Abstract
Water-deficit stress is one of the major global challenges in plant production. Biostimulants can help to reduce the harmful impacts of water deficit and improve the growth of the medicinal and aromatic plant species. This study is aimed to examine the role of pluramin metabolic activator and Pseudomonas fluorescens PF-135 on the performance, physiological properties and secondary metabolites of lemon balm (Melissa officinalis L.) under water-deficit stress condition. For this purpose, plants were subjected to water-deficit stress, followed by foliar application of pluramin and P. fluorescens PF-135 inoculation as biostimulants. The induced stress resulted in a remarkable reduction in a number of plant characteristics including total dry matter, chlorophyll content, and relative water content (RWC). After foliar application of pluramin and inoculation with P. fluorescens PF-135, a significant improvement in these characteristics was observed. Foliar application of pluramin and P. fluorescens PF-135 inoculation resulted in higher tolerance to the water-deficit stress condition due to the increase in RWC, antioxidant enzyme activity, and reduction in malondialdehyde and hydrogen peroxide contents. Furthermore, water-deficit stress resulted in higher caffeic acid, rosmarinic acid, essential oil content, proline, and total sugar contents, and the use of pluramin and P. fluorescens PF-135 inoculation showed a reduction in these compounds contents. After the continuous application of pluramin and P. fluorescens PF-135, plants initiated defense strategies against water-deficit stress condition in most of lemon balm samples, which indicted the interaction of pluramin and P. fluorescens PF-135 under water-deficit stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Lemon balm (Melissa officinalis L.) is a perennial herb from the Lamiaceae family. As one of the most important and popular medicinal plant, lemon balm has attracted the attention of the researchers especially due to its valuable essential oils (EOs). The aerial parts of this plant (i.e. leaves and flowers) have been used from ancient time until the now as traditional medicines as well as food flavoring herb [1].
Diverse biological processes by soil microorganisms, in particular the bacteria, can alter the nutrients of the soil and thus affecting the plant growth. Plant growth-promoting rhizobacteria is a famous example in this regard which can directly enhance plant growth and performance. The enhanced dissolution of low-soluble nutrients such as phosphorus, generation of 1‑aminocyclopropane-1-carboxylic acid deaminase, and plant growth hormones (e.g. auxin), nitrogen fixation, and production of siderophore are among the major mechanisms in improvement of plant growth and performance by plant growth-promoting rhizobacteria [2]. It is also very well known that inoculation with soil microorganisms can increase the plant resistance against abiotic stresses such as drought [3]. Several studies have shown that plant growth-promoting rhizobacteria can protect the plants against the detrimental impacts of environmental stresses [4].
Recently, the extensive application of mineral fertilizers has resulted in serious human health concerns. These concerns can be remarkably resolved by substituting the mineral fertilizers with the growth inducers or the actuating factor [5]. Environmental risks combined with serious concerns on the sustainability of the current agriculture systems have led to a rising tendency toward using natural products (including amino acids) to regulate plant growth and biosynthesis. Amino acids have exhibited direct and indirect influences on the physiological activities involved in plant growth. Similar to polyamines, pluramin metabolic activator can also serve as a metabolism stimulant and affect various metabolic processes such as photosynthesis, enzyme activities, meiosis, cell differentiation, gene expression, cell signalling, membrane rigidity, and ionic channel regulation [6]. Pluramin can also act as a secondary messenger promoting the plants' resistance against unsuitable environmental conditions [6]. Pluramin has been recommended for plants exposed to over-irrigation or drought as well as abiotic stresses.
As one of the major abiotic stresses, water deficiency will restrict the crops production, particularly in arid and semi-arid regions with irregular precipitation patterns. Plants respond to water deficiency by altering their cellular metabolism and defense mechanisms. Drought stress alters the phospholipid content of the root through enhancing the phosphatidylcholine level and at the same tome declining the phosphatidylethanolamine which result in incremented electrolyte leakage from the membrane [7].
Production of the secondary metabolites in the medicinal plants is mainly controlled by the plant’s genetic traits. Environmental factors such as stresses may influence the quality and quantity of EOs in the medicinal plants. Under the stress condition, the synthesis of these products in medicinal plants will be drastically enhanced. Elevated EOs have been reported under drought stress in various plants such as wormwood, chamomile, lavender, mint, sage, thymus and eucalyptus. This issue has highlighted the possible role of EOs in mechanisms involved in the drought-resistance through decremented transpiration [8].
Medicinal plants play a significant role in public health. Water-deficit stress can drastically affect the medicinal plant’s growth and performance and change its bioactive components. Furthermore, the plant metabolic activators and plant growth-promoting rhizobacteria can decrease the harmful impacts of environmental stresses. Therefore, the current research is mainly aimed to investigate the effect of water deficiency on growth and performance of lemon balm and also to determine the changes in some phytochemical composition of this plant upon spraying pluramin or Pseudomonas fluorescens PF-135 inoculation.
MATERIALS AND METHODS
Plant material. Lemon balm (Melisa officinalis L.) seeds were purchased from Research Institute of Forests and Rangelands, Karaj, Iran. Seeds were surface disinfected with sodium hypochlorite, washed three times with distilled water and put on filter paper (Whatman no. 1) in Petri dishes for initiating seed germination. Before the experiment, the viability of seeds was tested which was 97% on average. Then, ten uniform seeds were sown in plastic pots of 10 kg capacity of soil (25 cm in length and 30 cm in diameter). The seedlings were thinned later and only four plants were maintained after emergence in each pot. They were kept under light/dark cycle conditions of 16/8 h at 25°C and 75% relative humidity placed in a greenhouse. Soil analysis showed that the soil texture was loam (EC 1.04 dSm-1; pH 7.1; nitrogen 0.07%; phosphorus 10.2 ppm; potassium 126 ppm).
A factorial experiment in a randomized complete block design with three replications was performed. Factors were inoculation (i.e. inoculation with Pseudomonas fluorescens PF-135 strain and non-inoculation), pluramin metabolic activator and irrigation regimes. For inoculation P. fluorescens PF-135 was isolated from the Wheat (cultivar ‘Azar2’) rhizosphere which had significant plant growth promotion attributes such as production of indol-3-acetic acid and 1‑aminocyclopropane-1-carboxylate deaminase activity [9]. The bacteria were stored in 0.1 M magnesium sulphate (MgSO4·7H2O) solution at 4°C. Plant growth promoting activities of this strain has been identified. Different concentration of pluramin metabolic activator (0, 1 and 2.5 g/L water) that the concentration range of pluramin was selected according to Proteo International S.r.l. firm for industrial crops, (purchased from Proteco Company, Italy). The irrigation regimes included well-watered 80% and watering up to 50% of soil available water, representing non-stress and water-deficit stress treatments, respectively. From pre-flowering until the early stage of flowering (when 50% of the plants entered this phase), water-deficit stress applied to the experimental pots. Non-stressed pots were regularly irrigated on a normal basis through the entire experimental period.
Crop evapotranspiration was employed for assessing the irrigation requirements and planning. The total evapotranspiration requirements, crop coefficients for initial, development, and the middle stage of lemon balm were measured as 710, 0.68, 0.93, and 1.19 mm, respectively.
The soil moisture content was daily monitored by TRIME-TDR (PMS-714, Lutron, Taiwan) in which a 15-cm probe was employed for volumetric measurement of the soil moisture. The obtained data were confirmed using gravimetric moisture through the following equation:
where, ΘG and ΘV are the gravimetric water content and volumetric soil moisture, respectively. PW shows the water density and PS represents the soil density. The required water was supplied according to the available water.
Soil moisture at the filed capacity and wilting points were measured as 22.1 and 7.9%, respectively. The pressure plate apparatus was also applied to determine the soil water retention curve.
Pluramin is a powdery compound of amino acids which is rapidly absorbed and is recommended for the time when the plant is experiencing or under the stress conditions. Pluramin metabolic activator contains 14% total nitrogen, 13.8% organic nitrogen, 0.2% ammonium nitrogen, 92% organic materials and 90% total amino acid, therefore, it is a metabolic active ingredient [10]. The pluramin powder was dissolved in distilled water and sprayed manually at sunset for three consecutive days on the bushes until the solution started to leak from the leaves. This treatment was done through foliar spray, before initiation of water stress and flowering stage for three weeks. The control pots (not pluramin treated) were sprayed only with distilled water in away similar to the pluramin treated ones.
Harvesting was carried out when 50% of the plants entered flowering phase (110 days after planting). The aerial parts of the plants were shade dried and then weighed, for measuring the dry weight of shoots and for essential oil extraction a part of the shoots was set aside. The other portions of samples were frozen in liquid nitrogen and were kept at –80°C (Deep freezer, 88FD-4-95-A, Iran) for further measurement of the physiological parameters.
Relative water content (RWC). Using fully developed young leaf, RWC was determined. They were rapidly sealed into clear plastic bags and immediately after transferring to the laboratory the fresh weight (FW) of samples were recorded. Then, they were submerged in double distilled water within the covered Petri dish for 6 h at room temperature and the turgid weight (TW) was recorded. The leaf samples were oven dried at 70°C for 48 h and then the dry weight (DW) was determined. Ultimately, using the following formula, RWC was calculated:
Plastid pigment measurements. Shoot fresh tissue samples (0.1 g each sample) were grounded in 5 mL of acetone (80%; v/v) in order to extract photosynthetic pigments (i.e. chlorophyll a, b and carotenoids). The samples absorbance was measured at 645, 663, and 470 nm in a T80+ UV–Vis spectrophotometer (PG Instrument Ltd., United Kingdom) [11].
Determination of total soluble sugar. A 0.1 g of dry shoot was stored in 10 mL of alcohol for 1 h in an incubator. Then, the extract was poured into a 25 mL volumetric flask and the remainder was re-extracted. By adding alcohol, the final volume was reached to 25 mL. 1 mL aliquot was transmitted to a thick-walled test tube and 1 mL of 5% phenol was added to it and mixed. Then 5 mL of analytical grade sulphuric acid was added to it and thoroughly mixed by vertical agitation (using a glass rod). The test tube was cooled in the air for an exothermic reaction. Absorbance was recorded at 485 nm on T80+ UV–Vis spectrophotometer (PG Instrument). Using a glucose solution, the corresponding concentration was determined against a standard curve. The amount of sugar was expressed as mg/g dry weight [12].
Determination of hydrogen peroxide (H2O2) content. In order to determine H2O2 content in the shoot of lemon balm plants, 0.1 g fresh tissues were homogenized using 5 mL of 0.1% w/v trichloroacetic acid and centrifuged (12000 g for 15 min). Then, the supernatant (0.5 mL) was added to 0.5 mL of potassium phosphate buffer (10 mM, pH 7.0) and 1 mL of potassium iodide (1 M). The upper phase was aliquoted to read its absorbance at 390 nm. H2O2 was used for plotting the calibration curve in order to calculate H2O2 concentration [13]. Based on the standard curve, the H2O2 content was expressed as μmol/g fresh weight.
Assessment of malondialdehyde (MDA) content. Shoot fresh tissues (0.1 g each) were crushed and blended in 5 mL of trichloroacetic acid solution (0.1% w/v) and centrifuged (12 000 g for 15 min). 2 mL of the supernatant was added to 2 mL of the thiobarbituric acid (0.6% w/v). The mixture incubated for 30 min, at 95°C; the samples were then cooled down on the ice and were centrifuged (4000 g for 20 min). The absorbance of the supernatant was measured at 532 nm. The amount of MDA calculated based on Heath and Packer [14]. The MDA content was calculated using an extinction coefficient 155 mM–1 cm–1 and expressed in units of nmol/g fresh weight.
Determination of proline content. In order to determine the amount of proline free amino acid content in the shoot, 0.1 g of fresh tissues was homogenized with 10 mL of 3% aqueous sulfosalicylic acid and briefly centrifuged. 2 mL of the supernatant was blended with acid ninhydrin and glacial acetic acid (2 mL of each). The mixture in the test tube was kept in a water bath for 1 h at 100°C. The reaction mixture was extracted with toluene (4 mL). The absorbance of the mixture was determined at 520 nm after being cooled down to the room temperature. The standard calibration curve was plotted using appropriate proline concentrations [15]. Finally, based on the standard curve, proline content was calculated in μmol/g fresh weight.
Antioxidant enzymes assay. To estimate the antioxidant enzyme activities, the crude enzyme extract was utilized. Fresh leaf samples (0.5 g) were homogenized in 3 mL of 50 mM potassium phosphate buffer, pH 7.8, with 1 mM Na2S2O5 and 1% (w/w) polyvinylpyrrolidone, adding 1 mM ascorbate in the case of ascorbate peroxidase (APX) assay. The mixture was centrifuged at 15 000 g at 4°C for 20 min.
Superoxide dismutase (SOD) activity was determined spectrophotometrically based on measuring its capacity to inhibit the photochemical reduction of nitro-blue tetrazolium according to Beauchamp and Fridovich [16]. The reaction mixture included 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 0.1 mM EDTA, 2 μM riboflavin and 75 μM NBT and the required amount of enzyme extract. The solution absorbance was then measured at 560 nm.
To measure peroxidase (POX) activity, the Upadhyaya et al. [17] method was used. The reaction mixture included 2.5 mL of 50 mM potassium phosphate buffer (pH 6.1), 1 mL of guaiacol (1%), 1 mL of H2O2 (1%) and the required amount of enzyme extract. The increment in absorbance was recorded at 420 nm spectrophotometrically.
For catalase (CAT) activity assay, the procedure introduced by Upadhyaya et al. [17], was used. The reaction mixture involved 25 mM sodium phosphate buffer (pH 7.0), 10 mM H2O2 and the required amount of enzyme extract. The reduction in absorbance due to the reduction of extinction of H2O2 (extinction coefficient 39.4 mM–1 cm–1) was recorded at 240 nm.
To estimate APX activity, the method of Nakano and Asada [18] was applied after the reduction in A290 (extinction coefficient 2.8 mM–1 cm–1) for 1 min in 1 mL of a reaction mixture with 100 mM potassium phosphate buffer (pH 7.0), 2 mM H2O2, 0.5 mM ascorbate and the required amount of enzyme extract. To initiate the reaction, the crud enzyme extract was added to the reaction mixture. For the low non-enzymatic reduction of ascorbate by H2O2, corrections were performed.
Essential oil content. Plant aerial parts were shade-dried for a week. In order to extract their EOs, the dried samples were hydro-distilled in Clevenger apparatus for 3 h. The achieved aqueous EO was dehydrated by sodium sulfate. Then EO content was calculated based on its volume to the dry weight of the plant sample (w/w, %).
Caffeic and rosmarinic acid content. The extraction of phenolic acids was carried out according to the method described by Hazrati et al. [19] with some modification. When the leaf samples were ground, 0.5 g of leaf powder was weighed and 10 mL of methanol was added and the mixture was put in the Ultrasonic Device (Powersonic 405, Hwashing Technology, South Korea) for 40 minutes. Then the samples were filtered, and centrifuged for 2 min in 3000 rpm. Finally, the solution was kept in the dark small container at 4ºC until the HPLC analysis.
A waters liquid chromatography apparatus consisting of a Separations Module e2695 and a Dual λ Absorbance Detector 2487 (Waters, United States) were used for the HPLC analysis. The injection was done using Auto-sampler injector equipped with a 100 µL loop. Data acquisition and integration were performed with Millennium32 software. The chromatographic assay was performed on a 15 cm × 4.6 mm with pre-column, Eurospher 100-5 C18 analytical column provided by waters (sunfire) reversed phase matrix (3.5 μm) (Waters, USA). The elution was carried out in a gradient system with methanol as the organic phase (solvent A) and distilled water (solvent B) with the flow-rate of 0.5 mL/min. Peaks were monitored at 327 nm wavelength, and identified based on the retention time and spike method. Finally, the standard external method was applied to quantify the studied phenolic acids. Standard samples were purchased from Sigma-Aldrich, United States (powder, 98% purity). Injection volume was 20 µL and the temperature was maintained at 25°C [20].
Statistical Analysis. The obtained data were investigated by analysis of variance by SAS statistical software. Means were compared using Duncan’s Multiple Range Test at 0.01 probability level (P ≤ 0.01).
RESULTS
Total Dry Matter
Total dry matter was affected by the interactions between irrigation regime and pluramin application (Tables 1, 2). Total dry matter increased by pluramin application in both irrigation regimes. Moreover, the maximum dry matter was observed at 2.5 g/L pluramin (12.50 g/plant), while the lowest value (4.34 g plant–1) was recorded when no pluramin was applied under water-deficit stress. The dry matter of lemon balm showed a reduction under water-deficit stress condition. The observed decrease in dry matter of pluramin foliar treated plants was less than other treatments which may be due to the stimulatory effects of pluramin treatment (Table 3). The amount of total dry matter increased from 8.51 g/plant in non-inoculated samples to 9.31 g/plant in inoculated ones (Table 4).
Relative Water Content
Analysis of variance revealed two-way interactions between irrigation regime and Pseudomonas fluorescens PF-135 inoculation, irrigation regime and pluramin, and pluramin and P. fluorescens PF-135 inoculation which all those remarkably influenced the RWC (Table 1). Water deficiency significantly reduced RWC of the plants. However, the lower RWC was recorded in plants treated with P. fluorescens PF-135 inoculation and pluramin foliar application which was due to its stimulatory impacts on RWC under both water irrigation and normal condition (Tables 3, 5). Pluramin-treated plants exhibited higher RWC under water deficient situation (86.81%). The highest RWC (91.02%) was observed in sample treated by P. fluorescens PF-135 and pluramin foliar application (2.5 g/L) (Table 6).
Pigments Content
Irrigation regimes, P. fluorescens PF-135, and pluramin significantly affected leaf chlorophyll content (Table 1). The maximum (3.10 mg/g fr wt) and minimum (2.11 mg/g fr wt) total chlorophyll contents were observed in lemon balms inoculated under sufficient irrigation and non-inoculated ones under water-deficit stress conditions (Table 5). Moreover, higher chlorophyll b (21%) and total chlorophyll contents (14%) were recorded in samples that received 2.5 g/L foliar pluramin (Table 4). P. fluorescens PF-135-inoculated samples exhibited elevated chlorophyll content comparing to water-deficit stress and normal conditions (Table 5). In general, P. fluorescens PF-135 inoculation enhanced chlorophyll a, b, and total chlorophyll content under water deficiency as compared to the control treatment (in any case of irrigation regimes). Chlorophyll a content was significantly higher where both P. fluorescens PF-135 and pluramin were used. Maximum chlorophyll a (1.39 mg/g fr wt) was detected in plants treated with P. fluorescens PF-135 inoculation and pluramin foliar application together (2.5 g/L) (Table 6).
Irrigation regimes and P. fluorescens PF-135 inoculation had a significant effect on carotenoid contents (Table 1). Carotenoid content was reduced as a result of water deficiency. Furthermore, P. fluorescens PF-135 inoculation declined the carotenoid content in a way that the maximum carotenoid amount (0.81 mg/g fr wt) was recorded in well-irrigated plants (inoculated or non-inoculated), while lowest carotenoid content (0.46 mg/g fr wt) was observed in plants grown under water deficiency condition with no P. fluorescens PF‑135 inoculation (Table 5). A significant difference in carotenoid content was observed in the case of pluramin foliar application since the highest amount (0.78 mg/g fr wt) was recorded when 2.5 g/L pluramin was used (Table 4). Pluramin foliar application in different concentrations enhanced the photosynthetic pigments of lemon balm (Table 4).
Caffeic Acid and Rosmarinic Acid
Typical HPLC chromatograms of phenolic acid peaks recorded at 327 nm are shown in Fig. 1. A significant three-way interaction was observed among irrigation regime, P. fluorescens PF-135 inoculation, and pluramin concerning caffeic and rosmarinic acid content (Table 2). Water deficiency had different impacts on caffeic and rosmarinic acids which was dependent on the P. fluorescens PF-135 inoculation and pluramin metabolic activator (Table 7). Elevated levels of caffeic and rosmarinic acid were observed under drought stress as compared to the normal conditions. In pluramin foliar and P. fluorescens PF-135 inoculation treatments, caffeic and rosmarinic acid content were incremented under both irrigation plans. Maximum rosmarinic acid content (31.36 mg/g dry wt) was recorded under drought stress in the samples treated by the P. fluorescens PF-135 inoculation and pluramin foliar application (2.5 g/L); while the well-irrigated samples with no other treatment exhibited the lowest content of these components (10.49 mg/g dry wt). In the case of caffeic acid, the highest level (5.04 mg/g dry wt) was observed under water deficiency in samples treated by P. fluorescens PF-135 inoculation but no pluramin foliar application. Even lower caffeic and rosmarinic acid contents were detected in well-irrigated control samples (Table 7). In general, higher caffeic and rosmarinic acid contents were observed under water deficit condition as compared with well-irrigated lemon balm which were inoculated by P. fluorescens PF-135 and received pluramin foliar application (Table 7).
Essential Oil Content
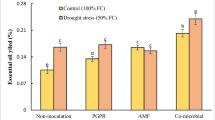
The ANOVA results for comparison of EO content among the treatments are listed in Table 2. P. fluorescens PF-135 inoculation, water-deficit stress, and their interaction significantly affected EO content. Enhanced content of EO was observed in response to more severe water deficiency. P. fluorescens PF-135 inoculation also increased the EO content, and the highest EO content (3.93%) was recorded under the drought stress in inoculated samples; while the lowest EO contents (2.85%) were found in samples grown under the normal condition without inoculation (Table 5). The EO content was also remarkably affected by pluramin foliar application (Table 2). The maximum EO content was observed at 2.5 g/L of pluramin foliar application (3.54%) (Table 4).
Hydrogen Peroxide (H2O2) Content
A significant three-way interaction was found among irrigation regime, P. fluorescens PF-135 inoculation, and pluramin concerning H2O2 content (Table 1). Under well-irrigation condition, the lower H2O2 contents were observed in the inoculated samples that received pluramin foliar application. Even lower H2O2 content was recorded in those samples received both pluramin and P. fluorescens PF-135 inoculation together. H2O2 content showed an increasing trend under water deficiency, however, pluramin foliar application and P. fluorescens PF-135 inoculation reduced H2O2 content. The maximum H2O2 content (4.613 μmol/g fr wt) was detected in non-treated samples grown under water deficiency; while the lowest H2O2 content (0.707 μmol/g fr wt) was observed in pluramin-treated (2.5 g/L) and P. fluorescens PF-135-inoculated samples under proper irrigation (Table 7). Co-application of P. fluorescens PF-135 and foliar pluramin resulted in lower H2O2 accumulation as compared with their individual application. In general, water deficiency dramatically increased the H2O2 content during the plant growth. Water deficiency also resulted in significant enhancement of H2O2 content, indicating the extent of its oxidative damage in plants.
Malondialdehyde Content
MDA content can be considered as a measure of lipid peroxidation. Under normal irrigation, P. fluorescens PF-135 and pluramin treatments, the MDA contents were declined. This reduction was especially intensified when the pluramin and P. fluorescens PF-135 were applied together (Tables 5, 6). In this case, P. fluorescens PF-135 was more effective than pluramin. Minimum MDA (0.75 nmol/g fr wt) content was detected in pluramin-treated samples which were inoculated with P. fluorescens PF-135 (Table 6). Foliar application of pluramin declined MDA accumulation under both irrigation regimes (Table 3). Besides, pluramin and P. fluorescens PF-135 also decremented the MDA amount (Table 6).
Proline Content
Irrigation, P. fluorescens PF-135, and pluramin significantly affected proline content of the samples (Table 1). Lemon balms grown under water deficiency contained more proline as compared with those planted in normal situations (Table 4). The co-application of P. fluorescens PF-135 and pluramin dramatically raised the proline content, which can be attributed to PGPR-induced regulation of the osmotic balance and conservation of the bioenergetics of the cells. In addition, pluramin foliar application significantly increased the proline content. The highest proline content (27.79 μmol/g fr wt) was recorded in plants which received 2.5 g/L pluramin (Table 4).
Total Sugar Content
Total sugar content was under the influence of three-way interactive effects of irrigation, P. fluorescens PF-135, and pluramin (Table 2). According to Table 7, the highest total sugar content (12.23 mg/g dry wt) was recorded in samples grown under water deficiency which were treated by P. fluorescens PF-135 incubation and foliar application of pluramin. Total sugar content also increased upon enhancing the exogenous application of pluramin concentration under both irrigation regimes. Co-application of pluramin and P. fluorescens PF-135 led to an increase the total soluble sugars of the samples as compared with their controls.
Antioxidant Enzyme Activity
This study aimed to examine different antioxidative responses of lemon balm to water deficiency and exogenous application of pluramin with or without P. fluorescens PF-135 inoculation. To this end, catalase (CAT), peroxidase (POX), superoxide dismutase (SOD), and ascorbate peroxidase (APX) activities of the samples subjected to water deficiency and P. fluorescens PF-135 and pluramin treatments were assessed. A statistically significant difference was detected in antioxidant enzymes behaviours under different irrigation regimes. POX, CAT, APX, and SOD exhibited similar trends when the plant exposed to water deficiency. Antioxidant activities enhanced significantly under water-deficit stress condition (Figs. 2–4). Upon P. fluorescens PF-135 inoculation, POX, CAT, APX, and SOD activities increased when plants encountered water deficiency (42, 37, 34, and 69%, respectively) (Fig. 2). Moreover P. fluorescens PF-135 inoculation enhanced antioxidants activities in both irrigation regimes. The maximum POX, CAT, APX and SOD activities were observed under water deficit condition in the P. fluorescens PF-135 inoculated samples (1.05, 19.20, 3.70 and 82.24 U/mg protein, respectively) (Fig. 2). Furthermore, pluramin application (regardless of the irrigation condition) resulted in an increase in both CAT and APX activities. The highest CAT and APX activities were observed in 2.5 g/L pluramin treatment under water-deficit stress (17.55 and 0.92 U/mg protein, respectively) (Fig. 3).
DISCUSSION
The pluramin metabolic activator can positively affect plant’s growth by enhancing its amino acid content which will act as a source of energy and carbon under sugar-scarce conditions. Pluramin releases organic acid and ammonia which are the building blocks of amino acids. Organic acids will then enter the Krebs cycle where they breakdown and release energy through respiration. On the other hand, amino acids offer a source of nitrogen, which can be more rapidly stored by the cells compared to inorganic nitrogen [6]. Amino acids are drastically involved in crop assimilation and protein metabolism which is vital for the formation of new cells; thus, they can enhance the dry matter yield. Furthermore, foliar application of amino acid has a positive influence on the chlorophyll content and its constituents [6] which can positively influence the chemical composition and dry matter.
P. fluorescens PF-135 inoculation also positively influences plant’s growth and dry matter. By colonizing the endo-rhizosphere/rhizosphere of plants, these useful plant growth-promoting rhizobacteria can enhance plant growth through various mechanisms [2]. One of these mechanisms is stimulating plants’ growth and yield through declining their ethylene production. Amino Cyclopropane-1-Carboxylate acid (ACC) is an ethylene forerunner that can be converted to ethylene through ACC-oxidase enzyme activity. ACC-deaminase enzyme bacteria employ ACC as the only source of nitrogen [21]. A decline in ethylene production and hence prevention from its accumulation at water-deficit stress can negatively affect the growth, yield, and the plant defence mechanism [4]. Numerous studies have shown that plant growth-promoting rhizobacteria inoculation can promote plant growth and development under water deficiency [21]. This study suggests the beneficial role of P. fluorescens PF-135 in the plants grown under both designed irrigation regimes demonstrated by elevated dry weight of the samples.
RWC is a useful measure to assess physiological traits of plants. Elevated RWC content results in lower stomata resistance and CO2 accessibility. Pluramin application promotes plant development as it supplies water by absorbing water molecules from the soil that can be released depending on the plants need. RWC is a suitable physiological indicator reflecting the strength of water-deficit stress. In this research, the highest dry matter was observed in samples with the maximum RWC. The RWC of drought-exposed samples was alleviated upon P. fluorescens PF-135 inoculation and exogenous pluramin treatment implying that these metabolic activators may help the plant to maintain its water stability despite the water deficiency. Such increase in RWC due to pluramin and P. fluorescens PF-135 treatment could be assigned to the enhanced water absorption. It is reported that PGPR inoculation also enhanced biomass, RWC, and water potential in drought-stressed Mentha pulegium [2] and Zea mays [3], through incrementing their proline content.
The dual inoculation led into a significant increase in total chlorophyll, chlorophyll a, and b content. Chlorophyll plays a decisive role in photosynthesis, thus can be regarded as a measure of water tolerance. The PGPR can elevate the photosynthetic pigments through increasing the stomatal conductance, photosynthetic potential, and water and ions absorption. These findings are consistent with the study by Levy and Krikun [22] who found that plant growth-promoting rhizobacteria can stimulate photosynthesis pigments and photosynthesis rate of several aromatic and medicinal species.
Pluramin can delay the chlorophyll loss thus increasing the light capture efficiency and consequently improve the net photosynthetic rate. Such increase in chlorophyll contents after pluramin foliar application could be related to higher levels of amino acids in the treated plants which can raise the chlorophyll content and increase the different growing characteristics. Moreover, this improving impact of pluramin can increase the thylakoid membrane rigidity [6]. Pluramin metabolic activator can bind to the charged phospholipid head groups of the membrane and affect the stability and permeability of the membrane. The pluramin contains substantial amount of auxins, cytokinins, and nitrogen, which can increase the chlorophyll content in the leaves [9].
Rosmarinic and caffeic acid are the 3,4-dihydroxy phenyl lactic acid esters abundant in the Lamiaceae. These phenolic acid compounds can help plants to preserve their water balance under water deficiencies. The increase in rosmarinic acid is assigned to the expression of the rosmarinic acid synthase gene in the in vitro lemon balm cultures. Such increase in rosmarinic acid contents in response to water deficiency has been previously reported in Thymus vulgaris L. plants [23].
Chickpea inoculation by plant growth-promoting rhizobacteria also resulted in enhanced phenolic compounds contents (including caffeic, tannic, chlorogenic, gallic, and cinnamic acids) [24]. del Rosario Cappellari et al. [25] showed that plant growth-promoting rhizobacteria inoculation can increase the total phenolic content in Tagetes minuta. Application of biotic elicitors has been considered as one of the approaches to enhance the content of major secondary metabolites in medicinal species. Plant growth-promoting rhizobacteria-generated molecules have a decisive role in the secondary metabolite formation pathways [24]. These compounds help to protect plants against various abiotic stresses. The results showed that pluramin and P. fluorescens PF-135 treatment increased the draught-resistance in lemon balm through production of phenolic compounds.
Elevated EO content under water deficiency may be associated to higher contents of terpenes as a result of poor carbon distribution for the growth process that leads to a trade-off between growth and resistance. The EO content showed a high dependency on the P. fluorescens PF-135 in both irrigation regimes. This can be attributed to the number of glandular hairs in the plants. Scanning electron microscopy description of leaves revealed an enhancement in glandular hairs in samples inoculated with P. fluorescens PF-135. These results are in line with the report of Copetta et al. [26] who stated that plant growth-promoting rhizobacteria inoculation of Ocium basilicum L. by Gigaspora margarita, Glomus mosseae, and Gigaspora rosea increased the number of glandular hairs in its leaves. Zolfaghari et al. [27] also documented an increase in the EO content in two Mentha species (piperita and arvensis) after plant growth-promoting rhizobacteria inoculation. They concluded that the increase in the EO content is related to the modifications in plant secondary metabolism and the number of glands per leaf. Such increase in the EO content can be also assigned to plant growth-promoting rhizobacteria inoculation. Moreover, according to Copetta et al. [26], the generation of terpenoids and constituents of EOs with fungicidal features can be a response to fungi colonization which increase the concentration of these metabolites in plant growth-promoting rhizobacteria-treated samples. del Rosario Cappellari et al. [25] reported an improvement in the biosynthesis of the main EO components after plant growth-promoting rhizobacteria inoculation (up to 70%) in Tagetes minuta. An enhanced EO content was also reported due to the use of amino acids as they are involved in signalling stress responses and secondary metabolism in aromatic plants [6].
The enzyme activities have been shown to improve the membrane lipid peroxidation as well as its injury, hence enhancing the water-resistance in lemon balm samples. The P. fluorescens PF-135 and pluramin treatments promoted the plant development and yield while declined its stress vulnerability. Furthermore, amino acids play a key role in glyoxylate formation pathways. Glyoxylate is capable of decreasing H2O2 content and hence it can reduce lipid peroxidation [6]. Proline, also plays a pivotal role in plants development under drought stress through osmoregulation and bioenergetics of the cells and promote the plants' drought-resistance by maintaining turgor through osmotic adjustment in stressed plants [2].
MDA content exhibited an increasing trend with abiotic stress which can be regarded as an index of lipid peroxidation or injury to organelle and plasma lemma membranes. MDA can be a considered as a sign of stress-induced damages. MDA increased in drought treatments implying the influence of water deficiency on the plant membranes. Higher MDA contents is an indication of decreased antioxidant enzyme resistance as well as an increased lipid peroxidation. The results showed that pluramin promoted the activity of antioxidant enzymes and decreased the oxidative stress. Pluramin evidently regulated the plant’s response to water deficiency [28]. Reduced MDA content in drought-stressed bio-fertilizer-inoculated samples is in line with the reports linking the declined lipid peroxidation to proper stress-resistance mechanisms of the plant [28]. Also, pluramin foliar spraying and P. fluorescens PF-135 inoculation relatively enhanced the proline content while decreased the MDA level in the water-deficient samples. This could be related to the membrane protection against the free radicals suggesting that proline responds to hydroxyl radicals by producing on-toxic hydroxyl proline.
Plants can accumulate sugar and proline to modulate cell’s damage as a result of low water potential. Sugar accumulation in drought stress helps the plant to maintain its membrane stability, protect the membrane fusion, and sustain the proteins function. These outcomes are in coincide with a report by Pirlak and Köse [29] that showed plant growth-promoting rhizobacteria enhanced the total sugar content.
Enzymatic antioxidants such as POD, CAT, SOD, and APX play a pivotal role in plant resistance against reactive oxygen species accumulation. Our results showed that pluramin treatment and P. fluorescens PF-135 inoculation significantly elevated the enzymatic antioxidant activities of the samples (Fig. 4). Furthermore, while P. fluorescens PF-135 can provide various defence mechanisms against water deficiency (e.g., by promoting plant growth), it could also change the plants gene expression and reduce the chance of exposure to these stresses [7]. For instance, different P. fluorescens PF-135 have been investigated to enhance the CAT, SOD and GPX activities to detoxify the reactive oxygen species. P. fluorescens PF-135 also increases the activity of reactive oxygen species-scavenging enzymes (e.g. APX and CAT) helping the plant to balance the adverse impacts of reactive oxygen species during water deficiency. In the present study, foliar application of pluramin resulted in a substantial increment in POD, POX, and CAT activities (Figs 2–4). Foliar application of spermidine also increased the activity of antioxidant enzymes in cucumber seedlings to modulate the temperature stress [30]. Enhanced antioxidant defense in pluramin-treated samples improved the cell membrane stability. Water deficiency signalling develops adaptive responses and a remarkable improvement in the activities of both non-enzymatic and enzymatic antioxidants.
To conclude, this study is the first report that analysed the effects of exogenous application of metabolic activator combined with P. fluorescens PF-135 inoculation on the drought-stressed medicinal and aromatic plants. The results indicated a positive impact of synthetic pluramin, alone or in combination with P. fluorescens PF-135, in lemon balm under water deficiency condition. Water deficit-stress elevated the MDA and H2O2 contents. The exogenous pluramin and P. fluorescens PF-135 inoculation treatment alleviated water stress-induced damages by declining the oxidants content. On the other hand, pluramin and P. fluorescens PF-135 inoculation increased the contents of soluble sugars, proline, caffeic acid, rosmarinic acid and EO content, as well as enzyme activities. The observed benefits of P. fluorescens PF-135 and metabolic activator in this study may offer a wide range of potentials to use them in agronomy (especially under stress condition). They may also contribute to resolving the climate change, drought, and food production issues. Moreover, further information on pathways involved in pluramin and P. fluorescens PF-135 induced changes in plants can be helpful to deal with inevitable water shortage problem in many parts of the world. Elucidating the involved mechanisms and pathways can greatly contribute to develop water deficiency tolerance in lemon balm as well as other crops.
REFERENCES
Miraj, S., Rafieian-Kopaei, and Kiani, S., Melissa officinalis L.: a review study with an antioxidant prospective, J. Evidence-Based Complementary Altern. Med., 2017, vol. 22, p. 385.
Asghari, B., Khademian, R., and Sedaghati, B., Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. (Amsterdam), 2020, vol. 263, p. 109.
Sandhya, V.S.K.Z., Ali, S.Z., Grover, M., Reddy, G., and Venkateswarlu, B., Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress, Plant Growth Regul., 2010, vol. 62, p. 21.
Glick, B.R., Cheng, Z., Czarny, J., and Duan, J., Promotion of plant growth by ACC-deaminase-producing soil bacteria, Eur. J. Plant Pathol., 2007, vol. 119, p. 329.
Shehata, S.M., Abdel-AzemHeba, S., Abou El-Yazied, A., and El-Gizawy, A.M., Effect of foliar spraying with amino acids and seaweed extract on growth chemical constitutes, yield and its quality of celeriac plant, Eur. J. Sci. Res., 2011, vol. 58, p. 257.
Bekebrede, A.F., Keijer, J., Gerrits, W.J.J., and Boer, V.C.J., The molecular and physiological effects of protein-derived polyamines in the intestine, Nutrients, 2020, vol. 12, p. 197.
Pereyra, M.A., Zalazar, C.A., and Barassi, C.A., Root phospholipids in Azospirillum-inoculated wheat seedlings exposed to water stress, Plant Physiol. Biochem., 2006, vol. 44, p. 873.
García-Caparrós, P., Romero, M.J., Llanderal, A., Cermeño, P., Lao, M.T., and Segura, M.L., Effects of drought stress on biomass, essential oil content, nutritional parameters, and costs of production in six Lamiaceae species, Water, 2019, vol. 11, p. 573.
Mohammadi, H., Dashi, R., Farzaneh M., Parviz, P., and Hashempour, H., Effects of beneficial root pseudomonas on morphological, physiological, and phytochemical characteristics of Satureja hortensis (Lamiaceae) under water stress, Braz. J. Bot., 2017, vol. 40, p. 41
Proteo International, 1995. http://www.proteoint.com.
Lichtenthaler, H.K. and Wellburn, A.R., Determination of total carotenoids and chlorophylls a and b in leaf extracts in different solvents, Biochem. Soc. Trans., 1983, vol. 11, p. 591.
Nielsen, S.S., Phenol-sulfuric acid method for total carbohydrates, in Food Analysis Laboratory Manual, Food Science Texts Ser., Boston, MA: Springer-Verlag, 2009, p. 47.
Velikova, V., Yordanov, I., and Edreva, A., Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines, Plant Sci., 2000, vol. 151, p. 59.
Heath, R.L. and Packer, L., Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation, Arch. Biochem. Biophys., 1968, vol. 125, p. 189.
Bates, L.S., Waldern, R.P., and Tear, I.D., Rapid determination of free proline for water stress studies, Plant Soil, 1973, vol. 39, p. 205. https://doi.org/10.1007/BF00018060
Beauchamp, C. and Fridovich, I., Superoxide dismutase: improved assays and an assay applicable to acrylamide gels, Anal. Biochem., 1971, vol. 44, p. 276.
Upadhyaya, A., Sankhla, D., and Davis, T.D., Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves, J. Plant Physiol., 1985, vol. 121, p. 453.
Nakano, Y. and Asada, K., Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts, Plant Cell Physiol., 1981, vol. 22, p. 867.
Hazrati, S., Ebadi, M.T., Mollaei, S., and Khurizadeh, S., Evaluation of volatile and phenolic compounds, and antioxidant activity of different parts of Ferulago angulata (schlecht.) Boiss, Ind. Crop Prod., 2019, vol. 140, p. 111589.
Wen, D., Li, C., Di, H., Liao, Y., and Liu, H., A universal HPLC method for the determination of phenolic acids in compound herbal medicines, J. Agric. Food Chem., 2005, vol. 53, p. 6624.
Pandey, S. and Gupta, S., ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants, Front. Microbiol., 2019, vol. 10, p. 1506.
Levy, Y. and Krikun, J., Effect of vesicular-arbuscular mycorrhiza on Citrus jambhiri water relations, New Phytol., 1980, vol. 85, p. 25.
Trócsányi, E., György, Z., Inotai, K., Szabó, K., Pluhár, Z., Radácsi, P., Malekzadeh, M., and Németh-Zámboriné, E., Enhanced rosmarinic acid accumulation and rosmarinic acid synthase gene expression under drought stress in thyme (Thymus vulgaris), Planta Med., 2015, vol. 81, p. 46.
Basha, S.A., Sarma, B.K., Singh, D.P., Annapurna, K., and Singh, U.P., Differential methods of inoculation of plant growth-promoting rhizobacteria induces synthesis of phenylalanine ammonia-lyase and phenolic compounds differentially in chickpca, Folia Microbiol., 2006, vol. 51, p. 463.
del Rosario Cappellari, L., Santoro, M.V., Nievas, F., Giordano, W., and Banchio, E., Increase of secondary metabolite content in marigold by inoculation with plant growth-promoting rhizobacteria, Appl. Soil Ecol., 2013, vol. 70, p. 16.
Copetta, A., Lingua, G., and Berta, G., Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var Genovese, Mycorrhiza, 2006, vol. 16, p. 485.
Zolfaghari, M., Nazeri, V., Sefidkon, F., and Rejali, F., Effect of arbuscular mycorrhizal fungi on plant growth and essential oil contents and composition of Ocimum basilicum L., Iran. J. Plant Physiol., 2013, vol. 3, p. 643.
Van Ha, C., Leyva-González, M.A., Osakabe, Y., and Tran, U.T., Positive regulatory role of strigolactone in plant responses to drought and salt stress, Proc. Natl. Acad. Sci. U.S.A., 2014, vol. 111, p. 851.
Pirlak, L. and Köse, M., Effects of plant growth promoting rhizobacteria on yield and some fruit properties of strawberry, J. Plant Nutr., 2009, vol. 32, p. 1173.
Tian, J., Wang, L., Yang, Y., Sun, J., and Guo, S., Exogenous spermidine alleviates the oxidative damage in cucumber seedlings subjected to high temperature, J. Am. Soc. Hortic. Sci., 2012, vol. 137, p. 11.
Funding
This study was funded by Azarbaijan Shahid Madani University, Tabriz, Iran (project no. 99/1510).
Author information
Authors and Affiliations
Contributions
H. Mohammadi and S. Saeedi designed the experiments. H. Mohammadi, S. Hazrati and S. Saeedi analyzed the data and wrote the manuscript which was further reviewed by M. Brestic. M. Brestic supervised the project.
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Mohammadi, H., Saeedi, S., Hazrati, S. et al. Physiological and Phytochemical Responses of Lemon Balm (Melissa officinalis L.) to Pluramin Application and Inoculation with Pseudomonas fluorescens PF-135 under Water-Deficit Stress. Russ J Plant Physiol 68, 909–922 (2021). https://doi.org/10.1134/S1021443721050125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443721050125