Abstract
Torrefaction is considered as a method for producing biofuels with improved characteristics compared to those of the “raw” biomass (higher calorific value, moisture resistance, better grindability). The torrefaction process is an endothermic process that is usually carried out in a gaseous atmosphere in the absence of oxygen. To reduce the required heat input, it is proposed to employ the oxidative torrefaction and conduct the process in a fluidized bed agitated with flue gases containing less than 6% oxygen. Preliminary studies of the oxidative torrefaction of sunflower husks, including thermogravimetric analysis of the treated material, have shown that the heat treatment time for the biomass should be at least 5 min. A fluidized bed is a reactor with ideal mixing of the treated material where uniform treatment of raw material particles cannot generally be attained. To overcome this disadvantage of the fluidization technique and achieve the required residence time for biomass in a fluidized bed during a continuous torrefaction process, it was proposed to equip a torrefaction reactor with a series of vertical baffles spaced at 50 mm. These baffles induce a loop-like flow of the processed biomass from the inlet to the outlet of the reactor. To investigate the residence time for husk particles in the reactor, a tracer, which was colored to husk particles' color with a water-soluble dye which did not change the weight and size of the particles, was injected into the bed of uncolored particles. Tracer samples were taken every 30 s at the outlet of the reactor and were analyzed using a special procedure to determine the fraction of colored particles in each sample. This enabled us to gauge the time during which the colored particles injected into the fluidized bed reached the point of their discharge from the bed. Studies performed in a “cold” model of the reactor showed that a series of vertical baffles in the bed can provide the required residence time for biomass in a reactor including commercial reactors. Plates can provide the necessary biomass residence time in the reactor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Biomass combustion is considered to release the same amount of СО2 as the plant absorbed during its growth, i.e., biomass combustion does not increase the content of greenhouse gases in the atmosphere [1, 2]. In addition, biomass is abundant since it is formed in a large amount at agricultural, forest, and wood-processing enterprises. However, wide application of biomass is hindered by the fact that it has a lower heating value in its initial state, a higher moisture content, and a lower bulk density than those of fossil fuels [3, 4].
Torrefaction is a method of low-temperature (at 200–300°С) treatment of biomass in an oxygen-free environment to cut down the moisture content of the biomass, improve its hydrophobic properties, increase the heating value, and also to render the biomass more suitable for further thermal processing by pyrolysis, gasification, or combustion [5–9].
At the same time, torrefaction is an energy-consuming process, and the use of an inert gas, nitrogen, for its implementation makes it also quite expensive. Using flue gases of a boiler or furnace rather than an inert gas is more efficient [10, 11]. The oxygen content in the flue gases can range from 6 to 14 vol % [11]. The oxidative torrefaction (OT) of biomass involves, in addition to the reactions of releasing some volatiles and oxygen, exothermic reactions reducing the required heat input and duration of the process. On the one hand, to obtain the same mass loss as that with the nonoxidative torrefaction, OT is carried out at lower temperatures [12, 13]. On the other hand, oxidative torrefaction is a more intricate process than torrefaction in an inert gas environment since this process occurs in a temperature range close to the onset of exothermic processes in the biomass during its heating and can lead to ignition of the biomass.
Since torrefied biomass is often a mixture of fine particles, it was proposed in [14] to perform oxidative torrefaction in a fluidized bed. This investigation dealt with OT of sawdust in a fluidized bed of 1.0 mm diameter glass beads in the temperature range from 240 to 300°С. The reactor feed-gas oxygen concentration varied from 0 to 9 vol %. The experiments suggest that increasing the torrefaction temperature and the feed-gas oxygen concentration considerably improves the characteristics of the biomass as a fuel (Table 1). This fact demonstrates that biofuels produced by oxidative torrefaction is more suitable for cocombustion with coal than fuels obtained in an inert atmosphere.
At the same time, biomass processing in a fluidized bed by the OT method brings about some problems. It is rather difficult to obtain uniform processing of raw materials in a fluidized bed, i.e., to attain conditions under which all heat-treated particles would have the same characteristics. This is due to the fact that the fluidized bed operates in a regime close to the ideal mixing one, i.e., the particles that have just been fed into the reactor for processing may be in the discharge zone after a short time.
Sawdust was supplied continuously into the reactor for torrefaction in [14]. The sawdust particles lost their weight as they were treated and were entrained from the fluidized bed by the gas flow. The torrefied sawdust particles were separated from the gas flow in a cyclone. However, it was found that, after 50 min of operation of the test setup, not all sawdust particles left the fluidized bed of glass beads. The sawdust accumulated in the fluidized bed increasing its height by 10–30%. The height of the fluidized bed increased at a very high rate at a torrefaction temperature ranging from 240 to 270°С. The sawdust sampled from the fluidized bed after 50 min of continuous oxidative torrefaction at a temperature of 300°С contained 56% of volatile matter and 43% of fixed carbon. At the same time, the sawdust sampled from the cyclone after the continuous oxidative torrefaction under the same conditions (50 min and 300°С) contains 78% of volatile matter and 22% of fixed carbon, i.e., the degree of uniformity of the biomass processing in the experiments of [14] was from 39 to 95%.
At present, the authors of this study are developing industrial equipment for the torrefaction of various biowastes, including sunflower husk for processing of which the method of oxidative torrefaction in a fluidized bed has been selected. To do this, we had to investigate the oxidative torrefaction of sunflower husk and design a reactor offering the required residence time for the material in the reactor to improve the uniformity of biomass treatment. The results of investigation are presented below.
EXPERIMENTAL SETUP
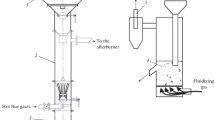
To study the process of oxidative torrefaction of sunflower husk in a fluidized bed, an experimental setup, whose schematic diagram is shown in Fig. 1, was constructed. It consists of a fluidized bed reactor with a jacket supplied with a high-temperature heat carrier headed in an electrical boiler to the required temperature. The reactor made of a 108 mm diameter steel pipe 1000 mm in height is equipped with a biomass batch feeder (at the reactor top) and a treated biomass discharge unit (at the reactor bottom).
According to [14] and [15], uniformity of biomass particles after heat treatment in a fluidized bed of inert material cannot be achieved. It is also difficult to separate biomass particles from particles of the inert material. Therefore, it was decided in this study to abandon the use of inert material. The fluidized bed in the experimental reactor is made up of ground particles of the biomass proper.
The biomass bed in the reactor is fluidized with flue gases directed to its bottom using an exhauster. The flue gases are supplied from a boiler (not shown in Fig. 1) fired with biofuel (pelletized sunflower husk).
The reactor outlet gases contain condensable and noncondensable gaseous products of the biomass torrefaction. These gases are cooled in a heat exchanger with water circulated by a pump (not shown in Fig. 1) installed between the heat exchanger and a water storage tank. Condensate formed in the heat exchanger is removed from the system, and noncondensable torrefaction gaseous products are cleaned mechanically in a cyclone and discharged into the atmosphere.
Prior to the experiment, the setup was purged with flue gases that were cooled in the heat exchanger and were analyzed after moisture removal for СО, СО2, Н2, and СН4 using a Vario Plus Industrial (SYNGAS) through-flow gas analyzer.
The torrefaction of ground sunflower husk was investigated since the husk in its original conditions could not be fluidized. These particles of the ground husk had the following fractional composition, %:
>5.0 mm | 0.02 |
From 2.0 to 5.0 mm | 6.48 |
From 1.0 to 2.0 mm | 30.84 |
From 0.4 to 1.0 mm | 50.54 |
From 0.2 to 0.4 mm | 9.72 |
From 0.09 to 0.2 mm | 2.40 |
Other characteristics of the husk and the experimental setup:
True density of husk particles, kg/m3 | 520 |
Minimum velocity of husk particles fluidization, m/s | 0.30 |
Operating gas velocity at reactor inlet, m/s | 0.45 |
The weight of ground particles of sunflower husk was measured before and after torrefaction with an Acom JW-1 laboratory balance, and the husk moisture content was measured with an Ohaus MB45 moisture analyzer.
The ash content and the volatile matter fraction were determined by a thermogravimetric analysis using a NETSCH STA 409 PC/PG thermal analyzer. Prior to measuring the ash content, volatile content and elemental composition, the samples were dried until a relative humidity of 0.1% was reached.
The elemental composition of the husk before and after heat treatment (content of C, H, N, S) was determined using an Elementar Vario Macro Cube elemental analyzer. The content of oxygen, wt %, on a dry basis was calculated from the following material balance:
where \({{C}_{{\text{C}}}},\)\({{C}_{{\text{H}}}},\)\({{C}_{{\text{N}}}},\) and \({{C}_{{\text{S}}}}\) are the content in a sample of carbon, hydrogen, nitrogen, and sulfur, respectively; \(AA\) is the raw material ash content. Both the content of elements and the raw material ash content are expressed in wt % on the dry basis.
The high heating value of the husk before and after treatment was determined using a BKS-2Kh combustion calorimeter.
In the experiments, a portion of biomass 218 g in weight was periodically loaded into the reactor. The experimental conditions were as follows:
Husk particles' stationary bed height in the reactor, mm | 100 |
Temperature of high-temperature heat carrier supplied to the reactor jacket, °C | 250 |
Temperature of flue gases at the reactor inlet, °C | 350–400 |
Temperature in the fluidized bed of ground husk particles, °C | 288 |
Biomass holding time in the reactor, min | 2.5, 5.0, 7.5 |
After holding in the reactor, the heat-treated biomass was unloaded into a tightly sealed container for subsequent analysis.
To attain the required residence time for the husk particles in the fluidized bed reactor, it was proposed to install a series of vertical baffles in the reactor. These baffles should be installed with a gap between them and the reactor wall. In addition, the first baffle in the husk particles from the reactor inlet was installed with a gap to a side wall, for example, the right wall. The next baffle had a gap with the left side wall, the third partition again had a gap with the right side wall, etc. This arrangement offered a loop like flowpath for the husk particles from the inlet to the outlet. The ideal mixing regime was observed between any two baffles with the overall reactor operating under plug flow conditions providing the required residence time for the husk particles in the reactor.
“COLD” REACTOR MODEL
A “cold” reactor model whose scheme is shown in Fig. 2 was prepared to simulate the husk particles flow in a fluidized bed reactor and obtain data required for designing an industrial reactor.
The “cold” reactor model was a 300 mm diameter cylinder 1640 mm in height. The reactor rests on an air distribution bed consisting of a 3-mm-diameter silica gel particle bed squeezed between two gratings. The silica gel bed was 70 mm thick. An air inlet chamber filled with short metal pipes 25 mm in diameter and 25 mm long is provided under the air distribution bed. The reactor model has a biomass feeder and a biomass discharge assembly. A cyclone is installed downstream of the fluidized bed reactor to separate biomass particles entrained from the fluidized bed from the air flow. The reactor was provided with a series of vertical baffles whose arrangement is shown in Fig. 3.
To investigate the residence time for the husk particles in this reactor, a tracer, which was colored to husk particles' color with a water-soluble dye, was injected into the bed of uncolored husk particles. After dye drying, these particles did not differ in weight and size from other particles of the bed.
The experiments were performed at an air velocity of 0.45 m/s corresponding to the free cross section of the reactor. The height of the stationary biomass particles' bed was 300 mm. With this bed height, the particles could not flow over the top of the baffles. Initially, the fluidized bed was made up of uncolored particles. A portion of colored particles was then loaded in a biomass hopper used to feed the fluidized bed reactor, and the colored particles were supplied continuously into the fluidized bed reactor at a rate of 500 g/min (30 kg/h). A mixture of colored and uncolored particles was sampled every 30 s at the reactor outlet. The sample volume was 40 dm3. Each experiment was repeated five times.
The samples were then processed by the procedure described in [15]. To do this, each sample was spilled in a thin, 1-particle thick layer and photographed. Each photo was then scanned from left to right and from top to bottom using a special software package to read each pixel (colored and uncolored) of the image. This yielded a fraction of colored particles in a given mixture. This enabled us to calculate the time for the colored particles injected into the fluidized bed to travel to the fluidized bed discharge assembly.
DISCUSSION OF THE RESULTS
Table 2 presents the results from chemical analysis of husk particles before and after the oxidative torrefaction in the fluidized bed at a temperature of 288°С.
As follows from Table 2, to make a high-calorie biofuel, the residence time for husk particles in the reactor may be limited to 5 min since a further increase in the torrefaction time does not considerably increase the husk heating value and noticeably reduce the volatile matter fraction. This conclusion is confirmed by the results of thermogravimetric analysis of sunflower husk samples (Fig. 4). The samples with a torrefaction time of 5 min (Fig. 4b) have nearly the same thermogravimetric characteristics as the husk samples with a torrefaction time of 7.5 min (Fig. 4c).
Figure 4 enables us to estimate the thermal effect observed in the thermogravimetric analysis of biomass. Each part of Fig. 4 demonstrates two peaks: left and right. The left peak is induced by the heat yield in the combustion of volatiles released from the husk during its heating, while the combustion of the coke residue (fixed carbon) is responsible for the right peak. The thermogravimetric analysis of the husk after OT yielded (see Figs. 4b, 4c) that the right peak becomes more gentle than that for the original husk. The area under this curve is larger than the area under the curve for the original husk, and, therefore, the thermal effect from the coke residue combustion will be greater. An increase in the OT duration from 5.0 to 7.5 min does not considerably increase the thermal residue of the heat-treated husk.
The oxidative torrefaction is a fluidized bed does not result in a noticeable change in the fractional composition of the husk. Thus, for example, a treatment of 7.5 min will yield the following particle size distribution, %:
>5.0 mm | 0.02 |
2.0 to 5.0 mm | 12.2 |
1.0 to 2.0 mm | 27.68 |
0.4 to 1.0 mm | 51.46 |
0.2 to 0.4 mm | 7.72 |
0.09 to 0.2 mm | 0.92 |
These data demonstrate that the husk particle loss due to entrainment is small.
Further, we examine the conditions that can offer the specified residence time for husk particles in the torrefaction reactor.
Figure 5 shows a photo sequence of a layer of particles from material samples taken at the “cold” model outlet.
Figure 6 shows a change in the concentration of colored particles in the samples taken at the “cold” reactor model outlet. It is evident from Fig. 6 that the percentage of colored particles in a sample taken at the model outlet does not exceed 15% 2.5 min after the onset of feeding colored particles into the “cold” reactor model. This means that, at a certain rate of particle discharge from the reactor (30 kg/h) and a certain air speed (0.3 m/s), 85% of the particles loaded into the reactor will reside in the reactor for at least 2.5 min. Five min after the onset of feeding the colored particles into the reactor, it can be guaranteed that more than 50% (more accurately 65%) of the particles will be in it for 5 min, while the residence time for the remaining particles in the apparatus will be less than 5 min. After 7.5 min from the onset of feeding the colored particles into the reactor, less than 50% (only 40%) of the particles will have a residence time of at least 7.5 min.
It should be noted that, on the one hand, the path length for the husk particles to travel along the baffles from the feeding point to the discharge point is proportional to the reactor diameter of the reactor. Therefore, increasing the reactor dimeter by a factor of 5 (up to 1500 mm), the residence time for the husk particles in the reactor will also be five times greater (while the number of baffles is kept). On the other hand, the husk particles' velocity along the baffles is proportional to the husk particles' discharge rate from the reactor. Therefore, having increased the reactor diameter by five times, we can increase the treated husk particles' discharge rate from the reactor by the same number of times, i.e., increase the reactor capacity by a factor of five. Thus, a 1.5-m diameter reactor can have a heat-treated biomass capacity of 150 kg/h. In this case, more than 50% of biomass particles will be in the reactor for a minimum of 5 min.
Further increasing the reactor capacity requires increasing the residence time for the particles in the reactor. To do this, for example, additional baffles can be installed between the existing ones to induce a loop-like low of the material along the vertical.
CONCLUSIONS
(1) The oxidative torrefaction of finely dispersed biomass, such as sunflower husk, can be carried out in a fluidized bed where the flue gases from a boiler fired with the same biomass is used as a fluidizing agent. This enables us to cut down the energy consumption in the torrefaction process with the processing time of only 5 min, which is sufficient for increasing the biomass heating value by 32.5%.
(2) It is difficult to have the required residence time for biomass in a fluidized bed reactor since this reactor operates in the ideal mixing regime.
(3) Installation of a series vertical baffles in a reactor producing a loop-like flow of the biomass from the feeding point to the discharge point is a simple design solution to make the operating conditions closer to those of a plug flow reactor without increasing the reactor’s overall dimensions.
REFERENCES
P. McNamee, P. W. R. Adams, M. C. McManus, B. Dooley, L. I. Darvell, A. Williams, and J. M. Jones, “An assessment of the torrefaction of North American pine and life cycle greenhouse gas emissions,” Energy Convers. Manage. 113, 177–188 (2016). https://doi.org/10.1016/j.enconman.2016.01.006
L. Jiang, X. Yuan, Z. Xiao, J. Liang, H. Li, L. Cao, H. Wang, X. Chen, and G. Zeng, “A comparative study of biomass pellet and biomass-sludge mixed pellet: Energy input and pellet properties,” Energy Convers. Manage. 126, 509–515 (2016). https://doi.org/10.1016/j.enconman.2016.08.035
S. Wang, G. Dai, H. Yang, and Z. Luo, “Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review,” Prog. Energy Combust. 62, 33–86 (2017). https://doi.org/10.1016/j.pecs.2017.05.004
H. Li, X. Liu, R. Legros, X. T. Bi, C. J. Lim, and S. Sokhansanj, “Pelletization of torrefied sawdust and properties of torrefied pellets,” Appl. Energy 93, 680–385 (2012). https://doi.org/10.1016/j.apenergy.2012.01.002
W.-H. Chen, J. Peng, and X. T. Bi, “A state-of-the-art review of biomass torrefaction, densification and applications,” Renewable Sustainable Energy Rev. 44, 847–866 (2015). https://doi.org/10.1016/j.rser.2014.12.039
M. A. Sukiran, F. Abnisa, W. M. A. Wan Daud, N. Abu Bakar, and S. K. Loh, “A review of torrefaction of oil palm solid wastes for biofuel production,” Energy Convers. Manage. 149, 101–120 (2017). https://doi.org/10.1016/j.enconman.2017.07.011
D. Chen, A. Gao, K. Cen, J. Zhang, X. Cao, and Z. Ma, “Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin,” Energy Convers. Manage. 169, 228–237 (2018). https://doi.org/10.1016/j.enconman.2018.05.063
D. Chen, Y. Li, M. Deng, J. Wang, M. Chen, B. Yan, and Q. Yuan, “Effect of torrefaction pretreatment and catalytic pyrolysis on the pyrolysis polygeneration of pine wood,” Bioresour. Technol. 214, 615–622 (2016). https://doi.org/10.1016/j.biortech.2016.04.058
D. Chen, J. Mei, L. Haiping, L. Yiming, L. Mengting, M. Tingting, and M. Zhongqing, “Combined pretreatment with torrefaction and washing using torrefaction liquid products to yield upgraded biomass and pyrolysis products,” Bioresour. Technol. 228, 62–68 (2017). https://doi.org/10.1016/j.biortech.2016.12.088
Y. Mei, R. Liu, Q. Yang, H. Yang, J. Shao, C. Draper, S. Zhang, and H. Chen, “Torrefaction of cedarwood in a pilot scale rotary kiln and the influence of industrial flue gas,” Bioresour. Technol. 177, 355–360 (2015). https://doi.org/10.1016/j.biortech.2014.10.113
Y. Uemura, V. Sellapah, T. H. Trinh, S. Hassan, and K. Tanoue, “Torrefaction of empty fruit bunches under biomass combustion gas atmosphere,” Bioresour. Technol. 243, 107–117 (2017). https://doi.org/10.1016/j.biortech.2017.06.057
W.-H. Chen, K.-M. Lu, W.-J. Lee, S.-H. Liu, and T.‑C. Lin, “Non-oxidative and oxidative torrefaction characterization and SEM observations of fibrous and ligneous biomass,” Appl. Energy 114, 104–113 (2014). https://doi.org/10.1016/j.apenergy.2013.09.045
C. Wang, J. Peng, H. Li, X. T. Bi, R. Legros, C. J. Lim, and S. Sokhansanj, “Oxidative torrefaction of biomass residues and densification of torrefied sawdust to pellets,” Bioresour. Technol. 127, 318–325 (2013). https://doi.org/10.1016/j.biortech.2012.09.092
Z. Wang, H. Li, C. J. Lim, and J. R. Grace, “Oxidative torrefaction of spruce-pine-fir sawdust in a slot-rectangular spouted bed reactor,” Energy Convers. Manage. 174, 276–287 (2018). https://doi.org/10.1016/j.enconman.2018.08.035
Z. Wang, Biomass Torrefaction in Slot–Rectangular Spouted Bed, Doctoral Dissertation in Engineering (Univ. of British Columbia, Vancouver, 2017).
Funding
This work was financially supported by the Ministry of Education and Science of the Russian Federation (agreement no. 074-11–2018–018 dated May 29, 2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by T. Krasnoshchekova
Rights and permissions
About this article
Cite this article
Isemin, R.L., Kuzmin, S.N., Konyakhin, V.V. et al. Development of a Fluidized-Bed Reactor for Oxidative Torrefaction of Biowastes. Therm. Eng. 67, 626–633 (2020). https://doi.org/10.1134/S0040601520090037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040601520090037