Abstract
Salinity is a global challenge issue that is drastically reducing agricultural production and limiting the uptake of essential nutrients such as zinc (Zn) in plants. A better understanding of wheat (Triticum aestivum L.) for improved yield and physiological responses may help in main programs, increasing growth and yield under stress conditions. Thus, factorial experiment was conducted based on a randomized complete block design with three replications under greenhouse conditions during 2020–2021. Experimental factors included salinity levels (no salinity or control, salinity 30, 60, and 90 mM NaCl) and Zn application methods (no Zn as control, soil applied Zn as ZnSO4, foliar application of nano Zn oxide (ZnO), and combined soil and foliar application). The results demonstrated that the maximum of shoot and stem dry matter remobilization, and the contribution of stem reserves to the grain yield and electrical conductance were observed in salinity severe stress and no Zn application. The combined soil and foliar application of Zn at no salinity increased chlorophyll index (69.83%), stomatal conductance (50.34%), quantum yield (45.78%), relative water content (RWC; 80.19%), leaf area index (46.89%), grain filling period (20.2%), effective grain filling period (29.22%), and grain yield (34.56%) compared to no Zn application at the highest salinity level. Based on the results of this study, it seems that combined soil and foliar application can increase the grain yield of wheat under salinity stress due to improving grain filling components and some physiological traits such as RWC, SPAD and stomatal conductance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Salinity is a global challenge that affects agricultural production worldwide. More than 800 million hectares of agricultural land suffer from soil salinity [1]. The increased salinity of agricultural land is expected to have destructive global impacts, resulting in the loss of up to half of the arable land by the middle of the twenty-first century [2]. The adverse effects of salinity have been attributed to the increase of Na+ and Cl–, which are considered to be the most important ions that induce several disorders in the physiological processes of different plants [3]. Salinity increases ion toxicity levels while reducing water availability to plants and absorption of essential nutrients [4].
Intense salinity stress may trigger the acceleration of senescence, enzymatic and metabolic dysfunctions, including a decline in photosynthetic carbon dioxide assimilation and electron transport activity, chlorophyll degradation, the accumulation of reactive oxygen species (ROS), and membrane damage, leading to plant death during prolonged exposure [2]. Khalilzadeh et al. [5] stated that salinity stress decreases chlorophyll content, quantum yield, relative water content (RWC) and grain filling parameters, but increases leaf electrical conductance (EC) and dry matter remobilization in wheat.
Zinc (Zn) is known as important micronutrients, and its deficiency is recognized as a critical problem in plants, especially grown in saline conditions with high pH values [6]. Zn nano-particles restrict the entry of Na+ and Cl– ions and reduce the accumulation of ROS and abscisic acid, in exchange for an increase in chlorophyll content, antioxidant activities, leaf gas exchange parameters, osmolytes, and hormone accumulation, resulting in a substantial improvement in plant growth and grain yield [7]. Zn ions are also known to be powerful inhibitors of enzymes generating oxygen radicals and protect salt-stressed plants from damaging attacks of these compounds [8].
In recent years, a considerable improvement in salinity tolerance has been achieved in some crop species by nanotechnology [9]. On the other hand, the application of nano-fertilizers is one of the proper ways for increasing resource use efficiency and plant production while reducing environmental pollution [10]. Babaei et al. [11] reported that the application of nano Zn-iron oxide increased nearly 17.40% from the grain yield in comparison with the lack of its application at the highest salinity level. Hence, Zn deficiency is frequently a major limiting factor for crops production worldwide, especially under salinity stress and high pH values. Shemi et al. [12] demonstrated that the application of Zn increased the grain yield of maize by increasing the RWC, stomatal conductance, chlorophyll content, and leaf area index (LAI), along with improving the photosynthetic processes of the plant. Sattar et al. [13] also reported that the foliar application of Zn improved the grain yield of wheat by improving RWC and stomatal conductance. Hence, a better understanding of wheat physiological responses under salinity may help in programs whose objective is to improve the grain yield under salinity stress. Therefore, this study sought to evaluate the effects of the foliar and soil application of Zn on grain filling components, yield, and some physiological traits of wheat under salinity stress.
MATERIALS AND METHODS
Experimental design, plant material and growth conditions. A factorial experiment was performed according to randomized complete block design with three replications under greenhouse conditions from 2020 to 2021. Experimental factors encompassed salinity at four levels [no salinity (S0) as control, salinity 30 (S1), 60 (S2), and 90 (S3) mM NaCl (equivalent to 1.02, 2.76, 5.53, and 8.3 dS/m, respectively) and Zn application methods at four levels [no application as control (Z1), soil application Zn as zinc sulfate (ZnSO4) (Z2), foliar application nano ZnO (Z3), combined soil, and foliar application of Zn (Z4)]. The soil application of Zn and foliar application of nano ZnO were 120 kg/ha as ZnSO4 at the planting stage and 1 g/L respectively. Saline irrigation water with three salinity levels (except for control) was prepared by adding sodium chloride to irrigation water to achieve the target salinity level. The temperature of the greenhouse varied between 20 and 30°C during the day and 18 to 21°C at night, and its maximum and minimum relative humidity levels were 60 and 67%, respectively. The soil type was silty with a pH rate of about 7.8. The wheat cultivar “Zagross” was used in the experiment. Its optimum density was 350 seeds/m2. Thus, 31 seeds were sown in every pot with a 2 cm depth and 40 cm diameter, filled almost with 18 kg soil. Foliar application with nano ZnO was performed in two stages of the growth period, namely (BBCH 13) and (BBCH 41–43). Nano ZnO was produced in China and prepared by Jahan Kimia Company (Iran). Nano ZnO had an average particle size of less than 30 nm and a special particle surface of more than 30 m2/g. Nano ZnO powder was added to deionized water and placed on ultra-sonic equipment (100 W and 40 kHz) on a shaker for a better solution [14]. To ensure the penetration of nano ZnO into the plant organ, the scanning electron microscopy images of the flag leaf of wheat under the foliar application of nano ZnO at different levels of salinity stress were obtained (Fig. 1) using a Leo-1430 VP Scanning Electron Microscope (Carl Zeiss AG, Germany). Details of the region are presented as a yellow circle, showing the presence of nanoparticles in flag leaf samples.
Relative water content. At the mid of the booting stage (BBCH 43), the flag leaves of the plants were selected to measure the chlorophyll index, stomata conductance, RWC, quantum yield and leaf EC. The RWC of the flag leaves was calculated based on the following formula [15]:
where FW—fresh weight, DW—dry weight, and TW—turgid weight.
Leaf electrical conductance. The EC of the flag leaf was determined based on the standard method of previous research [16]. From each pot, two developed flag leaves were randomly selected, and after placing them in aluminum foils, they were transferred to the laboratory very quickly. Then, the flag leaf samples were kept in flasks containing 25 mL of distilled water (with specific EC) for a period of time. It was placed at room temperature for 24 h, and then electrical conductivity was measured using an Mi180 Bench meter (Milwaukee Instruments Inc., USA). To measure the electrical conductivity of the flag leaf, it was attempted to homogenize the leaf samples.
Chlorophyll index (SPAD). The chlorophyll index of the flag leaves was calculated based on the method of a previous study [17]. A device chlorophyll meter SPAD-502 (Konica Minolta Sensing Inc., Japan) was used, and in each plant, measurements were taken at three spots on each leaf, two on each side of the midrib on the flag leaf.
Stomatal conductance. Stomata conductance was measured with an AP4 porometer system (Delta-T Devices Ltd., UK) according to the manual instructions. It was computed on the flag leaves of four different plants from each treatment.
Quantum yield of PSII electron transport (ØPSII). This trait was determined on the flag leaves by the uppermost fool expanded leaf using a chlorophyll fluorometer OS30P+ (Opti Sciences Inc., USA). For this purpose, the plants were adapted to darkness for 20 min by using one special clamp, and then the fluorescence amounts were measured in 1000 µM photon/m2 s using the following formula:
where Fm—is the maximum fluorescence after a saturated light pulse on plants adapted to darkness, and F0—represents the minimal fluorescence in the adapted light, which was determined by illumination with FR.
Leaf area index. This trait was measured at the heading stage using a leaf area meter LI-3000C (LI-COR Biosciences, USA).
Rate and grain filling period assay. Two plants in each pot were taken for studying grain filling parameters in each sampling. The first sampling was taken on day 8 after heading, and the other samplings steps were taken in 4-day intervals to determine the accumulation of grain weight. At each sampling, the grains were manually removed from spikes and dried at 75°C for 48 h. The total duration of grain filling for each treatment combination was calculated through fitting a bilinear model [18]:
where GW is the grain dry weight, a—the GW-intercept, gfr—the slope of grain weight indicating the grain filling rate, daa—the days after earring, and pm—physiological maturity.
Borrás et al. [19] determined grain filling using a bilinear model. The effective grain filling period (EGFD) was estimated from the following equation:
where HGW and RGF are highest grain weight (g) and ratio of grain filling (g/day), respectively.
Conversely, an increase in kernel weight in the filling period was calculated using the above-cited equation in statistical software SAS 9.2 via Proc NLIN and the DUD method.
Remobilization of stem reserves to grain yield assay. The dry matter and remobilization of stem reserves to grain yield were evaluated as follows [20]:
Overall, five plants of each pot were randomly harvested to measure grain yield per plant.
Statistical analysis. The analysis of variance and mean comparisons were performed using the SAS computer software package. The main effects and interactions were tested using the least significant difference test at the P < 0.05 probability level.
RESULTS
Considering that there were no significant differences between the two studied years (2020–2021), the averaged data from the two years were used for statistical analyses. The results indicated that the effect of Zn × salinity stress was significant on all traits such as RWC, chlorophyll index, stomata conductance, EC, quantum yield, LAI, maximum grain weight, and grain filling rate. The other affected parameters were effective grain filling period, grain filling period, dry matter remobilization from the stem, the contribution of remobilization from shoots to grain, stem reserve contribution in grain yield, and grain yield (Table 1).
Relative Water Content and Leaf Electrical Conductance
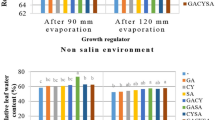
RWC decreased under salinity stress. The application of salinity as S4 decreased RWC by 18.7, 31.4, and 55.88% compared to its application as S3, S2, and S1, respectively (Fig. 2). RWC was 77.67% in combined soil and foliar application of Zn and decreased to 64.65% in control (Fig. 2). On the other hand, there was a decrease of about 20% in the application of Zn1 compared to Zn4. The interaction effect between salinity and Zn application demonstrated that the highest RWC (92.06%) was obtained in no salinity with Zn application as Zn4 while the lowest RWC (51.09%) was obtained at Zn1S4 (Table 2). Moreover, there was a decrease of about 80% RWC in the highest salinity level and no application of Zn (S4Zn1) in comparison with (S1Zn4) (Table 2).
Means comparison of the effects of salinity and zinc on some agro-physiological traits of wheat (mean of two years, 2020 and 2021). (a) Relative water content; (b) electrical conductivity; (c) chlorophyll index; (d) stomata conductance; (e) quantum yield; (f) leaf area index; (g) maximum of grain weight; (h) grain filling rate; (i) effective grain filling period; (g) grain filling period; (k) dry matter remobilization to grain; (l) dry matter remobilization from stem; (m) contribution of remobilization from shoots to grain; (n) stem reserve contribution in grain yield. ZnO—Zinc oxide.
Based on the results, the use of salinity as S4 increased EC by 153.8, 74.4, and 37% compared to its application as S1, S2, and S3, respectively (Fig. 2). Additionally, the application of Zn as Zn1 increased EC by 36.8, 19.5, and 7.1% compared to its application as Zn4, Zn3 and Zn2, respectively (Fig. 2). However, EC content significantly decreased when applying Zn was. The highest (180.1 µS/cm) and lowest (52.66 µS/cm) levels of EC were obtained in S4Zn1 and S1Zn4, respectively (Table 2). There was a decrease of about 242% in the EC content in S4Zn1 in comparison with S1Zn4 (Table 2).
Chlorophyll Index and Stomata Conductance
Chlorophyll index and stomata conductance were significantly affected by salinity stress, in other words, salinity decreased these two parameters (Fig. 2). The lowest chlorophyll index (35.67) was observed at 90 mM salinity. Salinity as S4 decreased the chlorophyll index by 13.17, 33.8, and 46.84%, respectively, in comparison with S3, S2, and no salinity (S1) (Fig. 2). The application of Zn increased the chlorophyll index under salinity stress (Fig. 2). Of course, the combined soil and foliar application of Zn (Zn4) increased this index by 5.25, 10.46, and 19.13% in comparison with the application of Zn as Zn3, Zn2, and Zn1 (Fig. 2). Conversely, stomatal conductance decreased with increasing salinity levels (Fig. 2) so that salinity 90 mM (S4) had the lowest value of stomatal conductance (22.02 mmol H20/m2 s). However, the application of Zn as Zn4 increased stomatal conductance by about 2.27, 13.98, and 15.21% in comparison with Zn3, Zn2, and Zn1 (Fig. 2). The interaction effect between salinity and Zn represented that salinity 90 mM decreased chlorophyll index and stomata conductance. The highest chlorophyll content (55.86) and stomata conductance (28.46 mmol H20/m2 s) were observed as S1Zn4 while the minimum levels of these values (32.89 and 18.93 mmol H20/m2 s respectively) were found as S4Zn1 (Table 2).
Quantum Yield and Leaf Area Index
Quantum yield and LAI decreased significantly under salinity stress (Fig. 2). The highest salinity level (S4) had the lowest value of quantum yield (0.6) so that 90 mM salinity decreased the quantum yield by 33.33% in comparison with no salinity condition (Fig. 2). Contrarily, the application of Zn increased the quantum yield (Fig. 2) so that the application of Zn as Zn4 increased the quantum yield by 5.71, 8.82, and 13.84% in comparison with Zn3, Zn2, and Zn1 (Fig. 2). LAI was progressively decreased with increasing salinity levels (Fig. 2). No salinity (S1) increased LAI about 25.26% in comparison with S4. Compared with the non Zn treatment (Zn1), the Zn treatment Zn2, Zn3, and Zn4 decreased LAI by 4.72, 13.47, and 25.77% respectively (Table 2).
Rate and Grain Filling Period
The results revealed that the highest grain filling rate was obtained under no salinity and application of Zn as Zn4 (Fig. 3, Tables 3 and 4). The highest level of salinity stress (90 mM) had the lowest grain filling components such as maximum grain weight (0.027 g), grain filling rate (0.0011 g/day), grain filling period (27.84 days), and effective grain filling period (23.52 days). On the other hand, salinity as S4 caused a decrease of 59.25, 36.36, 12.21, and 16.02%, respectively, in the maximum grain weight, grain filling rate, grain filling period, and effective grain filling period in comparison with no salinity (S1). The grain filling component was increased in response to Zn usage (Fig. 2). The application of Zn as Zn4 under the highest salinity level (Zn4S4) increased the maximum grain weight, grain filling rate, effective grain filling period, and grain filling period by approximately 25.1, 9.4, 14.3, and 10.5%, respectively, compared to no Zn application under the same salinity level (Zn1S4) (Table 4).
Effect of Zinc and salinity stress on grain filling of wheat (mean of two years, 2020 and 2021). (a) No salinity, (b) salinity 30 mM NaCl, (c) salinity 60 mM NaCl and (d) salinity 90 mM NaCl. (1) Estimated (application ZnSO4 and nano ZnO); (2) observed (application ZnSO4 and nano ZnO); (3) estimated (application nano ZnO); (4) observed (application nano ZnO); (5) estimated (application ZnSO4); (6) observed (application ZnSO4); (7) estimated (no application Zn); (8) observed (no application Zn). ZnO—Zinc oxide.
Dry Matter and Stem Reserves Mobilization to Grain Yield
The results showed that the highest salinity level (S4) increased dry matter remobilization from stem and shoots yield (38 and 50%, respectively) and the contribution of these processes to grain yield (43 and 70%, respectively) in comparison with no salinity. However, the Zn application demonstrated senescence and retarded such mobilization. The highest dry matter remobilization from the stem (0.205 g/plant), stem reserve contribution in the grain yield (27.15%), and the contribution of remobilization from shoots to grain (38.27%) was observed in S4Zn1 (Table 5). Contrarily, the lowest level of the mentioned traits was found in S1Zn4 (Table 5). On the other hand, there was an increase of about 71, 25, and 55%, respectively, in the contribution of remobilization from shoots to grain, dry matter remobilization from the stem, and stem reserve contribution in the grain yield at the 90 mM salinity level and Zn treated plants as Zn4 (S4Zn4) in comparison with S1Zn1 (Table 5).
Grain Yield
Grain yield was significantly affected by salinity stress levels (Fig. 2) and decreased under salinity stress (Fig. 2) so that salinity 90 mM (S4) had the lowest value of grain yield (0.796 g/plant). Salinity as S4 decreased 6.15, 22.73, and 23.36%, respectively, of the grain yield in comparison with S3, S2, and no salinity (S1) (Fig. 2). The application of Zn increased the grain yield under salinity stress (Fig. 2). Of course, the combined soil and foliar application of Zn (Zn4) could increase the grain yield by 4.54, 6.42, and 8.88% in comparison with the application of Zn as Zn3, Zn2, and Zn1 (Fig. 2). The highest grain yield (1.016 g/plant) was found as S1Zn4, whereas its minimum value (0.755 g/plant) was observed as S4Zn1 (Table 5).
DISCUSSION
Relative Water Content and Leaf Electrical Conductance
RWC decreased significantly under salinity stress (Fig. 2). These results may be attributed to the accumulation of toxic ions such as Na+ and Cl–, reducing leaf expansion, LAI and stomata conductance (Table 2) and apparently decreasing stomata conductance or stomata closure, leading to a reduction in intracellular CO2 partial pressure [21]. Chattha et al. [7] reported that salinity reduces the ability of plants to take up water and decrease in leaf RWC. Further, it could be related to low water availability under stress conditions or to root systems, which are unable to compensate for water lost by transpiration through a reduction of the absorbing surface [22]. They also concluded that the amelioration role of Zn in RWC maintenance might be attributed to the improvement of vascular tissue. Similarly, other researchers indicated that nano ZnO application decreased the leaf EC while increasing the leaf RWC of triticale [23]. Part of the increase in the RWC due to the application of Zn can be due to a decrease in EC (Table 2). Al-Zahrani et al. [24] also attributed the increase in RWC due to the application of Zn in salt stress conditions, to the reduction of electrolyte leakage.
Khalilzadeh et al. [5] found that under salinity stress, plant membranes are subject to changes often associated with increases in permeability and loss of integrity, but Zn application under such conditions decreased EC [23]. They demonstrated that the Zn application improved cell membrane stability in the triticale plant as a consequence of enhancing nutrient uptake, extension of the root system, and water status of the plants [23]. Al-Zahrani et al. [24] reported that salinity induced secondary oxidative stress by producing a raised level of ROS, damaged the membrane, and increased electrolyte leakage. The application of Zn increased the maintenance of plasma membrane integrity and thus controlled the toxic ions uptake under salinity stress [8].
Chlorophyll Index and Stomata Conductance
Altuntaş et al. [25] found that the reduction in the chlorophyll content under salinity stress was due to nutrient deficiency, diminished chlorophyll biosynthesis, and enhanced activity of chlorophyll destroying an enzyme named chlorophyllase enzyme. In saline conditions, reducing the RWC by disrupting the transpiration rate and photosynthetic processes causes a decrease in stomatal conductance [7]. It seems that the soil and foliar application of Zn by improving RWC and Fv/Fm (Table 2) has increased the chlorophyll index and stomatal conductance (Table 2). These results are in line with the findings of previous research [13], representing that the application of Zn by improving the RWC increases the chlorophyll content and stomatal conductance of wheat. Chattha et al. [7] concluded that the application of Zn increased the leaf chlorophyll content and stomatal conductance of wheat by improving the RWC and reducing electrolyte leakage. Rasouli et al. [26] attributed that the increase in the chlorophyll content due to the application of Zn under salinity stress conditions, to the improvement of antioxidant enzyme activity, an increase in Fv/Fm, and a reduction of electrolyte leakage.
Chattha et al. [7] indicated that the main reason for the decrease in the chlorophyll content may be the degradation by ROS. Low chlorophyll content under salinity stress was reported as a result of lower chlorophyll synthesis, destroying the PSII reaction center, inhibiting carbonic anhydrase and nitrate reductase activities, creating an imbalance in the ion flux inside plants, affecting the membrane stability index, and decreasing RWC [27]. The application of nano ZnO increased the chlorophyll, highlighting the Zn impact on reducing the stress effect; furthermore, it seems that the major reason for the reduction in chlorophyll may be degradation by ROS [11]. The completion and formation of chlorophyll are finally facilitated in the presence of Zn. Of course, the Zn is not directly effective in the formation of chlorophyll but may have an impact on the concentration of food elements involved in the formation of chlorophyll or substances that are part of the chlorophyll molecule, including Fe and Mg. In fact, Zn is required for the activity of enzymes that are involved in chlorophyll biosynthesis [28]. Shemi et al. [12] demonstrated that the application of Zn increased the stomatal conductance of maize by increasing the RWC and improving the photosynthetic processes of the plant. Sattar et al. [13] also reported that the foliar application of Zn improved the photosynthetic conditions and stomatal conductance of wheat leaves by improving the antioxidant defense system and increasing the chlorophyll content and RWC.
Quantum Yield and Leaf Area Index
Probably, the higher levels of salinity (S4) could decrease the chlorophyll index, RWC and stomatal conductance of the flag leaf which might have decreased the quantum yield (Fig. 2). In this regard, Khalilzadeh et al. [5] attributed the decrease in the quantum yield to the decline in the chlorophyll content, RWC, and stomatal conductance. Damage to PSII can lead to a change in chlorophyll fluorescence. Thus, chlorophyll fluorescence has been used as a powerful and reliable non-invasive method for assessing the changes in the function of PSII and reflecting the primary photosynthetic processes under environmental stress conditions [26]. Another common damage anticipated under stress conditions is the accumulation of excessive ROS [26], which can cause serious damage to organelles such as chloroplasts, mitochondria, and plasma membranes [29]. Photosynthetic pigments present in photosystems are believed to be damaged by stress factors, resulting in the reduced light-absorbing efficiency of both photosystems (PSI and PSII) and hence a reduced photosynthetic capacity. Babaei et al. [11] reported that the application of Zn on wheat increased the quantum yield. Rasouli et al. [26] also attributed the increase in the leaf quantum yield, due to the application of Zn under salt stress conditions, to the increase in the chlorophyll content and a decrease in electrolyte leakage.
Kherizadeh Arough et al. [30] stated that Zn treatment significantly increased LAI against the control. Moreover, it seems that the presence of high amounts of salt in the environment reduces the amount of water available for the plant and limits cell division, and causes a decrease in cell mass, thus reducing the leaf area [31]. Kheirizadeh Arough et al. [30] found that the foliar application of nano ZnO increased the LAI of triticale by modulating the effect caused by stress and increasing the supply of nutrients and photosynthetic materials. It seems that the soil and foliar applications of Zn have increased LAI by improving the chlorophyll content (Table 2). Lalarukh et al. [32] also attributed the increase in wheat LAI, due to the application of Zn under salt stress conditions, to the increase in the chlorophyll content.
A Zn-enhancement CA activity is highly beneficial for plants in order to facilitate the supply of CO2 from the stomatal cavity to the site of CO2 fixation [33]. Babaei et al. [11] concluded that Zn increased the quantum yield under salinity stress. Plants that grow under Zn deficiency conditions have low quantum yield performance, and damage to the components of chloroplast is irreparable [34]. Therefore, it seems that the application of Zn under salinity conditions can increase the quantum yield.
Rate and Grain Filling Period
The decrease in RWC, stomatal conductance, chlorophyll index, and quantum yield (Fig. 2) can have major effects on grain filling components (Fig. 2), which was consistent with the findings of other researchers, reporting that under salinity stress, low grain filling components of wheat are as a result of a decrease in RWC, stomatal conductance, chlorophyll content, and quantum yield [5]. Francois et al. [35] suggested that salinity reduces grain weight due to the shortage of the seed-filling period and accelerates the maturation of seeds. Munns and James [36] demonstrated that salinity stress decreases the duration of vegetative and reproductive growth of wheat genotypes, which reduces grain-filling components compared to normal conditions.
Although Zn is a micronutrient, Zn application can affect the susceptibility of plants to stress [37]. Seyed Sharifi et al. [38] indicated that the application of Zn increased grain filling components such as the effective grain filling period and grain filling rate of barley (Hordeum vulgare L.). Baniabbass et al. [39] represented that the reasons for the essential role of Zn in the structure of phospho-ethanol pyruvate carboxylase and its importance in the direct synthesis of growth material such as auxin cause photovoltaic power and consequently carbohydrate levels increase in the presence of Zn, leading to further production of dry matter and its storage in grain as a reservoir.
Dry Matter and Stem Reserves Mobilization to Grain Yield
The stress tolerance efficiency of cereals relies not only on the assimilation of dry matter and stem reserves but also on the effective partitioning of these reserves to grains. Khalilzadeh et al. [5] found that contributions become greater when plants are cultivated under salinity stress rather than no salinity treatment. The amount of dry matter remobilization and its contribution to grain yield are more affected by the source and sink relations and environmental conditions [40]. It seems that one rationale behind this may be improving the chlorophyll index (Fig. 2) under favorable conditions and accessibility to sufficient resources, which can increase current photosynthesis. However, accessibility to nutrient sources (e.g., Zn) is limited when the plants are under salinity conditions. In such conditions, an increase in the grain weight under the application of Zn is mainly attributed to the higher number of endosperm cells that cause reduced sink strength. Similar results have been reported by previous researchers, indicating that the application of nano ZnO decreased dry matter remobilization to grain, contributing stem reserves to grain [38].
Grain Yield
Babaei et al. [11] concluded that the application of Zn under salinity stress conditions increased the quantum yield, RWC, chlorophyll index, and grain yield. Kheirizadeh Arough et al. [23] also indicated that the application of Zn could increase chlorophyll content, quantum yield, RWC, and grain yield under stress conditions. Based on the findings of Seyed Sharifi et al. [38], the application of nano ZnO increased the grain filling components (e.g., effective grain filling period, grain filling period, and grain filling rate) of triticale. It seems that the soil and foliar application of Zn, by improving RWC, chlorophyll content, and stomatal conductance (Table 2) while reducing EC (Table 2), could increase the grain yield (Table 5). Chattha et al. [7] reported that the application of Zn under salinity stress conditions improved the rate of photosynthesis and increased grain yield of wheat by reducing electrolyte leakage while increasing quantum yield, chlorophyll content, and stomatal conductance.
The results revealed that the maximum of the shoot and stem dry matter remobilization, the contribution of stem reserves to the grain yield, and EC were observed in S4Zn1. Salinity severe stress as S4 decreased RWC, stomata conductance, chlorophyll index, LAI, and quantum yield (Fv/Fm), whereas the application of Zn improved these traits under salinity stress and normal conditions. Thus, the highest levels of RWC, stomata conductance, chlorophyll index, LAI, and quantum yield were observed in S1Zn4, which caused a 34.56% increase in the grain yield in comparison with S4Zn1. Hence, it seems that the application of Zn, especially combined soil and foliar application of Zn, can be recommended for profitable wheat production under soil salt stress conditions.
REFERENCES
Rengasamy, P., Soil processes affecting crop production in salt affected soils, Funct. Plant. Biol., 2010, vol. 37, p. 613. https://doi.org/10.1071/FP09249
Mahajan, S. and Tuteja, N., Cold, salinity and drought stresses: an overview, Arch. Biochem. Biophys., 2005, vol. 444, p. 139. https://doi.org/10.1016/j.abb.2005.10.018
Tavakkoli, E., Rengasamy, P., and McDonald, G.K., High concentrations of Na+ and Cl- ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress, J. Exp. Bot., 2010, vol. 61, p. 4449. https://doi.org/10.1093/jxb/erq251
Grattan, S.R. and Grieve, C.M., Mineral nutrient acquisition and response by plant grown in saline environments Agric. Ecosyst. Environ., 1999, vol. 38, p. 275. https://doi.org/10.1016/0167-8809(92)90151-Z
Khalilzadeh, R., Seyed Sharifi, R., and Jalilian, J., Growth, physiological status, and yield of salt-stressed wheat (Triticum aestivum L.) plants affected by bio fertilizer and cystocele applications, Arid Land Res. Manage., 2018, vol. 23, p. 71. https://doi.org/10.1080/15324982.2017.1378282
Cakmak, I., Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil, 2008, vol. 302, p. 1. https://doi.org/10.1007/s11104-007-9466-3
Chattha, M.U., Amjad, T., Khan, I., Nawaz, M., Ali, M., Chattha, M.B., Ali, H.M., Ghareeb, R.Y., Abdelsalam, N.R., Azmat, S., Barbanti, L., and Hassan, M.U., Mulberry based zinc nano-particles mitigate salinity induced toxic effects and improve the grain yield and zinc bio-fortification of wheat by improving antioxidant activities, photosynthetic performance, and accumulation of osmolytes and hormones, Front. Plant Sci., 2022, vol. 13, p. 920570. https://doi.org/10.3389/fpls.2022.920570
Weisany, W., Sohrabi, Y., Heidari, G., Siosemardeh, A., and Ghassemi-Golezani, K., Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.), Plant Omics J., 2012, vol. 5, p. 60.
Chen, H. and Yada, R., Nanotechnologies in agriculture: new tools for sustainable development, Trends Food Sci. Technol., 2011, vol. 22, p. 585. https://doi.org/10.1016/j.tifs.2011.09.004
Taha, R.A., Hassan, M.M., Ibrahim, E.A., Abou-Baker, N.H., and Shaaban, E.A., Carbon nanotubes impact on date palm in vitro cultures, Plant Cell, Tissue Organ Cult., 2016, vol. 127, p. 525. https://doi.org/10.1007/s11240-016-1058-6
Babaei, Kh., Seyed Sharifi, R., Pirzad, A., and Khalilzadeh, R., Effects of bio fertilizer and nano Zn-Fe oxide on physiological traits, antioxidant enzymes activity and yield of wheat (Triticum aestivum L.) under salinity stress, J. Plant Interact., 2017, vol. 12, p. 381. https://doi.org/10.1080/17429145.2017.1371798
Shemi, R., Wang, R., Gheith, E.M.S., Hussain, H.A., Hussain, S., Irfan, M., Cholidah, L., Zhang, K., Zhang, S., and Wang, L., Effects of salicylic acid, zinc and glycine betaine on morpho‑physiological growth and yield of maize under drought stress, Sci. Rep., 2021, vol. 11, p. 1. https://doi.org/10.1038/s41598-021-82264-7
Sattar, A., Wang, X., Ul-Allah, S., Sher, A., Ijaz, M., Irfan, M., Abbas, T., Hussain, S., Nawaz, F., Al-Hashimi, A., Al-Munqedhi, B.M., and Skalicky, M., Foliar application of zinc improves morpho-physiological and antioxidant defense mechanisms, and agronomic grain biofortification of wheat (Triticum aestivum L.) under water stress, Saudi J. Biol. Sci., 2022, vol. 29, p. 1699. https://doi.org/10.1016/j.sjbs.2021.10.061
Prasad, T.N., Sudhakar, P., Sreenivasulu, Y., Latha, P., Munaswamy, V., Raja Reddy, K., Sreeprasad, T.S., and Sajanlal, P.R., Effect of nanoscale Zinc oxide particles on the germination, growth and yield of peanut, J. Plant Nutr., 2012, vol. 35, p. 905. https://doi.org/10.1080/01904167.2012.663443
Chelah, M.K.B., Nordin, M.N.B., Musliania, M.I., Khanif, Y.M., and Jahan, M.S., Composting increases BRIS soil health and sustains rice production on BRIS soil, Sci. Asia, 2011, vol. 37, p. 291. https://doi.org/10.2306/scienceasia1513-1874.2011.37.291
Jodeh, S., Alkowni, R., Hamed, R., and Samhan, S., The study of electrolyte leakage from barley (Hordeum vulgare L.) and Pearl millet using plant growth promotion (PGPR) and reverse osmosis, J. Food Nutr. Res., 2015, vol. 3, p. 422. https://doi.org/10.12691/jfnr-3-7-3
Neufeld, H., Chappelka, A.H., Somers, G.L., Burkey, K.O., Davison, A.W., and Finkelstein, P., Visible foliar injury caused by ozone alters the relationship between SPAD meter readings and chlorophyll concentrations in cut leaf coneflower, Photosynth. Res., 2006, vol. 87, p. 281. https://doi.org/10.1007/s11120-005-9008-x
Borrás, L. and Otegui, M.E., Maize kernel weight response to post flowering source-sink ratio, Crop Sci., 2001, vol. 41, p. 1816. https://doi.org/10.2135/cropsci2001.1816
Borrás, L., Slafer, G.A., and Otegui, M.E., Seed dry weight response to source-sink manipulations in wheat, maize and soybean: A quantitative reappraisal, Field Crops Res., 2004, vol. 86, p. 131. https://doi.org/10.1016/j.fcr.2003.08.002
Inoue, T., Inanaga, S., Sugimoto, Y., An, P., and Eneji, A.E., Effect of drought on ear and flag leaf photosynthesis of two wheat Cultivars differing in drought resistance, Photosynthetica, 2004, vol. 42, p. 559. https://doi.org/10.1007/S11099-005-0013-2
Hasegawa, P.M., Bressan, R.A., Zhu, J.K., and Bohnert, H.J., Plant cellular and molecular responses to high salinity, Annu. Rev. Plant Physiol. Plant Mol. Biol., 2000, vol. 51, p. 463. https://doi.org/10.1146/annurev.arplant.51.1.463
Gadallah, M.A., Effect of indole-3-acetic acid and zinc on the growth, osmotic potential and soluble carbon and nitrogen components of soybean plants growing under water deficit, Alger. J. Arid Environ., 2000, vol. 44, p. 451. https://doi.org/10.1006/jare.1999.0610
Kheirizadeh Arough, Y., Seyed Sharifi, R., and Seyed Sharifi, R., Bio fertilizers and zinc effects on some physiological parameters of triticale under water limitation condition, J. Plant Interact., 2016, vol. 11, p. 167. https://doi.org/10.1080/17429145.2016.1262914
Al-Zahrani, H.S., Alharby, H.F., Hakeem, K.R., and Rehman, R.U., Exogenous application of zinc to mitigate the salt stress in Vigna radiata (L.) Wilczek–evaluation of physiological and biochemical processes, Plants, 2021, vol. 10, p. 1005. https://doi.org/10.3390/plants10051005
Altuntaş, C., Demiralay, M., Muslu, A.S., and Terzi, R., Proline-stimulated signaling primarily targets the chlorophyll degradation pathway and photosynthesis associated processes to cope with short-term water deficit in maize, Photosyn. Res., 2020, vol. 144, p. 35. https://doi.org/10.1007/s11120-020-00727-w
Rasouli, F., Asadi, M., Hassanpouraghdam, M.B., Aazami, M.A., Ebrahimzadeh, A., Kakaei, K., Dokoupil, L., and Mlcek, J., Foliar application of ZnO-NPs influences chlorophyll fluorescence and antioxidants pool in Capsicum annum L. under salinity, Horticulturae, 2022, vol. 8, p. 98. https://doi.org/10.3390/horticulturae8100908
Talaat, N.B. and Shawky, B.T., 24-Epibrassinolide ameliorates the saline stress and improves the productivity of wheat (Triticum aestivum L.), Environ. Exp. Bot., 2012, vol. 82, p. 80. https://doi.org/10.1016/j.envexpbot.2012.03.009
Lebedev, N. and Timco, P.M., Protochlorophyllide Photoreduction, Photosynth. Res., 1998, vol. 58, p. 5.
Moradi, F. and Ismaeil, M., Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice, Ann. Bot., 2007, vol. 99, p. 1161. https://doi.org/10.1093/aob/mcm052
Kheirizadeh Arough, Y., Seyed Sharifi, R., Sedghi, M., and Barmaki, M., Effects of biofertilizers and nano zinc oxide on remobilization and some growth indices of triticale under water limitation conditions, Crop Physiol. J., 2015, vol. 7, p. 37.
Wang, D., Shannon, M.C., and Grieve, C.M., Salinity reduces radiation absorption and use efficiency in soybean, Field Crops Res., 2001, vol. 69, p. 276. https://doi.org/10.1016/S0378-4290(00)00154-4
Lalarukh, I., Zahra, N., Al Huqail, A.A., Amjad, S.F., Al-Dhumri, S.A., Ghoneim, A.M., Alshahri, A.H., Almutari, M.M., Alhusayni, F.S., Al-Shammari, W.B., Almutari, M.M., Alhusayni, F.S., Al-Shammari, W.B., Poczai, P., Mansoora, N., et al. Exogenously applied ZnO nanoparticles induced salt tolerance in potentially high yielding modern wheat (Triticum aestivum L.) cultivars. Environ. Technol. Innovation, 2022, vol. 27, p. 102799. https://doi.org/10.1016/j.eti.2022.102799
Sasaki, H., Hirose, T., Watanabe, Y., and Ohsugi, R., Carbonic anhydrase activity and CO2 transfer resistance in Zn-deficient rice leaves, Plant Physiol., 1998, vol. 118, p. 929. https://doi.org/10.1104/pp.118.3.929
Chen, W., Yang, X., He, Z., Feng, Y., and Hu, F., Differential changes in photosynthetic capacity, 77 K chlorophyll fluorescence and chloroplast ultrastructure between Zn-efficient and Zn-inefficient rice genotypes (Oryza sativa L.) under low Zn stress, Physiol. Plant., 2008, vol. 132, p. 89. https://doi.org/10.1111/j.1399-3054.2007.00992.x
Francois, L.E., Grieve, C.M., Mass, E.V., and Lesch, S.M., Time of salt stress affects growth and yield components of irrigated wheat, Agron. J., 1994, vol. 86, p. 100. https://doi.org/10.2134/AGRONJ1994.00021962008600010019X
Munns, R. and James, R.A., Screening methods for salinity tolerance: a case study with tetrapod wheat, Plant Soil, 2003, vol. 253, p. 201. https://doi.org/10.1023/A:1024553303144
Sultana, N., Ikeda, T., and Kashem, M.A., Effect of foliar spray of nutrient solutions on photosynthesis, dry matter accumulation and yield in seawater-stressed rice, Environ. Exp. Bot., 2011, vol. 46, p. 129. https://doi.org/10.1016/S0098-8472(01)00090-9
Seyed Sharifi, R., Kamari, H., and Nagafi, Gh., Effects of salinity stress and foliar application of Nano-Zinc oxide on yield per plant and some morphophysiological traits of barley (Hordeum vulgare L.), Iranian J. Field Crops Res., 2015, vol. 13, p. 399.
Baniabbass, Z., Zamani, G., and Sayyari, M., Effect of drought stress and zinc sulfate on the yield and some physiological characteristics of sunflower (Helianthus annuus L.), J. Environ. Biol., 2012, vol. 6, p. 518.
Seyed Sharifi, R. and Nazarly, H., Effects of seed priming with plant growth promoting rhizobacteria (PGPR) on grain yield, fertilizer use efficiency and dry matter remobilization of sunflower (Helianthus annus L.) with various levels of nitrogen fertilizer, J. Sustainable Agric. Prod. Sci., 2013, vol. 23, p. 27.
ACKNOWLEDGMENTS
The authors are highly indebted to the Faculty of Agriculture and Natural Resources, University of Mohaghegh Ardabili for providing research facilities and technical assistance during the research work.
Funding
This research did not receive any specific funding. This experimental is part of thesis Hamed Narimani who is Ph.D student in Faculty of Agriculture and Natural Resources, University of Mohaghegh Ardabili via Research Grant Program.
Author information
Authors and Affiliations
Contributions
Hamed Narimani and Raouf Seyed Sharifi equally contributed to this work.
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any research involving people as objects of research.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Additional information
Abbreviations: EC—leaf electrical conductance; LAI—leaf area index; RWC—relative water content; SPAD—chlorophyll index.
Rights and permissions
About this article
Cite this article
Narimani, H., Seyed Sharifi, R. Effect of Foliar and Soil Application of Zinc on Grain Filling, Yield and Some Physiological Traits of Wheat (Triticum aestivum L.) under Salinity Stress. Russ J Plant Physiol 70, 133 (2023). https://doi.org/10.1134/S102144372360040X
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S102144372360040X