic absorption spectrophotometer (Shimadzu AA-7000, Kyoto, Japan) was used for the estimation of heavy metals present in the digested samples. Certified standard reference materials (Merck) were used to verify the obtained values.
Statistical analysis
Statistical analyses of the results were carried out according to the Duncan test at 5% probability level. Values of each experiments were expressed as mean ± SE and the data are an average of nine recordings. One-way ANOVA was applied using the SPSS software (Version 16.0, SPSS Inc., Chicago, IL, USA). Pearson’s correlation analysis was performed to evaluate the relationships between the most important variables.
Results
Chlorophylls and carotenoids content
Maize and rice seedlings subjected to CdCl2 and ZnSO4 exhibited a reduction in chlorophyll, as well as carotenoids contents (Table 1). The reduction in total chlorophyll content was 61–66% in maize subjected to CdCl2 and ZnSO4, but it was only 15–17% in rice. Similarly, the reduction in carotenoid content was also higher in maize (35–36%) than in rice (9%) as compared to the respective controls.
Chlorophyll a fluorescence induction curve and JIP parameters
The modifications in the light-dependent photosynthetic process of rice and maize under Zn and Cd toxicity were observed in the shape of induction curves of chlorophyll fluorescence (Fig. 1). Both metals did not affect the standard shape of OJIP chlorophyll a fluorescence transient curves in rice plants significantly (Fig. 1a). However, Cd caused a change in the OJIP curve of maize as compared to the control plants (Fig. 1b).
Transient curves of chlorophyll a fluorescence and relative variable chlorophyll a fluorescence and differential curve in rice and maize exposed to CdCl2 and ZnSO4 stress. Transient curves of rice (A) and maize (B); induction curve of relative variable chlorophyll fluorescence (Vt) resulting from the double normalization of the values in (A and B) to the FO and FM in rice (C) and maize (D); Differential curves of rice (E) and maize (F)
The transient of the double normalized fluorescent signal showed an initial rise in maize and rice subjected to both metal treatments and the peak was prominent in CdCl2 treated maize leaves (Fig. 1D). The bands of variable chlorophyll fluorescence at 2 ms (VJ) and variable chlorophyll fluorescence at 30 ms (VI) were increased in both treatments as compared to the control plants and this enhancement was higher in CdCl2 treated maize leaves and ZnSO4 treated rice leaves. The differential curve represents a strong positive peak in FO–FJ (FO-initial fluorescence, FJ-chlorophyll fluorescence transient at 2 ms) phase in rice subjected to Cd stress (Fig. 1e). On the contrary, the highest positive peak was observed after Zn treatments in maize (Fig. 1f). The K-band was observed in the differential induction curve and it was prominent in Zn- treated leaves; even though the intensity was different in rice and maize, the shape was the same in both plants. But only in the case of maize (Fig. 1f), a strong K-peak was exhibited on CdCl2 treatment but not in rice. Negative peaks in FI–FP (FI -chlorophyll fluorescence transient at 30 ms, FP-maximal fluorescence) phase was also observed in both Cd and Zn treatments. A shoulder peak in the FO–FJ region was evidently seen in the differential curve of Zn treated rice (Fig. 1e) and maize plants (Fig. 1f).
Pronounced changes during CdCl2 and ZnSO4 treatments were also observed in the radar plot representing different parameters including yield parameters and performance index (Fig. 2). However, the most obvious negative influence was observed in the case of maize plants after CdCl2 treatment where the reduction in DF (driving force) was prominent (Fig. 2b). The relative pool size of plastoquinone was determined by the parameter SM and it was observed that by the influence of both metals, the plastoquinone pool in rice (Fig. 2a) as well as in maize (Fig. 2b) was reduced.
Radar plots of different JIP parameters deduced from chlorophyll a fluorescence induction curves of O. sativa (A) and Z. mays (B) exposed to CdCl2 and ZnSO4 stress and all values are shown as percent of control. The green, red, and yellow lines represent control, CdCl2 and ZnSO4 treated plants, respectively
Reduction in area above the induction curve and maximum efficiency of water splitting complex (FV/FO) was higher in ZnSO4 treated rice plants as compared to CdCl2 treated rice plants (Fig. 2a). However, a drastic reduction in these two parameters was observed in CdCl2 treated maize plants as compared to ZnSO4 treated maize plants (Fig. 2b). The decrease in tFM (time taken to reach FM) and Kn (turn over number of QA) were visualized in the radar plot of these parameters in CdCl2 and ZnSO4 treated rice and maize plants. Yield parameters also showed significant variations during CdCl2 treatment and prominent variations were observed in φPo, φ(Eo) φ(Do) and ψo. Of these, φPo, φ(Eo) and ψo were decreased, but φ(Do) increased in both plants during CdCl2 and ZnSO4 treatments. All these modifications in the yield parameters were predominantly observed in the CdCl2 treated maize plants as compared to rice plants (Fig. 2a, b).
Analysis of these parameters revealed that CdCl2 affects maize plants photosynthetic efficiency more than ZnSO4. On the contrary, photosynthetic performance of rice plants subjected to ZnSO4 was more affected than CdCl2 (Table 2). This was confirmed from the results represented in Fig. 2b.
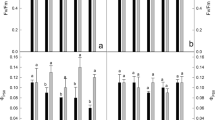
Phenomenological energy pipeline models help to visualize the intensity of Cd- and Zn-induced modifications and it also interprets the stepwise flow of energy through PSII at the reaction centre (RC) level (ABS/RC, TR/RC, ET/RC, and DI/RC) as well as at the level of a cross-section (CS) (ABS/CSM, TRo/CSM, ETo/CSM, and DIo/CSM). Here the RC represents photochemically active reaction centre of PSII that can reduce QA, whereas the CS indicates the surface of the excited photosynthetic sample, so the latter includes the photosynthetic response of both active and inactive reaction centres. The phenomenological energy flux parameters such as ABS/CSM, TRo/CSM, ETo/CSM and DIo/CSM were lower in the plants exposed to CdCl2 and ZnSO4 stress (Figs. 3 and 4), but a drastic reduction of these parameters was observed in maize (Fig. 3) as compared to the respective treatments of rice (Fig. 4). The closure of reaction centres was represented as dark circles and it was maximum in maize leaves exposed to Cd as compared to other treatments (Fig. 4c).
Leaf pipeline model showing the proportion of phenomenological energy flux parameters (calculated per cross section approximated by maximal fluorescence, CSM and per reaction centre, RC) of the heavy metal treated rice plant (A and B,control; C and D, CdCl2; and E and F, ZnSO4 treated plants). The width of the arrow represents the relative values of each parameter; empty and dark circles represent active and non active reaction centers, respectively
Leaf pipeline model showing the proportion of phenomenological energy flux parameters (calculated per cross section approximated by maximal fluorescence, CSM and per reaction centre, RC) of the heavy metal treated maize plant (A and B, control; C and D, CdCl2; and E and F, ZnSO4 treated plants). The width of the arrow represents the relative values of each parameter; empty and dark circles represent active and non active reaction centers, respectively
The specific membrane model indicates the response of active (QA-reducing PSII) reaction centres towards metal stress and ABS/RC, TRo/RC, and ETo/RC reduced in CdCl2 and ZnSO4 treated plants, but DIo/RC was increased in maize plants subjected to CdCl2 and ZnSO4 treatments (Fig. 3d, f). These changes were most prominent in CdCl2 treated maize plants (Fig. 4d), but insignificant in rice plants (Fig. 3d).
Activity of photosystems (PSI and PSII)
The results related to the activity of both photosystems (PSI and PSII) supported the data obtained from chlorophyll fluorescence analysis. Both PSI and PSII activities were severely affected by Cd and Zn toxicity, and a prominent reduction was observed in maize (40–65%) as compared to the control, at the same time the reduction was only 17–40% in rice plants (Fig. 5a, b).
ROS accumulation
ROS accumulation and membrane degradation were significantly increased during CdCl2 and ZnSO4 treatments as compared to the control seedlings of rice as well as maize. The increase in O2− content of rice was 98 and 67% under CdCl2 and ZnSO4 stress, respectively, but it was 73 and 54% in maize (Fig. 6a). H2O2 showed a drastic increase (91 and 134% under Cd and Zn toxicity respectively) in maize, and the increase was 11 and 62% in rice under Cd and Zn toxicity (Fig. 6b). As compared to Zn, Cd toxicity leads to the accumulation of ROS (O2− as well as H2O2) in rice and maize. The O2− content was higher in rice, but H2O2 content was higher in maize under Cd and Zn toxicity (Fig. 6a, b).
An insignificant negative correlation was observed between ROS (O2− and H2O2 content) and PIABS (R2 = − 0.830 and − 0.828, respectively) in CdCl2 and ZnSO4 treated rice plants. MDA content of the leaf tissue also showed a significant increment when rice and maize seedlings were subjected to CdCl2 and ZnSO4 and an extreme increase (419%) was observed in ZnSO4 treated maize plants (Fig. 6c). An insignificant negative correlation was observed between MDA content and PIABS (R2 = − 0.334) in CdCl2 and ZnSO4 treated maize plants (Supplementary data Table 2).
Ascorbate accumulation and APX activity
Cd and Zn toxicity increased ascorbate content of rice and maize plants (Fig. 7). The increase was prominent in maize plants (threefold) under CdCl2 and ZnSO4 stress. But, the increase was only 73% in rice plants exposed to CdCl2 and was insignificant in ZnSO4 treated rice plants. Simultaneously, an increase was observed in APX activity. The increase was 172 and 29% in rice plants exposed to CdCl2 and ZnSO4 stress, respectively, but it was 191% in maize plants exposed to CdCl2 stress and insignificant in ZnSO4 treated maize plants.
Cd, Zn and Mg accumulation
Under the exposure of heavy metal stress, rice plants showed higher accumulation of Cd (0.748 ± 0.05 mg g−1d.w) and Zn (0.366 ± 0.04 mg g−1d.w) as compared to the Cd (0.609 ± 0.077 mg g−1d.w) and Zn (0.122 ± 0.073 mg g−1d.w) accumulation in maize leaves (Table 3). In maize, the Mg accumulation showed an 82% increase under Cd stress and it was 30% under Zn stress. But rice did not exhibit a significant modification in Mg content as compared to the control. A significant positive correlation was observed between the leaf Mg to Cd (R2 = − 0.974) and Zn (R2 = − 0.958) content in maize plants, but there was no significant correlation in rice (Supplementary data Table 3).
Discussion
Both heavy metals, Cd and Zn affects the integrity of photosynthetic apparatus and reduce the rate of photochemical reactions by inducing the degradation of chlorophyll molecules in rice and maize as reported in Cucumis sativus (Feng et al. 2010), Trigonella foenum-graecum (Bashri and Prasad 2015), Elsholtzia argyi (Li et al. 2015) and Triticum aestivum (Ci et al. 2010). The reduction in total chlorophyll content in rice and maize under Cd and Zn toxicity can be attributed to the reduction in the rate of chlorophyll biosynthesis, due to the metal induced inhibition of protochlorophyllide reductase and δ-aminolevulinic acid dehydrogenase (Kasim, 2007; Rana, 2015). As compared to rice, a drastic reduction in total chlorophyll content and carotenoids was observed in the maize leaves on the exposure to CdCl2 and ZnSO4 causing photosynthetic inefficiency and was one of the prime reasons for decreased tolerance of maize towards these metals.
The antenna complex gets affected with elevated metal concentrations and it was reflected as delays in energy migration to the reaction centres. ABS/RC and ABS/CSM are the two important parameters indicating the efficiency of antennae complexes and reduced values of both these parameters in rice and maize points towards Cd- and Zn-induced disturbances in the antennae complexes. Similar to our results, Arabidopsis arenosa and Arabidopsis halleri showed Cd- and Zn-induced reduction in absorption flux (Szopiński et al. 2019). The very distinguishable reduction in the efficiency of antennae complexes in maize acts as the primary limiting factor for successful photosynthesis and for this reason, it is considered as highly vulnerable to Cd and Zn stress. Initial fluorescence (FO), represents the yield of fluorescence when all reaction centres are open or oxidised and it was reduced under Cd toxicity due to the degradation of chlorophyll molecules (Vassilev and Manolov 1999). As the results show, the reduction of FO in CdCl2 treated maize leaves could be considered as an indicator for the susceptibility of the same towards Cd and the minimal difference in FO value of rice with that of control indicates the better adaptation of this plant with Cd. The reduction in FM could also be correlated to the reduced chlorophyll a concentration under Cd and Zn exposure (Paunov et al. 2018). In the differential curves, a prominent K-peak was found in the OJ rise of rice and maize. The presence of positive K-peak in the maize plants under Cd and Zn stress indicates a downfall in the electron donation from OEC (oxygen-evolving complex) to the oxidized RC. At the same time, in the differential curve of rice exposed to ZnSO4, K-peak was not visualized, which indicates higher stability of OEC in this plant under Zn toxicity. Yusaf et al. (2010) reported the development of K-peak in Brassica juncea under the exposure of Cd ions and this K-peak can be used as the tool to analyse the metal-induced inactivation of the donor side of PSII RCs (Strasser 1997; Yusaf et al. 2010). Significantly higher reduction of Fv/Fo in maize plants over rice plants indicates the higher functional impairment the activity of the water-splitting complex on the donor side of PS II. Similarly, Fv/Fo was reduced in Sedum alfredii and Sorghum-sudangrass hybrid under Cd and Zn stress, respectively (Zhou and Qiu 2005; Oh and Koh 2016). Cd- and Zn-induced damage to the water-splitting complex of maize plants was reflected in the reduced PSII activity, seen as reduced evolution of oxygen.
The efficiency of PSII RC is one of the important factors determining the efficiency of electron flow in noncyclic photophosphorylation. The response of rice and maize plants towards Cd and Zn was seen to cause a prominent reduction in RC/CSM and it was similar to that observed in durum wheat (Paunov et al. 2018). When comparing the reduction in the RC/CSM, it was very high in CdCl2 treated maize plants indicating a very low level of tolerance as compared to rice. In the case of rice, reduction in RC was prominent in ZnSO4 treated plants and the impairment of photosynthesis was also significant in ZnSO4 treated plants as compared to the CdCl2 treated plants. FV/FM, and PIABS were also reduced in metal treated maize and rice leaves, indicating that the efficiency and charge separation capacity of PSII was affected by the high concentration of metal ions. The vulnerability of maize plants towards Cd and Zn was higher as compared to rice and it was deduced from the higher reduction of FV/FM and PIABS in maize plants. Metal toxicity-induced reduction in FV/FM and PIABS was observed in Phaseolus vulgaris (Wael et al. 2015), durum wheat (Paunov et al. 2018), Helianthus annuus (Azevedo et al. 2005) and Brassica juncea (Yusuf et al. 2010). DF indicates the importance of individual components in driving the processes in PSII. The drastic reduction in the DF of CdCl2 treated maize plants was mainly due to the reduction in the partial driving force for the conversion of excitation energy required for the electron transport beyond QA (Krüger et al. 2014). It indicates the higher vulnerability of maize towards Cd, and the lower reduction of the same parameter in rice can be attributed to the Cd and Zn tolerance potential of the crops. In oxygen evolution experiments, the reduction in PSII activity observed in maize and rice plants under metal exposure attributed to the inefficiency of OEC, inhibition in electron transport and structural damage to PSII due to the elevated metal ions. The observations support the findings of the study conducted in barley plants exposed to Cd stress (42 mg kg−1) (Vassilev et al. 2004). The higher level of Cd and Zn stress tolerance of rice was prominently observed in the results of PSII activity, where the Cd- and Zn-induced reduction in PSII activity was lesser than that of the maize.
The efficiency of electron migration depends on the rate of oxidation and reduction of plastoquinone. FJ depends on the availability of oxidized PQ-molecules bound to the QB-site of PSII and this could be also utilized to quantify the oxidoreduction state of QA and QB (Zhu et al. 2005; Tóth et al. 2007). In the present study, FI and FP were decreased in CdCl2 and ZnSO4 treated rice and maize plants which correspond to the state of QA−QB2− and QA−QB2−/PQH2, respectively. But FJ (corresponds to QA−QB/QA−QB− state) showed some species-specific response towards Cd, and it was reduced in maize and increased in rice as compared to the respective controls. This reduction in the FJ value denotes the Cd-induced degradation of plastoquinone pool and OEC and thus the primary photochemical events of maize plants were extremely sensitive to the Cd toxicity as compared to the rice. A closed reaction centre in the phenomenological energy pipeline model represents a PSII reaction centre in which the associated QA is reduced and the density of closed reaction centres was maximum in the maize leaves treated with Cd which indicates the permanent loss in function of the QA.
The efficiency of the acceptor side of PSII molecule enhances with an enhancement in the turnover number (N), but Cd and Zn cause a reduction of the same. This reduction in turnover number possibly indicates fewer exciting electrons (Sayyad-Amin et al. 2014). CdCl2 treated rice plants keep up the efficiency of electron migration by ensuring the turnover number same as that of the control, but the inefficiency of maize to maintain the turn over number makes it more sensitive towards Cd and Zn. The reduction in φEo value and ETo/RC also represents the Cd- and Zn-induced impairment of e− transfer from QA− to plastoquinone.
The total e− transfer efficiency of ETC was represented as PITOTAL, it takes into account the capacity of PSI as well as PS II (Yusuf et al. 2010). A reduction of this parameter indicates a loss in the ability of energy conservation as observed in the Cd-affected maize and rice plants as well as in Zn-affected rice plants. Previous studies showed related results in T. foenum-graecum due to Cd stress (Bashri and Prasad 2015) and the insignificant reduction of all these parameters in rice under CdCl2 stress is an implication of the high level of tolerance of rice towards Cd.
The polarographic measurements corresponding to the O2 consumption indicates a reduction in the activity of PSI under Cd and Zn toxicity in maize and rice. Similar results were observed in the experiments with barley plants exposed to different concentrations of Cd (Vassilev et al. 2004). The prominent reduction in PSI activity observed in maize plants exposed to CdCl2 and ZnSO4 was again indicative of the low metal tolerance potential of maize as compared to rice.
The negative peaks observed in the IP phase of maize and rice directly correlate to the reduction in the density of PSI in both CdCl2 and ZnSO4 treatments (Schansker et al. 2003). More than this, the negative peak in the IP phase indicates a high affinity of electron acceptors due to the inefficiency of electron transport from PSII to PSI (Ceppi et al. 2012; Paunov et al. 2018). It also represents the high sensitivity of the PSI reaction centre of maize plants towards Cd, where the IP phase exhibited a dramatic reduction. δ(Ro), which indicates the rate of electron migration to the acceptor side of PSI, showed species-specific response which was increased in maize under both metal treatment but an insignificant reduction was observed in the case of rice. It shows a metal-induced variation in the intersystem electron transport and also the reduction of terminal electron acceptor (NADP). tFM has a strong correlation with the PSII/PSI ratio and the size of the PSI acceptor side pool (Kalaji et al. 2017). This parameter was increased in ZnSO4 treated maize plants, but it was decreased in CdCl2 treated rice and maize plants as well as in ZnSO4 treated rice plants. The reduction in FM and an associated reduction in tFM was reported in Solanum tuberosum as observed in this study (Olechowicz et al. 2018). In the present study, Cd- and Zn-induced a reduction in the area of induction curves of rice and maize plants which indicates changes in the redox state of ETC or changes in the stoichiometry of PSII and PSI acceptor side (Schansker et al. 2011; Kalaji et al. 2016, 2017). The reduction in the efficiency of electron transfer was predominantly observed in CdCl2 treated maize and ZnSO4 treated rice plants which had a negative impact on the rate of photosynthesis.
The permanent inactivation of RCs was also high in CdCl2 treated maize as compared to CdCl2 and ZnSO4 treated rice plants The reduction of ABS/CSM, TRo/CSM, and ETo/CSM in maize and rice was related to the increased density of inactive reaction centres and the inefficiency of PSII and it strongly indicates the vulnerability of the plant towards elevated Cd and Zn content. Dissipation was decreased when it was analysed in CS, but at the same time, it was increased per active RC in CdCl2 treated maize plants indicating its high sensitivity. The increase in the DIo/RC was associated with increased dissipation of absorbed light as heat which specified the inefficiency in the utilization of energy (Kalaji et al. 2011; Faseela et al. 2019). But, the reduction in the DIo/CSM is proportional to the reduction in the absorption due to the increase in the inactive RCs and this phenomenon was also prominently observed in maize plants (Zushi et al. 2012). The percentage reduction or enhancement in OJIP parameters was represented as Supplementary Table 1.
Of the different JIP parameters, area, FV/FO, FV/FM and RC/CSM showed significant changes and could be used to assess the intensity of stress as well as the tolerance potential of rice as well as maize under Cd and Zn toxicity. At the same time, the development of K peak in the differential curve is species-dependent, which corresponds to the differential response of rice and maize towards these metal toxicity.
The generation of oxygen radicals in photosynthesis is a normal part of plant metabolism, but the overproduction of ROS because of Cd and Zn stress may cause oxidation of D1 protein and entire PSII protein damage (Marshall et al. 2002). The higher O2− in rice indicates the inefficiency in the transfer of absorbed energy to PSII. The over-accumulation of H2O2 in maize was due to the inadequate activity of peroxidase and this accumulated H2O2 further reduced the rate of photosynthesis by inhibiting the electron transport (Samuilov et al. 2001). H2O2 induced inhibition in the electron transfer was studied in cells of Anabaena variabilis and found that H2O2 potentially reduces the photosynthetic O2 evolution and electron transport (Samuilov et al. 2001). The outbreak of ROS was predominantly observed in maize as compared to rice and it points to the low Cd and Zn tolerance potential of maize and also the inefficiency of photosynthesis. O2− overproduction showed a strong negative correlation with FV/FM in rice and maize indicating the greater impact of this ROS in photosynthesis. Moreover, H2O2 overproduced in maize plants caused a high reduction in photosynthesis and it may be due to the formation of OH•. H2O2 reacts with O2¯ to form more reactive OH• in the presence of trace amount of Fe or Cu and this OH• initiates self-propagating reactions leading to degradation of lipids and proteins which finally leads to the structural deformation of chloroplast (Subba et al. 2014).
Apart from this, ROS also elicit the peroxidation of lipid and from the present study, it was evident in the elevated MDA (product of lipid peroxidation) content in maize and rice (Pospíšil et al. 2016). Similar to these results, Zn-induced accumulation of ROS and MDA was reported in Citrus reticulate (Subba et al. 2014). The strong negative correlation observed between the photosynthetic efficiency (FV/FM) and MDA content in rice content indicates the crucial role of membrane stability in photosynthesis.
The antioxidant defence system of plants was also elicited due to the overaccumulation of ROS under Cd and Zn stress. Increase in the ascorbate is a strong indicator of stress as its biosynthesis depends on the quantity ROS in the cell (Bielen et al. 2013; Roy et al. 2017). Here, maize showed a drastic increase in ascorbate content which indicates a higher imbalance in ROS production and scavenging. But, maize plants under Cd stress showed a prominent increase in the activity of APX, but the higher accumulation of H2O2 and ascorbate together indicates the inadequate activity of APX in maize to overcome the oxidative stress induced by Cd (Sofo et al. 2015).
Cd and Zn accumulation potential of rice and maize was reported earlier (Saengwilai et al. 2017) and in this study too both Cd and Zn content was higher in the leaves of rice plants as compared to maize plants The increase in the metal uptake of rice was due to the enhanced phloem and xylem transportation with the aid of more virulent transporters than maize (Uraguchi et al. 2009). Even then, the rice leaves maintained the photosynthetic efficiency better than maize plants under Cd and Zn stress indicating the high tolerance level of the rice. Mg has different roles in photosynthesis; it acts as the structural component of chlorophyll, activates different enzymes involved in CO2 fixation and exports carbohydrates from source to sink sites (Yang et al. 2012). In the present study, leaf Mg content increased with an increase in Cd and Zn content in maize leaves and it indicates that elevated concentration of Cd and Zn stimulated the Mg absorption and translocation to leaves. The results clearly implicate a co-uptake mechanism of Cd/Zn along with Mg. Therefore, the Mg deficiency was not the reason for the elevated impairment of photosynthesis in maize over rice under Cd and Zn stress. But in rice, a significant increase in Mg content was not found. Similarly, earlier reports pointed out that Mg content in rice showed positive and non-significant correlation with Cd and Zn stress (Liu et al. 2003; Yu et al. 2010).
Conclusion
The differential responses in the chlorophyll a fluorescence of rice (variety Varsha) and maize (variety CoHM 6) towards Cd and Zn toxicity could be related to the level of metal tolerance of these crop plants. Therefore, this technique can be very well utilized to analyse the metal tolerance of crop plants by a noninvasive method. The potential of rice plants to maintain the photochemical events including the activity of oxygen-evolving complex and electron transport process under Zn and Cd stress enabled it to effectively encounter the heavy metal stresses more better than maize plants. Moreover, the limited ROS generation and an efficient antioxidant defence system improved the Cd and Zn tolerance level of rice plants as compared to maize plants.
Author contribution statement
EJ, JTP and HK designed the experimental setup, EJ performed the analysis, JTP and HK processed the experimental data and interpreted the results. EJ wrote the initial draft of the manuscript JTP and HK critically revised the manuscript. All authors read and approved it for submission.
References
Allan JE (1969) The preparation of agricultural samples for analysis by Atomic Absorption Spectrometry, Varian Techtron Bulletin SIS Edn. 12–69.
Anjum SA, Tanveer M, Hussain S, Bao M, Wang L, Khan I et al (2015) Cadmium toxicity in Maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ Sci Pollut Res 22:17022–17030
Arnon DI (1949) Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1
Azevedo H, Glória Pinto CG, Fernandes J, Loureiro S, Santos C (2005) Cadmium effects on sunflower growth and photosynthesis. J Plant Nutr 28:2211–2220
Barwick M, Maher W (2003) Biotransference and biomagnification of selenium copper, cadmium, zinc, arsenic and lead in a temperate seagrass ecosystem from Lake Macquarie Estuary, NSW, Australia. Mar Environ Res 56:471–502
Bashri G, Prasad SM (2015) Indole acetic acid modulates changes in growth, chlorophyll a fluorescence and antioxidant potential of Trigonella foenum-graecum L. grown under cadmium stress. Acta Physiol Plant 37:49
Bielen A, Remans T, Vangronsveld J, Cuypers A (2013) The influence of metal stress on the availability and redox state of ascorbate, and possible interference with its cellular functions. Int J Mol Sci 14:6382–6413
Björkman O, Demmig-Adams B (1995) Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. In: Schulze ED, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Heidelberg, pp 17–47
Bussotti F, Desotgiu R, Pollastrini M, Cascio C (2010) The JIP test: a tool to screen the capacity of plant adaptation to climate change. Scand J For Res 25:43–50
Ceppi MG, Oukarroum A, Çiçek N, Strasser RJ, Schansker G (2012) The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: a study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol Plant 144:277–288
Chao YY, Chen CY, Huang WD, Kao CH (2010) Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil 329:327–337
Chen JX, Wang XF (2002) Guide to plant physiological experiments. South China University of Technology Press, Guangzhou, pp 123–127
Ci D, Jiang D, Wollenweber B, Dai T, Jing Q, Cao W (2010) Cadmium stress in wheat seedlings: growth, cadmium accumulation and photosynthesis. Acta Physiol Plant 32:365–373
de Carvalho AC, Salvador JP, Pereira TDM, Ferreira PHA, Lira JCS, Veiga TA (2016) Fluorescence of chlorophyll a for discovering inhibitors of photosynthesis in plant extracts. Am J Plant Sci 7:1545
Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol 23:345–357
Faseela P, Sinisha AK, Brestič M, Puthur JT (2019) Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 57:108–115
Feng J, Shi Q, Wang X, Wei M, Yang F, Xu H (2010) Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci Hortic 123:521–530
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Junglee S, Urban L, Sallanon H, Lopez-Lauri F (2014) Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am J Analyt Chem 5:730
Kalaji HM, Bosa K, Kościelniak J, Żuk-Gołaszewska K (2011) Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. J Exp Bot 73:64–72
Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA et al (2016) Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant 38:102
Kalaji HM, Schansker G, Brestic M, Bussotti F, Calatayud A, Ferroni L et al (2017) Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth Res 132:13–66
Kanu AS, Ashraf U, Bangura A, Yang DM, Ngaujah AS, Tang X (2017) Cadmium (Cd) stress in rice; phyto-availability, toxic effects, and mitigation measures—a critical review. IOSR-JESTFT 11:07–23
Kasim WA (2007) Physiological consequences of structural and ultra-structural changes induced by Zn stress in Phaseolus vulgaris. I. Growth and photosynthetic apparatus. Int J Botany 3:15–22
Kong X, Liu T, Yu Z, Chen Z, Lei D, Wang Z et al (2018) Heavy metal bioaccumulation in rice from a high geological background area in Guizhou Province, China. Int J Environ Res Public Health 15:2281
Krüger GHJ, De Villiers MF, Strauss AJ, De Beer M, Van Heerden PDR, Maldonado R, Strasser RJ (2014) Inhibition of photosystem II activities in soybean (Glycine max) genotypes differing in chilling sensitivity. S Afr J Bot 95:85–96
Küpper H, Šetlík I, Spiller M, Küpper FC, Prášil O (2002) heavy metal-induced inhibition of photosynthesis: targets of in vivo heavy metal chlorophyll formation. J Phycol 38:429–441
Li S, Yang W, Yang T, Chen Y, Ni W (2015) Effects of cadmium stress on leaf chlorophyll fluorescence and photosynthesis of Elsholtzia argyi—a cadmium accumulating plant. Int J Phytoremed 17:85–92
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents.
Liu JG, Liang JS, Li KQ, Zhang ZJ, Yu BY, Lu XL et al (2003) Correlations between cadmium and mineral nutrients in absorption and accumulation in various genotypes of rice under cadmium stress. Chemosphere 52:1467–1473
Marshall JA, Hovenden M, Oda T, Hallegraeff GM (2002) Photosynthesis does influence superoxide production in the ichthyotoxic alga Chattonella marina (Raphidophyceae). J Plankton Res 24:1231–1236
Mirshad PP, Puthur JT (2016) Arbuscular mycorrhizal association enhances drought tolerance potential of promising bioenergy grass (Saccharum arundinaceum retz.). Environ Monit Assess 188:425
Mishra P, Mishra M (2018) Risk Assessment of heavy metal contamination in paddy soil, plants, and grains (Oryza sativa L.). In: Varma A (ed) Hashmi M, Zaffar. Environmental pollution of paddy soils, Springer, Cham, pp 165–178. https://doi.org/10.1007/978-3-319-93671-0
Moradi F, Ismail AM (2007) Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann Bot 99:1161–1173
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Oh S, Koh SC (2016) Growth, Photosynthesis and Zinc Elimination Capacity of a Sorghum-Sudangrass Hybrid under Zinc Stress. J Environ Sci Int 25:1143–1153
Olechowicz J, Chomontowski C, Olechowicz P, Pietkiewicz S, Jajoo A, Kalaji MH (2018) Impact of intraspecific competition on photosynthetic apparatus efficiency in potato (Solanum tuberosum) plants. Photosynthetica 56:971–975
Paunov M, Koleva L, Vassilev A, Vangronsveld J, Goltsev V (2018) Effects of different metals on photosynthesis: Cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int J Mol Sci 19:787
Pospíšil P (2016) Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front Plant Sci 7:1950
Raj H, Sharma SD (2009) Integration of soil solarization and chemical sterilization with beneficial microorganisms for the control of white root rot and growth of nursery apple. Sci Hortic 119:126–131
Rana S (2015) Plant response towards cadmium toxicity: an overview. Ann Plant Sci 4:1162–1072
Roy S, Arora A, Chinnusamy V, Singh VP (2017) Endogenous reduced ascorbate: an indicator of plant water deficit stress in wheat. Indian J Plant Physiol 22:365–368
Saengwilai P, Meeinkuirt W, Pichtel J, Koedrith P (2017) Influence of amendments on Cd and Zn uptake and accumulation in rice (Oryza sativa L.) in contaminated soil. Environ Sci Pollut Res 24:15756–15767
Samuilov VD, Bezryadnov DB, Gusev MV, Kitashov AV, Fedorenko TA (2001) Hydrogen peroxide inhibits photosynthetic electron transport in cells of cyanobacteria. Biochem (Mosc) 66:640–645
Sayyad-Amin P, Borzouei A, Jahansouz MR (2014) Assaying the photosynthetic performance of salt-affected soybean using chlorophyll-a fluorescence transients. Int J Plant Anim Environ Sci 4:677–689
Schansker G, Srivastava A, Strasser RJ (2003) Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct Plant Biol 30:785–796
Schansker G, Tóth SZ, Kovács L, Holzwarth AR, Garab G (2011) Evidence for a fluorescence yield change driven by a light-induced conformational change within photosystem II during the fast chlorophyll a fluorescence rise. Biochim Biophys Acta Bioenerget 1807:1032–1043
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16:13561–13578
Song X, Yue X, Chen W, Jiang H, Han Y, Li X (2019) Detection of cadmium risk to the photosynthetic performance of Hybrid Pennisetum. Front Plant Sci 10:798
Strasser BJ (1997) Donor side capacity of photosystem II probed by chlorophyll a fluorescence transients. Photosynth Res 52:147–155
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) chlorophyll a fluorescence. Springer, Dordrecht, pp 321–362
Subba P, Mukhopadhyay M, Mahato SK, Bhutia KD, Mondal TK, Ghosh SK (2014) Zinc stress induces physiological, ultra-structural and biochemical changes in mandarin orange (Citrus reticulata Blanco) seedlings. Physiol Mol Biol Plants 20:461–473
Szopiński M, Sitko K, Gieroń Ż, Rusinowski S, Corso M, Hermans C, Małkowski E (2019) Toxic effects of Cd and Zn on the photosynthetic apparatus of the Arabidopsis halleri and Arabidopsis arenosa pseudo-metallophytes. Front Plant Sci 10:748
Tóth SZ, Schansker G, Strasser RJ (2007) A non-invasive assay of the plastoquinone pool redox state based on the OJIP-transient. Photosynth Res 93:193
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Vassilev A, Lidon F, Scotti P, Da Graca M, Yordanov I (2004) Cadmium-induced changes in chloroplast lipids and photosystem activities in barley plants. Biol Plant 48:153–156
Vassilev A, Manolov P (1999) Chlorophyll fluorescence of barley (H. vulgare L.) seedlings grown in excess of Cd. Bulg J Plant Physiol 25:67–76
Wael MS, Mostafa MR, Taia AAEM, Saad MH, Magdi TA (2015) Alleviation of cadmium toxicity in common bean (Phaseolus vulgaris L.) plants by the exogenous application of salicylic acid. J Hortic Sci Biotechnol 90:83–91
Yang GH, Yang LT, Jiang HX, Li Y, Wang P, Chen LS (2012) Physiological impacts of magnesium-deficiency in Citrus seedlings: photosynthesis, antioxidant system and carbohydrates. Trees 26:1237–1250
Yu K, Zou J, Zou J (2010) Effects of cadmium stress on antioxidant enzyme system and absorption of mineral elements in maize seedlings. Nong Ye Huan Jing Ke Xue Xue Bao 291:050–1056
Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Sarin NB (2010) Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. Biochim Biophys Acta Bioenerget 1797:1428–1438
Zhou W, Qiu B (2005) Effects of cadmium hyperaccumulation on physiological characteristics of Sedum alfredii Hance (Crassulaceae). Plant Sci 169:737–745
Zhu XG, Baker NR, Desturler E, Ort DR, Long SP (2005) Chlorophyll a fluorescence induction kinetics in leaves predicted from a model describing each discrete step of excitation energy and electron transfer associated with photosystem II. Planta 223:114–133
Zushi K, Kajiwara S, Matsuzoe N (2012) Chlorophyll a fluorescence OJIP transient as a tool to characterize and evaluate response to heat and chilling stress in tomato leaf and fruit. Sci Hortic 148:39–46
Acknowledgments
The authors greatly acknowledge Centre for Plant Breeding and Genetics, Department of millets, Tamil Nadu Agriculture University (TNAU), Coimbatore, India and Regional Agricultural Research Station (RARS) of Kerala Agricultural University, Pattambi, Kerala, India for providing seeds essential to coduct the experiments.
Funding
This work was supported by the University Grant Commission (UGC), India in the form of JRF under grant 319492.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by V. P. Singh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Janeeshma, E., Kalaji, H.M. & Puthur, J.T. Differential responses in the photosynthetic efficiency of Oryza sativa and Zea mays on exposure to Cd and Zn toxicity. Acta Physiol Plant 43, 12 (2021). https://doi.org/10.1007/s11738-020-03178-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03178-x