Abstract
Solid polymer electrolytes (SPE) based on poly-(vinyl alcohol) (PVA)0.7 and sodium iodide (NaI)0.3 complexed with sulfuric acid (SA) at different concentrations were prepared using solution casting technique. The structural properties of these electrolyte films were examined by X-ray diffraction (XRD) studies. The XRD data revealed that sulfuric acid disrupt the semi-crystalline nature of (PVA)0.7(NaI)0.3 and convert it into an amorphous phase. The proton conductivity and impedance of the electrolyte were studied with changing sulfuric acid concentration from 0 to 5.1 mol/liter (M). The highest conductivity of (PVA)0.7(NaI)0.3 matrix at room temperature was 10−5 S cm−1 and this increased to 10−3 S cm−1 with doping by 5.1 M sulfuric acid. The electrical conductivity (σ) and dielectric permittivity (ε′) of the solid polymer electrolyte in frequency range (500 Hz–1 MHz) and temperature range (300–400) K were carried out. The electrolyte with the highest electrical conductivity was used in the fabrication of a sodium battery with the configuration Na/SPE/MnO2. The fabricated cells give open circuit voltage of 3.34 V and have an internal resistance of 4.5 kΩ.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Studies on the electrical properties of polymer electrolyte have attracted much attention in view of their application in electronic devices like solar cells, fuel cells, and solid-state batteries. Electrical conduction in polymers has been studied aiming to understand the nature of the charge transport prevalent in these materials. The electrical properties of polymer can be suitably modified by the addition of dopants. Poly-(vinyl alcohol) is an exceptional polymer; PVA is a potential material having high dielectric strength, good charge storage capacity, and dopant-dependent electrical and optical properties. It has a carbon chain backbone with hydroxyl groups attached to methane carbons. These OH-groups can be a source of hydrogen bonding and hence assist the formation of polymer electrolytes [1–6]. Recent studies have dominated lithium ion conducting systems for their potential use in solid-state batteries with high cell voltages and energy densities. A few attempts have tried polymer electrolytes based on sodium ion complexed films [7–9]. The main advantage of using sodium metal ion is its availability in abundance at a cheaper cost than lithium. Further, more softness of the material makes it easier to achieve good contact with other components in the battery. V. Rao et al. [6] have a solid polymer electrolyte development of PVA complexed with NaI systems for sodium battery. They have got the conductivity of up to 1.12 × 10−5 S cm−1, from PVA/NaI wt.% ratio of 70:30.
Sulfuric acid H2SO4, is a strong mineral acid that has a high electrical conductivity due to an intra-molecular proton-switch mechanism, its incorporation in a polymer system may be expected to enhance its electrical performance [1, 10]. We propose to develop a radically new, alternative ionic-conducting electrolyte (or membrane) that is based on compounds whose chemistry and properties are intermediate between those of a normal acid, such as H2SO4, and a normal salt, such as NaI and not a hydrated polymer. Thus, composite membranes will be developed, in which a solid acid is embedded in an inert polymer matrix, with the polymer providing mechanical support and enhancing chemical stability. Hence the current work is aimed to improve the electrical and electrochemical properties of (PVA)0.7(NaI)0.3 through doping in different proportions of sulfuric acid. Structure and ionic conductivity studies are performed on the solid polymer electrolyte (SPE). With a SPE of optimum composition, solid-state Na/SPE/MnO2 cell is assembled, and its electrochemical performances will be briefly examined to evaluate the applicability of the solid polymer electrolyte to solid-state sodium batteries.
Experimental
Preparation of SPE
PVA with molecular weight of ~1,800 was obtained from QualiKems chemical company (India). The PVA-based membranes were prepared by dissolving 0.7 g of PVA in distilled water, to get a 10 wt.% solution. The solution was left for 24 h at 50 °C in order to obtain a transparent low-viscous liquid. At this point, approximately 0.3 g of NaI (QualiKems) was added to the solution. The solution was left to stir for 20 min. Then 1 cm3 of x M H2SO4(GPR-ADWIC; x = 0, 1.7, 3.4, and 5.1 M) solution was separately added to the beakers. The solution was left to stir for 24 h. The transparent solution was then cast on a Petri-glass dish for 2 weeks. The final product was vacuum-dried thoroughly.
Characterization
In order to investigate the nature of these polymer electrolyte films, X-ray diffraction studies were carried out using Bruker X-ray diffractometer. The diffraction system based with Cu tube anode of wave length \( {{\text{K}}_{\alpha 1}} = 1.5460\;{\AA} \) and \( {{\text{K}}_{\alpha 2}} = 1.54439\;{\AA} \). The start angle (2θ) was 4°, and the end angle was 50°.
Samples of diameter 1 cm were taken and silver was deposited on both surfaces of the film to ensure good contacts in electrical measurements. Silver-coated samples were sandwiched between the two similar brass electrodes of a spring-loaded sample holder. The whole assembly was placed in a furnace monitored by a temperature controller. The rate of heating was adjusted to be 2 K/min. Dielectric and electrical measurements were carried out in the temperature range 303–373 K using Hitester 3532 programmable automatic RCL (Hioki) meter. The measurements were carried out over a frequency range 500 Hz–1 MHz.
Na/SPE/MnO2 cell
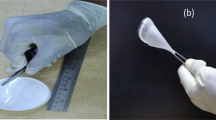
Sodium battery was fabricated from the film PVA-NaI-x M (SA) that gave the highest electrical conductivity. A 0.4 g γ-MnO2 powder (domestic source) and 0.1 g of graphite (QualiKems) powder were mixed with 10% PVA solution as binder to form cathode pellet of the battery, while 0.25 g sodium metal (domestic source) was used to form the anode pellet. A hydraulic press (2 tons cm−2) was used to compress the pellets. The cells were then assembled by sandwiching the SPE between the two electrodes, Fig. 1. Current drains ranging from 30 to 250 μA were used to plot the current–voltage (I–V) and current density–power density (J–P) curves. The average of each battery’s voltage was monitored for each current drain after 10 s of operation.
Results and discussion
X-ray diffraction analysis
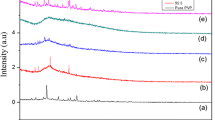
The crystallinity of the polymer film with respect to pure and SA-doped (PVA)0.7(NaI)0.3 complexes has been examined by X-ray diffraction. The diffraction pattern for pure (PVA)0.7(NaI)0.3 is shown in Fig. 2. The sample is partially crystalline with a broad peak at 2θ = 20.5°. The broad peak becomes less intense as the SA content is increased to up 5.1 M. This could be due to the disruption of the (PVA)0.7(NaI)0.3 semi crystalline structure by SA. XRD shows that the sample with (PVA)0.7(NaI)0.3:5.1 M SA is the least crystalline.
AC conductivity studies
The ionic conductivity values of the electrolytes are calculated by using the equation:
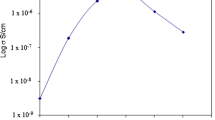
where l and A are the thickness and known area of the electrolyte film and R is the resistance of the electrolyte film at different ac frequencies. The conductivity of pure (PVA)0.7(NaI)0.3 is ≈10−5 S cm−1 [6], and it increases sharply to ≈10−3 S cm−1 on complexing the (PVA)0.7(NaI)0.3 with 5.1 M SA. Figure 3 shows the variation of conductivity, σ (at zero frequency) with the amount of SA added. It can be seen that the conductivity decreases with the amount of SA added at 1.7 M after which the conductivity increases. The results depicted in Fig. 3 seem to agree with the results obtained from XRD analysis. The degree of crystallinity affects the electrical conductivity of the samples. The amorphous nature produces greater ionic diffusivity in accordance with the high ionic conductivity, which can be obtained in amorphous polymers that have a fully flexible backbone [11].
We have attempted to explain the variation in conductivity with SA content in terms of the preparation of the films, where different amounts of SA were added to each solution. Hence, the volume of the host matrix ((PVA)0.7(NaI)0.3) was the same for all films. Two characteristic regions can be easily distinguished. The initial low-concentration region (I) in which a decrease in conductivity is observed has been ascribed to ion pair formation [12]. The subsequent linear region (II), showing an increase in conductivity beyond the minimum, could be a result of triple ion formation or redissociation effect [12].
Figure 4 represents the temperature dependence of ionic conductivity for all compositions of ((PVA)0.7(NaI)0.3)/x M SA polymer electrolytes at frequency 1 kHz. It has been found that the ionic conductivity of the membranes show two regions, the first at relatively low temperature. The ionic conductivity show relatively temperature-independent behavior. While the second region indicating Arrhenius type thermally activated process given by the relation
where σ 0 is the pre-exponential factor, E a the activation energy and k is the Boltzmann constant.
Druger et al. [5] have attributed the increase in conductivity with temperature in solid polymer electrolyte to segmental (i.e., polymer chain) motion, which results in an increase in the free volume of the system. Thus, the segmental motion either permits the ions to hop from one site to another or provides a pathway for ions to move. In other words, the segmental movement of the polymer facilitates the translational ionic motion. From this, it is clear that the ionic motion is due to translational motion/hopping facilitated by the dynamic segmental motion of the polymer. As the amorphous region increases the polymer chain acquires faster internal modes in which bond rotations produce segmental motion to favor inter- and intra-chain ion hopping, and thus the degree of conductivity becomes high. The activation energy (combination of the energy of defect formation and the energy for migration of ion) is calculated for all the prepared polymer electrolytes by linear fit of the Arrhenius plot. It has been found that activation energies increase with increasing SA concentration, table 1. This can be attributed to SO4 group need more energy to execute translational motion at relatively higher temperature.
The variation of ac conductivity with frequency for different concentrations of SA doped ((PVA)0.7(NaI)0.3 at 303 K is shown in Fig. 5. The variation of σ ac is negligible at lower frequency and shows rapid change at higher frequencies. The total conductivity σ tot can be interpreted with the help of the following equation [13]:
where σ dc is the dc conductivity, A is frequency independent constant,ω is the angular frequency and n is the index which is characteristic of the type of conduction mechanism/relaxation mechanism dominant in amorphous materials. The values of n evaluated from curves fitting between 500 kHz and 1 MHz of Fig. 5 is around 0.2 both at lower and higher temperatures. The calculated values of n as a function of SA concentration in the polymer electrolyte are listed in Table 1. It can be seen that the low value of n (n < 1) indicate that conduction mechanism via translational hopping and/or reorientation motion.
Dielectric studies
From Fig. 6, it is evident that dielectric permittivity increases with the increase of temperature for PVA/NaI/x M SA polymer electrolyte system. This behavior is typical of polar dielectrics in which the orientation of dipoles is facilitated with the rising temperature and thereby the permittivity is increased [14].
The frequency dependence of ε′ for different values of SA concentration in the (PVA)0.7(NaI)0.3 system at room temperature are shown in Fig. 7. From the plots, it is clear that the permittivity decreases monotonically with increasing frequency and attains a constant value at higher frequencies. Similar behavior was observed in other materials [1]. This is because, for polar materials, the initial value of the dielectric permittivity is high, but as the frequency of the field is raised the value begins to drop which could be due to the dipoles not being able to follow the field variation at higher frequencies and also due to the polarization effects [14]. The low-frequency dispersion region is attributed to the charge accumulation at the electrode–electrolyte interface. At higher frequencies, the periodic reversal of the electric field occurs so fast that there is no excess ion diffusion in the direction of the field. Hence ε′ decreases with the increase of the frequency in all the samples of PVA polymer electrolytes.
Battery characterization
The result of higher chemical activity for sodium metal is difficult to deal with for long periods without regular interaction with the normal air and due to our limited lab. We will be satisfied with the measurement of I–V and J–P characteristics.
The open circuit voltage (OCV) of the Na/SPE/MnO2 battery was estimated by 3.34 V. It seems that the high value of OCV qualifies this type of cells for very important applications like lithium batteries. Figure 8 shows the I–V and J–P characteristics for the Na/SPE/MnO2 battery at room temperature. The I–V curve had a simple linear form at low current which indicates that the polarization on the electrode was primarily dominated by ohmic contributions. The plot of the operating J–P suggests that the contact between electrolyte/electrodes was good. The voltage of the battery dropped to a short circuit current density of 0.17 mAcm−2 and the maximum power density was determined to be 0.25 mWcm−2. The internal resistance of the cell was then calculated by using equation:
where V is the voltage, E the electromotive force, I the current and r is the internal resistance. The internal resistance of the battery was obtained from the gradient of the I–V graph, which was 4.5 kΩ.
Conclusion
The information obtained is summarized as follows: electrical and electrochemical properties of (PVA)0.7(NaI)0.3 solid acid polymer electrolyte have been improved due to doping by sulfuric acid. The (PVA)0.7(NaI)0.3 electrolyte with 5.1 M SA has ionic conductivity of 1.7 × 10−3 S cm−1 at room temperature. This solid acid polymer electrolyte can be used to fabricate Na–MnO2 solid acid battery. Further extensive investigations are required to reduce internal resistance of Na–MnO2 solid acid battery for practical applications. Eventually, Na–MnO2 solid acid battery was characterized physicochemically and electrochemically in this study. The studies suggest Na–MnO2 solid acid battery a promising cell.
References
Sheha E, El-Mansy MK (2008) J Power Sources 185:1509
Hirankumar G, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2005) J Power Sources 144:262
Jeong S-K, Jo Y-K, Jo N-J (2006) Electrochimica Acta 52:1549
Mohamad AA, Arof AK (2007) Mater Lett 61:3096
Hema M, Selvasekerapandian S, Sakunthala A, Arunkumar D, Nithya H (2008) Phys B Condens Matter 403:2740
Balaji Bhargav P, Madhu Mohan V, Sharma AK, Rao VVRN (2007) Ionics 13:173
Subba Reddy CHV, Jin AP, Zhu QY, Mai LQ, Chen W (2006) Eur Phys J E 19:471
Anantha PS, Hariharn K (2003) J Phys Chem Solids 64:1131
Sreekanth Reddy T, Jaipal Reddy M, Ramalingaiah S, Subbarao UV (1999) J Power Sources 79:105
Martinelli A, Matic A, Jacobsson P, Börjesson L, Navarra MA, Fernicola A, Panero S, Scrosati B (2006) Solid State Ionics 177:2431
Mohamad AA, Mohamed NS, Yahya MZA, Othman R, Ramesh S, Alias Y, Arof AK (2003) Solid State Ionics 156:171
Johnston SF, Ward IM, Cruickshank J, Davies GR (1996) Solid State Ionics 90:39
Saravanan S, Joseph Mathai C, Anantharaman MR, Venkatachalam S, Prabhakaran PV (2006) J Phys Chem Solids 67:1496
Tareev B (1979) Physics of dielectric materials. MIR Publications, Moscow
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badr, S., Sheha, E., Bayomi, R.M. et al. Structural and electrical properties of pure and H2SO4 doped (PVA)0.7(NaI)0.3 solid polymer electrolyte. Ionics 16, 269–275 (2010). https://doi.org/10.1007/s11581-009-0391-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-009-0391-8